Abstract
Methicillin-resistant Staphylococcus pseudintermedius (MRSP) is an important companion animal pathogen, but few published studies have evaluated its epidemiology in primary care settings. This study determined MRSP prevalence on hand- and animal-contact surfaces in 11 small animal primary care hospitals in Washington and Idaho, USA. Overall, MRSP was isolated from at least 1 sample from 7 of 11 hospitals (64%) and from 36 of 374 total samples (10%) with no difference in prevalence between hand- and animal-contact surfaces (P = 0.51). Strain typing by pulsed-field gel electrophoresis indicated high within-hospital similarity of MRSP strains, but minimal similarity between strains from different hospitals. Indistinguishable MRSP strains were present on handand animal-contact surfaces within individual hospitals. A questionnaire was administered to a representative from each hospital. Respondents reported that animal-contact surfaces were cleaned and disinfected more frequently than hand-contact surfaces (P < 0.001). Improving hand hygiene and disinfection of hand-contact surfaces may decrease exposure of veterinary patients to MSRP.
Résumé
Prévalence de Staphylococcus pseudintermedius résistant à la méthicilline sur des surfaces en contact avec les mains et des surfaces en contact avec les animaux dans des hôpitaux de première ligne pour animaux de compagnie. Staphylococcus pseudintermedius résistant à la méthicilline (MRSP) est un agent pathogène important chez les animaux de compagnie, mais peu d’études publiées ont évalué son épidémiologie dans les sites de soins de première ligne. Dans la présente étude on détermina la prévalence de MRSP sur les surfaces de contact avec les mains et les surfaces de contact avec les animaux dans 11 hôpitaux de première ligne pour animaux de compagnie dans les états de Washington et de l’Idaho, USA. De manière globale, le MRSP fut isolé à partir d’au moins un échantillon dans 7 des 11 hôpitaux (64 %) et de 36 des 374 échantillons (10 %) sans noter de différence dans la prévalence entre les contacts main-surface ou animal-surface (P = 0,51). Le typage des souches par électrophorèse en champs pulsés indiqua une similarité intra-hôpital élevée des souches de MRSP, mais une similarité minimale entre les souches provenant d’hôpitaux différents. Des souches indistinguables de MRSP étaient présentes sur les surfaces de contact avec les mains et les animaux dans un même hôpital. Un questionnaire fut soumis à un représentant de chaque hôpital. Les répondants rapportèrent que les surfaces de contact avec l’animal étaient nettoyées et désinfectées plus fréquemment que les surfaces de contact avec les mains (P < 0,001). Une amélioration de l’hygiène des mains et de la désinfection des surfaces en contacts avec les mains pourraient diminuer l’exposition de patients vétérinaires au MSRP.
(Traduit par Dr Serge Messier)
Introduction
Methicillin-resistant Staphylococcus pseudintermedius (MRSP) is a rapidly emerging pathogen of concern in companion animals. Staphylococcus pseudintermedius colonizes the canine mouth, pharynx, nares, perineal area, and rectum and is the most frequently isolated organism from surgical site infections following canine tibial plateau leveling osteotomy procedures and from dogs presenting with pyoderma (1–6). Methicillin-resistant S. pseudintermedius is not necessarily more pathogenic than methicillin-susceptible S. pseudintermedius (MSSP), but limited antimicrobial therapy options can prolong or prevent successful treatment of MRSP infections compared to MSSP. Methicillin-resistant S. pseudintermedius is characterized by the staphylococcal cassette chromosome mec (SCCmec), which includes the mecA gene (7). This gene confers resistance to virtually all β-lactam antimicrobials including cephalosporins by coding for an altered penicillin binding protein 2a (PBP-2a) (8). Co-resistance to other important classes of antimicrobials is increasingly common (4).
Prevalence of MRSP in healthy companion animal populations has been reported to range from 0% to 4%, whereas prevalence of MRSP in dogs presented to a dermatology referral service can be as high as 40.5% (6,9). Common carriage and infection sites include external body locations that may come into contact with surfaces in the hospital environment. Staphylococci are transmitted via direct or indirect contact and can persist on surfaces for extended periods of time (10). Close human-animal relationships include behavioral interactions such as play, petting, and sharing furniture (11). In veterinary settings human-dog interactions often involve hands for such activities as performing examinations and procedures, petting, or giving treats. Hands are therefore likely to be important vehicles for indirect transmission between patients. Video surveillance in small animal hospitals found that overall hand hygiene compliance is 14% (12). This, in conjunction with the transmission route, suggests that humans, animals, and the environment play instrumental roles in movement and persistence of MRSP in veterinary hospitals.
The epidemiology of methicillin-resistant staphylococci in companion animals has primarily been reported from teaching or referral hospitals rather than community small animal primary care veterinary practices (13,14). The few studies in primary care practices have limited numbers and data on MRSP epidemiology (15,16). Small animal primary care practices may have less support than teaching or referral hospitals for infection control resources such as dedicated infection control teams, availability of continuing education, or monitoring and surveillance protocols and practices.
As a commensal organism of many healthy dogs, it is likely that MRSP is shed into any environment in which multiple dogs are present on a daily basis. In these environments, it is expected that the hands of individuals would be periodically contaminated with MRSP and might transfer the bacteria to inanimate hand-contact surfaces. In the absence of rigorous infection control practices, this contamination would contribute to the persistence of MRSP in the health care environment and increase patient exposure to this potential pathogen. We hypothesized that hand-contact surfaces in small animal primary care veterinary hospitals would be contaminated with MRSP at the same frequency as animal-contact surfaces and that the strains isolated from surfaces would be identical within an individual hospital.
Materials and methods
Hospitals
The study population consisted of 11 veterinary hospitals, 10 in Washington State and 1 in western Idaho. Hospitals were eligible for inclusion if they principally provided primary veterinary medical and surgical care to small companion animal patients. Mixed animal practices were included only if most of the patients seen were small companion animals and if the small and large animal hospitals were physically separated. Teaching, specialty, and referral hospitals were excluded. Hospitals were recruited May 2015 through January 2016 via a series of e-mails that invited participation in an observational, cross-sectional study to examine regional prevalence and epidemiology of MRSP. The recruited hospitals were purposively selected to ensure a geographically dispersed sample population. The mean distance between each hospital pair was 330.2 km (median: 342 km, range: 6 to 630 km) (Table S1, available from the authors). Summary results of environmental sampling, including the bacterial species and type of surface from which organisms were isolated, were provided to all hospitals as soon as they were available and each hospital received detailed results specific to their hospital. The Institutional Review Board of Washington State University determined that this project satisfied the criteria for exempt research.
Sample collection
Hospital visits for sample collection occurred between July 2016 and January 2017 during their regular business hours. An average of 34 samples (range: 33 to 35 samples) was collected from environmental surfaces within each hospital, half from predominantly hand-contact surfaces and half from predominantly animal-contact surfaces. Specific surface types that were sampled are identified in Table 1. Samples were collected using Swiffer electrostatic cloths (Procter & Gamble, Cincinnati, Ohio, USA); a new cloth and examination glove were used for each sample (17). The dry electrostatic cloth was wiped across the desired surface, immediately placed into a sterile sampling bag with a flat wire closure (Fisherbrand; Thermo Fisher Scientific, Waltham, Massachusetts, USA), and placed into a chilled cooler with freezer packs for transport to the laboratory. The surface areas sampled were not uniform in size across surface types because the available areas varied widely (e.g., light switch versus floor); however, despite hospital diversity, similar surfaces were sampled using a consistent technique across hospitals. For example, although makes and models of computer keyboards varied between hospitals, the method of wiping lightly across the entire top of the keyboard was constant.
Table 1.
Types of hand- and animal-contact surfaces sampled within participating hospitals.
| Hand-contact surfaces | Animal-contact surfaces |
|---|---|
| Door handlesa | Clinic floora |
| Light switchesa | Clipper bladesa |
| Computer keyboards/micea | Examination tables (examination room, treatments area, clean and dirty surgery, radiology)a |
| Kennel/cage door handles | Inside surfaces of kennels/cagesa |
| Clipper handlesa | Muzzles/Elizabethan collarsa |
| Faucet handlesa | Stethoscope diaphragm |
| Otoscope handlesa | Endotracheal tubesa |
| Drawer/cabinet handlesa | Nail clippers |
| Supply containers | Warming padsa |
| Phones/printers/fax machinesa | Food/water bowlsa |
| IV pumps (buttons/pole)a | Leashes |
| Overhead light handles | Carts/gurneysa |
| Medical charts | Oxygen monitorsa |
| Ultrasound machine (buttons/knobs) | |
| Counter tops |
Surfaces about which respondents were questioned concerning the frequency of cleaning and disinfection.
Culture and isolation
Bacterial culture and isolation were carried out at the Washington State University Paul G. Allen Center for Global Animal Health. Tryptic soy broth (TSB) (CRITERION; Hardy Diagnostics, Santa Maria, California, USA) with 2.5% NaCl (90 mL) was added to each sample bag, massaged thoroughly to evenly submerge the cloth, then incubated overnight at 35°C. The next day samples were streaked on mannitol salt agar (MSA) plates (CRITERION; Hardy Diagnostics) with 2 μg/mL of oxacillin (SIGMA-ALDRICH; Saint Louis, Missouri, USA) and incubated for 24 to 48 h at 35°C. Next, 1 to 3 yellow colonies were sub-cultured onto Columbia blood agar plates (CBA) (CRITERION; Hardy Diagnostics) and incubated for 18 to 24 h at 35°C. Isolates that exhibited beta-hemolysis on the CBA plates were submitted to the Washington Animal Disease and Diagnostic Laboratory (WADDL) for species confirmation using matrix-assisted laser desorption/ionization — time of flight (MALDI-TOF) mass spectrometry. Minimum inhibitory concentration (MIC) antimicrobial susceptibility testing of 16 antimicrobials (Table 2) was completed using broth dilution according to the Clinical Laboratory Standards Institute (CLSI) protocol and isolates were tested for inducible clindamycin resistance using a D-test (18). Only results for methicillin-resistant coagulase-positive staphylococci were requested from WADDL. For each sampling event, an unused electrostatic cloth was also placed in TSB with 2.5% NaCl (90 mL) and incubated overnight at 37°C, streaked on MSA supplemented with 2 μg/mL of oxacillin, and incubated for 24 to 48 h at 37°C. If yellow colonies appeared they were streaked on CBA and beta-hemolytic colonies were submitted to WADDL for bacterial identification using MALDI-TOF and MIC.
Table 2.
Antimicrobial susceptibilities of environmental methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolates.
| Antimicrobial | Antimicrobial class | Resistant isolates — n (%) | Susceptible isolates — n (%) | Intermediate isolates — n (%) |
|---|---|---|---|---|
| Amoxicillin/Clavulanic acid | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Ampicillin | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Cefazolin | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Cefpodoxime | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Cephalothin | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Oxacillin + 2% NaCl | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Penicillin | β-lactam | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Enrofloxacin | Quinolone | 19 (52.8) | 13 (36.1) | 4 (11.1) |
| Marbofloxacin | Quinolone | 19 (52.8) | 17 (47.2) | 0 (0.0) |
| Doxycycline | Tetracycline | 14 (38.9) | 22 (61.1) | 0 (0.0) |
| Tetracycline | Tetracycline | 14 (38.9) | 19 (52.8) | 3 (8.3) |
| Chloramphenicol | Phenicol | 1 (2.8) | 29 (80.6) | 6 (16.7) |
| Clindamycin | Lincosamide | 19 (52.8) | 17 (42.7) | 0 (0.0) |
| Gentamicin | Aminoglycoside | 21 (58.3) | 10 (27.8) | 5 (13.9) |
| Rifampin | Ansamycin | 0 (0.0) | 36 (100.0) | 0 (0.0) |
| Trimethoprim/Sulfamethoxazole | Folate pathway inhibitor | 20 (55.6) | 16 (44.4) | 0 (0.0) |
Minimum inhibitory concentration antimicrobial susceptibility testing using broth dilution was performed on all MRSP isolates (n = 36). Shown here are the proportions of isolates that were resistant, susceptible, or intermediate to 16 antimicrobials from 8 classes using CLSI breakpoints (18).
mecA detection
Methicillin resistance was confirmed through polymerase chain reaction (PCR) detection of the mecA gene in all coagulase-positive methicillin-resistant Staphylococcus species identified through MALDI-TOF and MIC. DNA was extracted using the boiled cell lysate method and PCR amplification was performed using a Bio-Rad T100 Thermal Cycler (Bio-Rad Laboratories, Hercules, California, USA). The reaction mixture contained forward primer 5′ATACTTAGTTCTTTAGCGAT-3′ and reverse primer 5′-GATAGCAGTTATATTTCTA-3′ (Eurofins Scientific, Louisville, Kentucky, USA) and the reaction conditions were 95°C for 3 min, 96°C for 30 s (30 cycles), 49°C for 30 s, 72°C for 30 s, 72°C for 5 min.
Genotyping
Pulsed-field gel electrophoresis (PFGE) was performed on all laboratory-confirmed MRSP isolates using a slightly modified Centers for Disease Control and Prevention unified pulsed-field gel electrophoresis protocol for Gram-positive bacteria (19). The restriction enzyme ApaI was used instead of SmaI and the run conditions were as follows: initial switch time of 1 s, final switch time of 11 s, and run time of 18 h. Gel images were obtained using a Bio-Rad Universal Hood III Imaging System (Bio-Rad Laboratories) and imported into Bionumerics software (Version 6.6; Applied Maths, Austin, Texas, USA). Cluster analysis was performed using the Dice similarity coefficient and the unweighted pair group method with arithmetic mean (UPGMA) algorithm with an optimization of 0%, and a tolerance no greater than 2.5% in Bionumerics (Applied Maths).
Questionnaire
A questionnaire was developed for veterinary personnel to assess perceptions about infection control behavior in veterinary hospitals (survey provided in S2, available from the authors). It was beta-tested by veterinarians at the Washington State University College of Veterinary Medicine and a co-author (DM) provided survey expertise (20,21). One author (AP) carried out an in-person interview with 1 hospital representative immediately following environmental sample collection during each hospital visit. The interviewee was the employee identified by the owner, lead veterinarian, or manager at each hospital as the best suited to answer questions about basic hospital infection control practices. Most questions were structured with balanced interval-level scale responses and a few were multiple choice or unstructured open-ended questions (S2, S3, available from the authors) (22).
Statistical methods
To detect a 10% difference in the prevalence of MRSP on handand animal-contact surfaces with an α = 0.05 (2-sided) and a power of 80%, a total of 306 samples and 10 hospitals were required (23–25). The Fisher’s exact test was used to examine potential associations using R and a significance cut-off of P = 0.05 was used (26). Ordinal variables were dichotomized. The effect of missing data was determined by carrying out a sensitivity analysis. For example, Fisher’s exact tests were carried out using both possible dichotomous response options for missing values.
Results
Environmental cultures
Forty-two hospitals received invitations and 11 agreed to participate. Samples were collected from 374 surfaces in 11 hospitals; 190 samples were obtained from hand-contact surfaces and 184 from animal-contact surfaces (Table 3). Methicillin-resistant S. pseudintermedius was isolated from at least 1 sample from 7 of 11 hospitals (64%) and from 36 of 374 total samples (10%). Within-hospital prevalence varied from 0% to 39% with a median of 3%. A small number of other coagulase positive methicillin-resistant staphylococci were recovered: Staphylococcus aureus (MRSA) was isolated from 2% (8/374) of total surfaces sampled and from at least 1 surface in 4 of 11 hospitals (36%) and Staphylococcus schleiferi subspecies coagulans was isolated from 0.8% (3/374) of total surfaces sampled and from 2 of 11 hospitals (detailed sampling results are provided in Table S4, available from the authors). Of the 36 samples from which MRSP was isolated 58.3% were hand-contact surfaces and 41.7% were animal-contact surfaces; this difference was not statistically significant (P = 0.38).
Table 3.
Isolation of methicillin-resistant Staphylococcus pseudintermedius (MRSP).
| Hand-contact surfaces | Animal-contact surfaces | All surfaces | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Hospital | Number | MRSP positive | Number | MRSP positive | Number | MRSP positive |
| A | 17 | 4 (23.5%) | 17 | 1 (5.9%) | 34 | 5 (14.7%) |
| B | 18 | 4 (22.2%) | 17 | 4 (23.5%) | 35 | 8 (22.9%) |
| C | 17 | 0 (0.0%) | 17 | 0 (0.0%) | 34 | 0 (0.0%) |
| D | 18 | 0 (0.0%) | 17 | 0 (0.0%) | 35 | 0 (0.0%) |
| E | 17 | 0 (0.0%) | 17 | 0 (0.0%) | 34 | 0 (0.0%) |
| F | 17 | 1 (5.9%) | 17 | 0 (0.0%) | 34 | 1 (2.9%) |
| G | 17 | 5 (29.4%) | 16 | 8 (50.0%) | 33 | 13 (39.4%) |
| H | 17 | 1 (5.9%) | 16 | 0 (0.0%) | 33 | 1 (3.0%) |
| I | 17 | 0 (0.0%) | 17 | 0 (0.0%) | 34 | 0 (0.0%) |
| J | 17 | 4 (23.5%) | 16 | 2 (12.5%) | 33 | 6 (18.2%) |
| K | 18 | 2 (11.1%) | 17 | 0 (0.0%) | 35 | 2 (5.7%) |
Prevalence of methicillin-resistant Staphylococcus pseudintermedius isolated from environmental surfaces of participating hospitals.
Antimicrobial resistance
All MRSP isolates (n = 36) were tested against a panel of 16 antimicrobial drugs representing 8 antimicrobial classes (Table 2). Isolates were resistant to all 7 of the β-lactams that were tested. Co-resistance to at least 1 other antimicrobial was identified in 86% (31/36) of isolates, and 72% (26/36) were multi-drug resistant (MDR, resistant to ≥ 3 classes). Five isolates (14%) were resistant to only β-lactam antimicrobials, 8 isolates (22%) were resistant to 2 or 3 antimicrobial classes, and 23 isolates (64%) were resistant to between 4 and 8 antimicrobial classes. Rifampin was the single ansamycin on the panel and was the only antibiotic to which all isolates were susceptible. Three isolates (8%) from 2 separate facilities (Hospitals A and B) were susceptible to only rifampin. All MRSP isolates were negative for inducible clindamycin resistance, but 3 of the MRSA isolates were positive. Methicillin resistance was confirmed through PCR detection of the mecA gene in all MALDI-TOF identified coagulase positive Staphylococcus isolates in this study (n = 47) that exhibited a methicillin-resistant phenotype (MIC).
Questionnaire responses
When asked about frequency of disinfection of hand- and animal-contact surfaces, 25% of responses indicated daily disinfection of hand-contact surfaces compared to 90% of responses that indicated daily disinfection of animal-contact surfaces (P < 0.001). Cleaning and disinfection of these surfaces was otherwise reported to occur on a weekly basis (or less often). In response to the question “How many veterinarians, technicians/assistants, and non-technical staff are scheduled to work on a regular business day?” it was reported that hospitals were staffed with a mean of 15 people per day (median: 14.5, range: 7 to 28). Hospitals staffed with 15 or more people per day (n = 5 hospitals) were associated with significantly higher environmental MRSP prevalence (P < 0.001). When asked about the general frequency of hand antisepsis after touching common animal-contact surfaces compared to after touching common hand-contact surfaces, 64% of respondents reported that employees always or often (compared to sometimes or rarely) practiced hand antisepsis after touching animal-contact surfaces while 9% reported that employees always or often practiced hand antisepsis after touching hand-contact surfaces (P = 0.02). In response to the question “What type of disinfectant is primarily used in your clinic: bleach, accelerated hydrogen peroxide, quaternary ammonium, unknown, or other?” the primary disinfectant was identified as a quaternary ammonium product in 5/11 hospitals, a chlorhexidine solution in 4/11 hospitals, and an accelerated hydrogen peroxide product in 2/11 hospitals. Most respondents (6/11) simply reported the product brand name and were not aware of the active ingredient in the product. The researcher (AP) confirmed the active ingredient during the questionnaire process. Few hospitals were able to report annual caseload so those data were omitted from analysis; however, 9/11 hospital representatives reported greater than 20 patient visits on a regular business day, 1 hospital reported between 16 and 20, and 1 hospital reported between 6 and 10. Number of patient visits on a regular business day was not associated with hospital staffing numbers (P = 0.45). Ten hospitals reported that they had designated isolation rooms and this was confirmed by observation. Among those 10, 4 were not prepared for immediate use because they were being used for storage or for housing staff owned animals. Resident cats were observed to roam freely indoors, including in operating theaters, in 4/11 hospitals. Significant outcomes were all robust enough to withstand vulnerabilities of the sensitivity analysis.
Genotyping
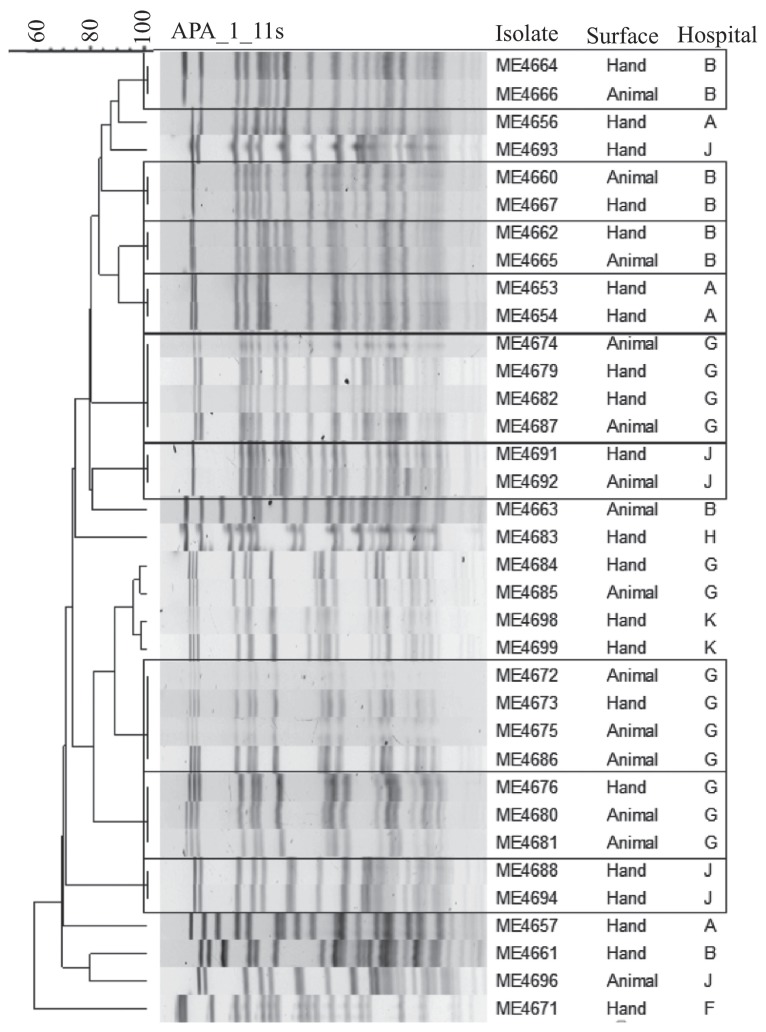
Analysis of PFGE profiles resulted in 9 clusters of indistinguishable isolates. One cluster of two indistinguishable isolates came from within hospital A, 3 clusters of 2 came from within hospital B, 1 cluster of 3 and 2 clusters of 4 came from within hospital G, and 2 clusters of 2 came from within hospital J. Every cluster of indistinguishable MRSP isolates included at least 1 that was recovered from a hand-contact surface. Indistinguishable isolates were within, but not between hospitals, but the 2 hospitals nearest to one another (approximately 6 km) (Table S1, available from the authors) had isolates with PFGE profiles that differed by a single visible band (hospitals G and K) (Figure 1). One isolate from Hospital A was unavailable for PFGE.
Figure 1.
Unweighted Pair Group Method using Arithmetic averages (UPGMA) cluster analysis of Dice similarities based on pulsed-field gel electrophoresis (PFGE) of MRSP isolates. Indistinguishable strains are enclosed in boxes.
Discussion
Our finding of MRSP contamination of an average of 10% of surfaces in 64% of small animal primary care hospitals suggests that environmental prevalence in these settings is much higher than previously thought, and that companion animal patients have multiple opportunities for exposure to MRSP in these settings. This is a critical finding for addressing the problem of MRSP because most research and interventions hitherto have focused on referral hospitals such as teaching hospitals, in which patients are referred from primary care settings such as those sampled in this study. A 2010 study of companion animal hospital environments in southern Ontario found that the hospital-level prevalences of MRSP and MRSA were 7% and 9%, respectively, which is lower than the hospital-level prevalence observed in our study (MRSP 64%, MRSA 36%) (16). Additionally, the Ontario study found a higher hospital-level prevalence of MRSA than MRSP, while our study found the opposite. The high hospital-level prevalence in this study compared to the 2010 study in Ontario could be due to geographical variation or to our smaller sample size; however, with our smaller sample size detection should have been less likely. It is also possible that our results simply reflect increased prevalence and distribution of methicillin-resistant staphylococci, a trend that has been observed over the last decade (4). In addition to the unexpectedly high environmental prevalence, we found that, while hand-contact surfaces (surfaces unlikely to have contamination introduced directly by animals) have the same high prevalence as animal-contact surfaces, hospitals reported consistently that hand-contact surfaces were cleaned infrequently if at all. These findings are novel and indicate potentially fruitful directions for research and interventions to mitigate the problem of MRSP in veterinary settings.
Hands of healthcare workers are the main way pathogens are transmitted between patients within hospitals (27,28). American Animal Hospital Association infection control guidelines include practicing hand hygiene before and after touching patients and surfaces in the patient’s environment as well as cleaning and disinfecting intensively used patient contact surfaces between each patient such as examination tables and scales (29). In the current study, veterinary hospital representatives consistently reported that hand hygiene occurred more often after touching common animal-contact surfaces compared to hand-contact surfaces and that animal-contact surfaces were cleaned and disinfected more frequently than hand-contact surfaces. These 2 findings suggest perceived differences in risk associated with the different types of surfaces because animal contact surfaces may be considered “dirtier.” They also suggest that the importance of cleaning and disinfection of hand-contact surfaces may be under-emphasized in training and continuing education for veterinary professionals.
Chlorhexidine, quaternary ammonium, and accelerated hydrogen peroxide were identified as primary hospital disinfectants. Compared with other disinfectants, accelerated hydrogen peroxide is fast acting, very safe, and highly effective against a broad spectrum of microorganisms (29). Chlorhexidine (a biguanide) and quaternary ammonium disinfectants generally have a more limited spectrum of activity and longer required contact times, respectively. They are not always effective against some organisms of veterinary concern such as Pseudomonas, bacterial spores, or non-enveloped viruses such as canine parvovirus (29–32). Despite the availability of a safer and broader-spectrum disinfectant such as accelerated hydrogen peroxide, most hospitals were using less effective agents such as chlorhexidine and quaternary ammonium compounds. The high number of hospitals reporting chlorhexidine as a primary disinfectant was of particular concern. Although chlorhexidine has antiseptic benefits such as residual activity and being non-irritating to the skin and mucous membranes, it is not typically recommended as an environmental disinfectant because it is only effective in a narrow pH range, becomes inactive in the presence of certain cleaning products, and can be an environmental toxin if improperly disposed of (29,30). The American Animal Hospital Association recommends that veterinary employees be familiar with disinfectant product labels as a crucial part of hospital biosafety (29). The frequently reported use of less effective alternative disinfectants, particularly chlorhexidine solutions, may be attributed to low cost, convenience, or availability. Alternatively, it may indicate unfamiliarity with disinfectants and industry standards. In either case there is a need for training and education, including knowledge of the spectrum of activity, appropriate dilutions, contact time, removing debris, applying soap or detergent, and allowing surfaces to dry before applying the disinfectant.
The observed resistance to β-lactam antimicrobials was consistent with the presence of the SCCmec and mecA gene, but the high level of co-resistances and the MDR profile of most of the MRSP isolates recovered suggest that MRSP can acquire resistance to many drugs used as alternatives to β-lactams, for example fluoroquinolones and aminoglycosides, which are important for human and veterinary medicine. The detection of MRSP isolates in veterinary hospital environments that are susceptible to only rifampin increases concerns about the diminishing number of safe and effective therapeutic options and increases the importance of prevention measures.
There were several important limitations to this study. Although participating hospitals were geographically dispersed across Washington and Idaho to be representative of regional hospitals, they may not be nationally or globally representative. Because this study relied on voluntary participation, the potential bias of self-selection must be considered. Hospitals with recently identified cases of MRSP may have been more likely to participate out of a desire for more knowledge, which could result in an overestimation of true prevalence. Conversely, such hospitals may be less willing to participate if concerned that identification of contaminated surfaces could damage the hospital’s reputation or would force them to implement potentially costly control measures, a scenario that could lead to underestimates of true prevalence. The finding of high hospital-level prevalence suggests that the latter did not affect the results. As field studies rely on volunteers it would be difficult to prevent this self-selection. Due to the cross-sectional study design, study investigators could not monitor hand hygiene compliance so we had to rely on reports by a single hospital representative. It is possible that hand hygiene practices varied between staff members in each hospital; however, responses regarding hand hygiene frequency were consistent across hospitals. It is likely that hand hygiene frequency was exaggerated due to self-reporting. Although most veterinary personnel agree that hand hygiene is important, video surveillance in small animal hospitals has demonstrated that actual hand hygiene compliance is very low (12,33). The discordance between self-reported versus directly monitored frequency of hand hygiene in these settings warrants further investigation. Animal healthcare providers and their patients may benefit from hand hygiene campaigns tailored towards veterinary care similar to those that are prominent in human medicine (34). The cross-sectional study design has an important role in epidemiological investigations, but has limited power to detect temporal relationships, which could be addressed with a longitudinal study. These data do, however, provide a snapshot of the high variability among hospitals and, given the cross-sectional design, suggests a high prevalence of surface contamination with MRSP. Finally, although we found equivalent prevalence of contamination on hand- and animal-contact surfaces, and identical PFGE strain types from different sites within hospitals, it is important to point out that environmental contamination does not necessarily mean that transmission and new acquisition by patients are occurring. The main objective of this study was to determine frequency of MRSP on hand- versus animal-contact surfaces as a preliminary step to inform future larger sample-sized and longitudinal studies.
The prevalence of MRSP on environmental surfaces in small animal primary care hospitals has not been thoroughly explored. For many companion animals, primary care hospitals may be the only type of veterinary facility visited. Sick or injured animals may visit a primary care hospital once or several times before being referred to a specialist, particularly in cases of non-healing or recurrent infections. Although MRSP is rarely pathogenic in humans, humans may act as transient carriers and/or transmission vectors to companion animals, particularly dogs, which are more frequently infected with MRSP (10,35). Our findings suggest that MRSP is frequently present in regional small animal primary care hospitals. Many of the isolates expressed co-resistance to multiple non β-lactam antimicrobials important for both humans and animals. Although the reservoir of MRSP is known to be small companion animals, particularly dogs, contamination was not restricted to animal-contact surfaces within the sample population. The equivalency in frequency of MRSP recovery between hand- and animal-contact surfaces suggests there is opportunity for improvement of infection control protocols in these settings, especially in areas such as hand hygiene and cleaning and disinfection. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This research was funded in part by the Washington State University College of Veterinary Medicine and the Morris Animal Foundation D17CA-019 ‘Evaluation of genotyping methods for Staphylococcus pseudintermedius at local, regional, and global scales.’
References
- 1.Devriese LA, Depelsmaecker K. The anal region as a main carrier site of Staphylococcus intermedius and Streptococcus canis in dogs. Vet Rec. 1987;121:302–303. doi: 10.1136/vr.121.13.302. [DOI] [PubMed] [Google Scholar]
- 2.Iverson SA, Brazil AM, Ferguson JM, et al. Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin-resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI) Vet Microbiol. 2015;176:202–208. doi: 10.1016/j.vetmic.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Misic AM, Davis MF, Tyldsley AS, et al. The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome. 2015 Jan 23;3:2. doi: 10.1186/s40168-014-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris DO, Loeffler A, Davis MF, Guardabassi L, Weese JS. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017 Jun;28(3):304–e69. doi: 10.1111/vde.12444. [DOI] [PubMed] [Google Scholar]
- 5.Nazarali A, Singh A, Moens NMM, et al. Association between methicillin-resistant Staphylococcus pseudintermedius carriage and the development of surgical site infections following tibial plateau leveling osteotomy in dogs. J Am Vet Med Assoc. 2015;247:909–916. doi: 10.2460/javma.247.8.909. [DOI] [PubMed] [Google Scholar]
- 6.Beck KM, Waisglass SE, Dick HLN, Weese JS. Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma. Vet Dermatol. 2012;23:369–375. doi: 10.1111/j.1365-3164.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 7.Black CC, Solyman SM, Eberlein LC, Bemis DA, Woron AM, Kania SA. Identification of a predominant multilocus sequence type, pulsedfield gel electrophoresis cluster, and novel staphylococcal chromosomal cassette in clinical isolates of mecA-containing, methicillin-resistant Staphylococcus pseudintermedius. Vet Microbiol. 2009;139:333–338. doi: 10.1016/j.vetmic.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Tian W, Lin D, et al. Prevalence and characterization of methicillin-resistant Staphylococcus pseudintermedius in pets from South China. Vet Microbiol. 2012;160:517–524. doi: 10.1016/j.vetmic.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. 2010;140:418–429. doi: 10.1016/j.vetmic.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 10.van Duijkeren E, Kamphuis M, van der Mije IC, et al. Transmission of methicillin-resistant Staphylococcus pseudintermedius between infected dogs and cats and contact pets, humans and the environment in households and veterinary clinics. Vet Microbiol. 2011;150:338–343. doi: 10.1016/j.vetmic.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz A, Hecht J. Examining dog-human play: the characteristics, affect, and vocalizations of a unique interspecific interaction. Animal Cognition. 2016;19:779–788. doi: 10.1007/s10071-016-0976-3. [DOI] [PubMed] [Google Scholar]
- 12.Anderson MEC, Sargeant JM, Weese JS. Video observation of hand hygiene practices during routine companion animal appointments and the effect of a poster intervention on hand hygiene compliance. BMC Vet Res. 2014 May 7;10:106. doi: 10.1186/1746-6148-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoet AE, Johnson A, Nava-Hoet RC, et al. Environmental methicillin-resistant Staphylococcus aureus in a veterinary teaching hospital during a nonoutbreak period. Vector-Borne Zoonotic Dis. 2011;11:609–615. doi: 10.1089/vbz.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nienhoff U, Kadlec K, Chaberny IF, et al. Methicillin-resistant Staphylococcus pseudintermedius among dogs admitted to a small animal hospital. Vet Microbiol. 2011;150:191–197. doi: 10.1016/j.vetmic.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Worthing KA, Brown J, Gerber L, Trott DJ, Abraham S, Norris JM. Methicillin-resistant staphylococci amongst veterinary personnel, personnel-owned pets, patients and the hospital environment of two small animal veterinary hospitals. Vet Microbiol. 2018;223:79–85. doi: 10.1016/j.vetmic.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Murphy CP, Reid-Smith RJ, Boerlin P, et al. Escherichia coli and selected veterinary and zoonotic pathogens isolated from environmental sites in companion animal veterinary hospitals in southern Ontario. Can Vet J. 2010;51:963–972. [PMC free article] [PubMed] [Google Scholar]
- 17.Ruple-Czerniak A, Bolte DS, Burgess BA, Morley PS. Comparison of two sampling and culture systems for detection of Salmonella enterica in the environment of a large animal hospital. Equine Vet J. 2014;46:499–502. doi: 10.1111/evj.12193. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 3rd ed. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 19.Unified Pulsed-Field Gel Electrophoresis (PFGE) Protocol for Gram positive Bacteria. CDC; [Last accessed March 24, 2020]. Available from: https://www.cdc.gov/hai/pdfs/labsettings/unified_pfge_protocol.pdf. [Google Scholar]
- 20.Moore DA, Leach DA, Bickett-Weddle D, et al. Evaluation of a biological risk management tool on large western United States dairies. J Dairy Sci. 2010;93:4096–4104. doi: 10.3168/jds.2010-3272. [DOI] [PubMed] [Google Scholar]
- 21.Pires A, Peterson A, Baron J, Adams R, Martinez-Lopez B, Moore D. Small-scale and backyard livestock owners needs assessment in the western United States. PLoS One. 2019;14:e0212372. doi: 10.1371/journal.pone.0212372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillman D, Smyth J, Christian L. Internet, Phone, Mail, and Mixed-Mode Surveys: the Tailored Design Method. 4th ed. Hoboken, New Jersey: John Wiley and Sons; 2014. [Google Scholar]
- 23.Abramson JH. WINPEPI updated: Computer programs for epidemiologists, and their teaching potential. 2011. [Last accessed March 24, 2020]. Available from: https://epi-perspectives.biomedcentral.com/articles/10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed]
- 24.Sahai H, Khurshid A. Formulae and tables for the determination of sample sizes and power in clinical trials for testing differences in proportions for the two-sample design: A review. Stat Med. 1996;15:1–21. doi: 10.1002/(SICI)1097-0258(19960115)15:1<1::AID-SIM134>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Zar J. Biostatistical Analysis. 4th ed. Upper Saddle River, New Jersey: Prentice Hall; 1998. [Google Scholar]
- 26.Core Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Last accessed March 24, 2020]. Available from: https://www.R-project.org/ [Google Scholar]
- 27.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings — Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MEC. Contact precautions and hand hygiene in veterinary clinics. Vet Clin North Am Small Anim Pract. 2015;45:343–360. doi: 10.1016/j.cvsm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Stull JW, Bjorvik E, Bub J, Dvorak G, Petersen C, Troyer HL. 2018 AAHA infection control, prevention, and biosecurity guidelines. J Am Anim Hosp Assoc. 2018;54:297–326. doi: 10.5326/JAAHA-MS-6903. [DOI] [PubMed] [Google Scholar]
- 30.Morley P, Burgess B, Van Metre D, Ouyang B Committee MotJLVIC. Infection Control and Biosecurity Standard Operating Procedures (SOP) Fort Collins, Colorado: Colorado State University; [Last accessed March 24, 2020]. Available from: http://csu-cvmbs.colostate.edu/vth/diagnostic-and-support/biosecurity-and-infection-control/Pages/area-protocol.aspx. [Google Scholar]
- 31.McDonnell GE. Antisepsis, Disinfection, and Sterilization Types, Action, and Resistance. Washington DC: ASM Press; 2007. p. 361. [Google Scholar]
- 32.Rutala WA, Weber DJ. Disinfection, sterilization, and antisepsis: An overview. Am J Infect Control. 2016;44:E1–E6. doi: 10.1016/j.ajic.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Anderson MEC, Weese JS. Self-reported hand hygiene perceptions and barriers among companion animal veterinary clinic personnel in Ontario, Canada. Can Vet J. 2016;57:282–288. [PMC free article] [PubMed] [Google Scholar]
- 34.SAVE LIVES: Clean Your Hands. Organization WH: World Health Organization; [Last accessed March 24, 2020]. Available from: http://www.who.int/gpsc/5may/en/ [Google Scholar]
- 35.Starlander G, Borjesson S, Gronlund-Andersson U, Tellgren-Roth C, Melhusa A. Cluster of infections caused by methicillin-resistant Staphylococcus pseudintermedius in humans in a tertiary hospital. J Clin Microbiol. 2014;52:3118–3120. doi: 10.1128/JCM.00703-14. [DOI] [PMC free article] [PubMed] [Google Scholar]