Abstract
PURPOSE
In oncology trials, the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) is the standard tool for reporting adverse events (AEs), but it may underreport symptoms experienced by patients. This analysis of the NRG Oncology RTOG 1203 compared symptom reporting by patients and clinicians during radiotherapy (RT).
PATIENTS AND METHODS
Patients with cervical or endometrial cancer requiring postoperative RT were randomly assigned to standard 4-field RT or intensity-modulated RT (IMRT). Patients completed the 6-item patient-reported outcomes version of the CTCAE (PRO-CTCAE) for GI toxicity assessing abdominal pain, diarrhea, and fecal incontinence at various time points. Patients reported symptoms on a 5-point scale. Clinicians recorded these AEs as CTCAE grades 1 to 5. Clinician- and patient-reported AEs were compared using McNemar’s test for rates > 0%.
RESULTS
Of 278 eligible patients, 234 consented and completed the PRO-CTCAE. Patients reported high-grade abdominal pain 19.1% (P < .0001), high-grade diarrhea 38.5% (P < .0001), and fecal incontinence 6.8% more frequently than clinicians. Similar effects were seen between grade ≥ 1 CTCAE toxicity and any-grade patient-reported toxicity. Between-arm comparison of patient-reported high-grade AEs revealed that at 5 weeks of RT, patients who received IMRT experienced fewer GI AEs than patients who received 4-field pelvic RT with regard to frequency of diarrhea (18.2% difference; P = .01), frequency of fecal incontinence (8.2% difference; P = .01), and interference of fecal incontinence (8.5% difference; P = .04).
CONCLUSION
Patient-reported AEs showed a reduction in symptoms with IMRT compared with standard RT, whereas clinician-reported AEs revealed no difference. Clinicians also underreported symptomatic GI AEs compared with patients. This suggests that patient-reported symptomatic AEs are important to assess in this disease setting.
INTRODUCTION
The standard tool used in oncology trials to report adverse events (AEs) has been the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). There are 3 general categories of AEs in the CTCAE: observable events (eg, injection site reaction), laboratory abnormalities (eg, anemia), and symptomatic events (eg, fatigue). Standard practice is for physicians or research staff to report all of these categories, but there is a growing body of evidence that physician reporting may not accurately reflect the incidence or severity of symptomatic AEs as experienced by patients.1-4 There is also evidence that patient-reported AEs complement physician-reported AEs in predicting clinical outcomes.5,6
The NRG Oncology RTOG 1203 trial is a phase III randomized trial comparing standard 4-field pelvic radiotherapy (RT) with pelvic intensity-modulated RT (IMRT) in patients with cervical or endometrial cancer requiring postoperative RT, with the primary end point of change in acute GI toxicity as reported by patients using a validated patient-reported outcome (PRO) instrument. One of the secondary objectives of the study was to assess the reporting of symptomatic AEs and their severity by patients and clinicians during RT. Patient reporting of 3 common GI symptoms during pelvic RT was performed using a 5-item PRO-CTCAE questionnaire,7 with physician reporting of the same symptoms using the CTCAE (version 4.0).8 The aim of our analysis was to compare symptom reporting by patients and clinicians during RT as well as patient-reported AEs by treatment arm.
PATIENTS AND METHODS
Study Design
Patients with cervical or endometrial cancer with indications for postoperative RT after hysterectomy based on pathologic risk factors were eligible for enrollment in this phase III multicenter randomized controlled trial. Patients were randomly assigned 1:1 to receive either standard 4-field pelvic RT or pelvic IMRT. Patients were treated to 45 or 50.4 Gy on the basis of physician preference. Five cycles of cisplatin 40 mg/m2 per week were administered at physician discretion according to predefined pathologic criteria. Patients were stratified by dose (45 v 50.4 Gy), use of chemotherapy (yes v no), and disease site (cervix v endometrium). RT details are described in detail elsewhere.9 The study protocol was approved by the institutional review board of each participating center and was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT00331760). The primary end point was a change in acute GI toxicity from baseline to 5 weeks measured with the bowel domain of the Expanded Prostate Cancer Index Composite PRO instrument, and the results of this analysis are reported elsewhere.9
Toxicity Assessments
Patient-reported toxicity was evaluated before treatment, after 23 to 25 fractions (at 5 weeks), and at 4 to 6 weeks and 1 and 3 years after completion of RT. One of the instruments used to assess patient-reported toxicity was the PRO version of the CTCAE (PRO-CTCAE).7 The PRO-CTCAE is a validated10 library of plain English questions developed to characterize the frequency and severity of treatment toxicities and the extent to which these toxicities interfere with daily activities.11 Patients completed a 5-item PRO-CTCAE GI toxicity questionnaire assessing severity and interference of abdominal pain, frequency of diarrhea, and frequency and interference of fecal incontinence over a recall period of 7 days. Patients reported toxicity on a 5-point Likert scale with regard to severity (none to very severe), interference (not at all to very much), or frequency (never to almost constantly) with 0 indicating none, not at all, or never. Patients were also asked about how many antidiarrheal medications were taken on average over the past 7 days.
Toxicity occurring after the start of treatment was reported by treating physicians using CTCAE (version 4.0) at weeks 3 and 5 of RT and then 4 to 6 weeks after RT, followed by every 6 months from the start of RT for the first 2 years, annually for the next 5 years, and at progression/relapse and death.
Statistics
The frequencies of patient- and physician-reported toxicities were tabulated as the percentage with a PRO-CTCAE score or CTCAE grade ≥ 1 and ≥ 3 after starting RT, because the occurrence and reporting of symptomatic toxicities were of interest. For the PRO-CTCAE, the highest score for each question after baseline was used for the comparison, and for the CTCAE, the highest grade was used. Physician-reported severe toxicity (CTCAE grade ≥ 3) was compared with patient-reported toxicity with high scores for severity, frequency, and/or interference (PRO-CTCAE score of 3 or 4). Physician-reported any-grade toxicity (grade ≥ 1) was compared with reports by patients of at least a little bit of interference, mild severity, and rarely occurring toxicity (score ≥ 1). McNemar’s test was used for comparisons between PRO-CTCAE and CTCAE when rates exceeded 0%. The χ2 test and, if cell counts were < 5, Fisher’s exact test were used for PRO-CTCAE between-arm comparisons. Correlations were assessed using Spearman correlation coefficients. Given that this was an exploratory analysis, a 2-sided significance level of .05 was used for all comparisons without multiplicity adjustment.
RESULTS
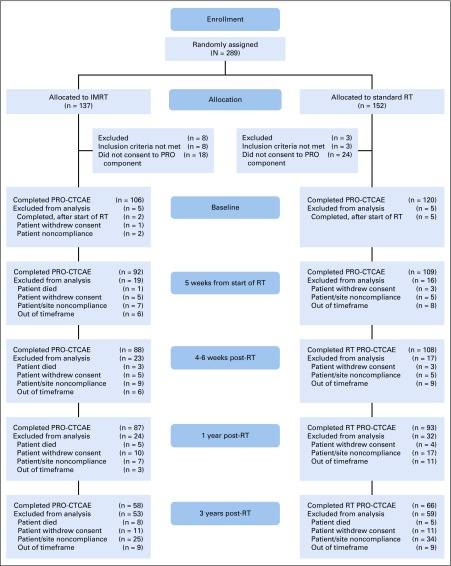
Of the 279 eligible patients, 236 consented to participate in the quality-of-life (QOL) component of this trial, which included the PRO-CTCAE questionnaire, and 234 patients had PRO-CTCAE data at ≥ 1 time points and were observed for CTCAE reporting. PRO-CTCAE patient-reporting compliance was generally high at all time points (95.8% at baseline, 85.2% at week 5 of RT, 83.1% at 4-6 weeks after RT, 76.3% at 1 year after RT, and 52.5% at 3 years after RT; Fig 1). The median follow-up time for all patients was 37.8 months (range, 0.33-66.2 months).
FIG 1.
CONSORT diagram. PRO-CTCAE, patient-reported outcomes version of Common Terminology Criteria for Adverse Events; IMRT, intensity-modulated radiotherapy; RT, radiotherapy.
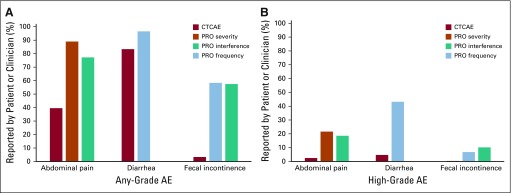
Overall, patients reported more AEs than clinicians (Fig 2). The clinician-reported any-grade CTCAE abdominal pain rate was 35.6%, compared with 80.1% of patients reporting at least mild abdominal pain and 69.5% reporting that it interfered with usual activities at least a little bit (P < .0001 for both). The grade ≥ 3 CTCAE toxicity rate was 2.5%, whereas 21.6% of women reported severe or very severe abdominal pain and 18.6% reported that their abdominal pain interfered with their activities quite a bit or very much (P < .0001 for both). CTCAE abdominal pain was correlated with the corresponding PRO-CTCAE items (severity: ρ = 0.26; P < .001; interference: ρ = 0.25; P < .001).
FIG 2.
(A) Comparison of any-grade clinician-reported (by Common Terminology Criteria for Adverse Events [CTCAE]) and patient-reported (by patient-reported outcomes version of CTCAE [PRO-CTCAE]) symptomatic adverse events (AEs). Includes AEs of any CTCAE grade or PRO-CTCAE score ≥ 1. All comparisons between PRO-CTCAE and CTCAE were P < .05. (B) Comparison of high-grade clinician-reported (CTCAE) and patient-reported (PRO-CTCAE) symptomatic AEs. Includes AEs of CTCAE grade or PRO-CTCAE score ≥ 3. PRO-CTCAE score of 3 or 4 represents AE frequency of frequently or almost constantly, AE severity of severe or very severe, or AE interference with usual or daily activities of quite a bit or very much. All comparisons between PRO-CTCAE and CTCAE were P < .05, except for fecal incontinence.
The rate of any-grade CTCAE diarrhea was 75%, compared with 86.9% of patients reporting diarrhea occurring at least rarely (P = .0002). The grade ≥ 3 CTCAE diarrhea toxicity rate was 4.7%, whereas the rate of patients reporting diarrhea that occurred frequently or almost constantly was 43.2% (P < .0001). Diarrhea reported by the physician was correlated with that reported by the patient (ρ = 0.30; P < .001).
The rate of any-grade CTCAE fecal incontinence was 3.0%, compared with 52.5% of patients reporting fecal incontinence occurring at least rarely and 51.7% of patients reporting that their fecal incontinence interfered with their usual activities at least rarely (P < .0001 for both). The rate of grade ≥ 3 CTCAE fecal incontinence was 0%, whereas 6.8% of women reported frequent or almost constant fecal incontinence and 10.2% reported that their fecal incontinence interfered with their usual activities frequently or almost constantly. Because of the low reporting of fecal incontinence by the physicians, the correlation with the corresponding PRO-CTCAE items was not significant (frequency: ρ = 0.10; P = .13; interference: ρ = 0.12; P = .071).
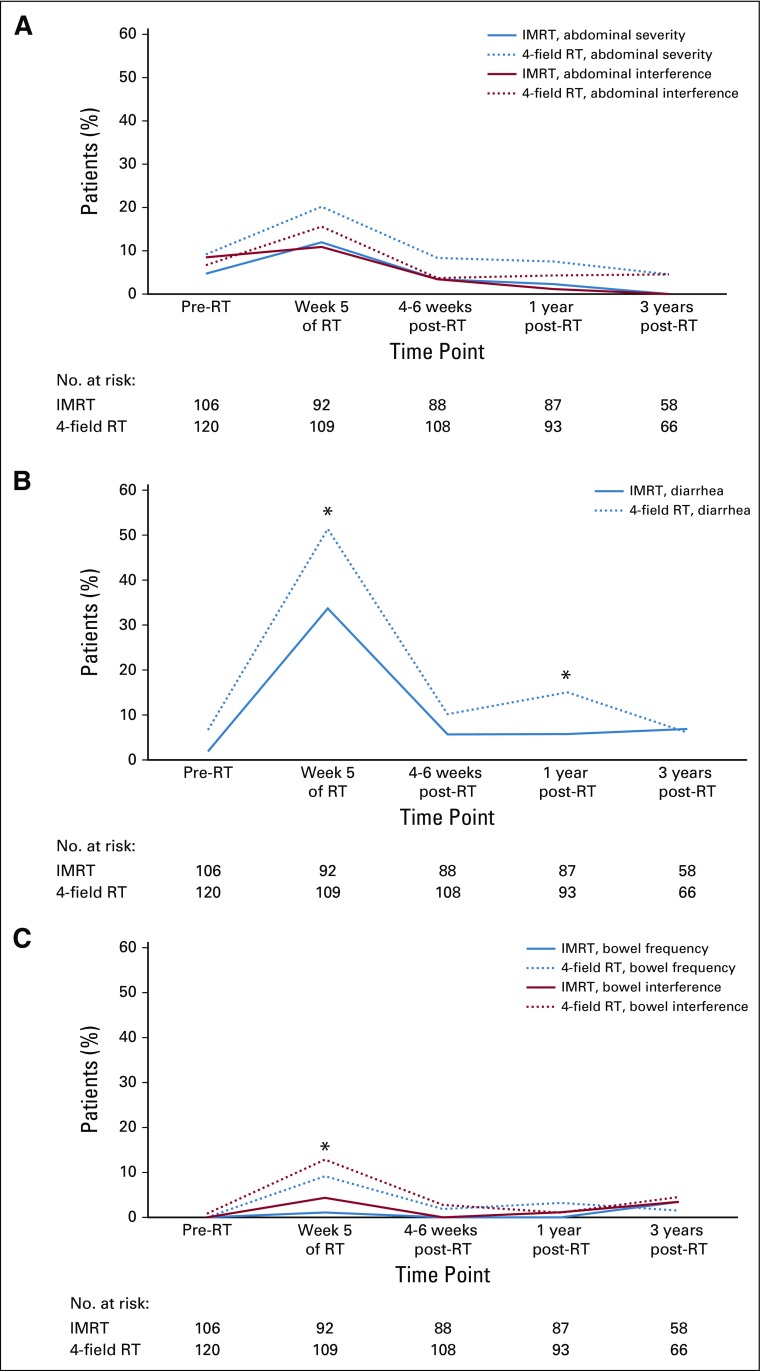
Between-arm comparisons of whether a patient had a PRO-CTCAE score ≥ 3 (AE frequency rating of frequently or almost constantly, severity rating of severe or very severe, or interference with usual or daily activities rating of quite a bit or very much) were conducted at multiple time points (Fig 3). There were no between-arm differences at baseline. At 5 weeks, patients who received IMRT experienced significantly less toxicity than patients who received 4-field pelvic RT in terms of frequency of diarrhea (33.7% v 51.9%, respectively; P = .01), frequency of fecal incontinence (1.1% v 9.3%, respectively; P = .01), and interference of fecal incontinence (4.4% v 12.9%, respectively; P = .04). There were no differences for severity or interference of abdominal pain at 5 weeks. There were no significant between-arm differences at 4 to 6 weeks or 3 years after RT. At 1 year after RT, more patients in the 4-field pelvic RT arm reported a higher frequency of diarrhea as compared with patients in the IMRT arm (15.1% v 5.8%; P = .042). There was also a significant difference at 1 year in the number of women requiring antidiarrheal medication ≥ 2 times per day (4.6% with IMRT v 13.0% with 4-field pelvic RT; P = .036), suggesting a small improvement in early toxicity with IMRT, which disappeared by 3 years after RT.
FIG 3.
(A) Percentage of patients with high-grade (score ≥ 3) abdominal pain severity and interference by arm. (B) Percentage of patients with high-grade diarrhea frequency by arm. (C) Percentage of patients with high-grade fecal incontinence frequency and interference by arm. IMRT, intensity-modulated radiotherapy; RT, radiotherapy. (*) P < .05.
DISCUSSION
Our analysis of the NRG Oncology RTOG 1203 toxicity data revealed that clinician-reported GI AEs significantly underrepresented the symptomatic AEs experienced and reported by patients during pelvic RT. To our knowledge, this is the first reporting of a large-scale multi-institutional phase III trial using both PRO-CTCAE and clinician-reported CTCAE scores to assess symptomatic AEs. In this trial, physician-reported AEs revealed no difference between arms,9 whereas patient-reported AEs showed a reduction in symptoms with IMRT compared with standard RT. These findings suggest that patient-reported symptomatic AEs may be more sensitive than physician-reported AEs, which is important when comparing toxicity between 2 treatments.
Other smaller studies have shown similar results. A phase II study of chemotherapy for metastatic prostate cancer compared physician- with patient-reported symptoms and found that physician reporting was neither sensitive nor specific in detecting common chemotherapy AEs.12 Another phase II study of RT in oropharyngeal cancer found that the severity of symptoms reported by physicians was lower than that reported by patients.13 RTOG 0126, the only other phase III trial to use patient-reported AEs to compare 2 treatment modalities, yielded different results from our study. The trial was designed to compare RT doses for prostate cancer but also analyzed patient-reported AEs for men in the high-dose RT arm treated with either 3-dimensional conformal RT (3DCRT) or IMRT. Unlike our trial, however, RTOG 0126 actually showed no difference in patient-reported symptoms between 3DCRT and IMRT,1 whereas physician-reported toxicity showed a reduction in acute grade ≥ 2 combined GI/genitourinary toxicity and late grade ≥ 2 GI toxicity in patients treated with IMRT.14 Several possible explanations may account for the discrepancy in our results. First, in RTOG 0126, patients were not randomly assigned to IMRT or 3DCRT. Instead, treatment modality was determined at physician discretion, which may have introduced sampling bias. Second, the PRO instrument used in RTOG 0126 (Functional Alterations Due to Changes in Elimination [FACE]) was administered to patients beginning at 3 months, meaning that any differences in patient-reported acute toxicity between treatment modalities were not measured. Also, the FACE instrument does not address rectal bleeding, which was the most common physician-reported late GI toxicity in RTOG 0126.
Although the PRO-CTCAE questions mirror the CTCAE reporting items, there are challenges in directly comparing the 2 tools. For example, a patient who loses control of bowel movements once a day may report frequent fecal incontinence, which would correspond to high-level toxicity in this comparison. By CTCAE criteria, however, this symptom would correspond to grade 1 or 2 fecal incontinence, depending on the number of pads required (grade 1, occasional use of pads required; grade 2, daily use of pads required). The PRO-CTCAE questions ask patients to grade the severity, frequency, and bother of their own symptoms, a process that is inherently subjective. The corresponding CTCAE items and grades are designed for clinicians to objectively grade the severity of symptoms that patients are experiencing. And in fact, for many of the symptomatic AEs, the patient must first communicate to the clinician that he or she is having a specific symptom, which can also lead to discordant comparisons. Therefore, there is no straightforward way to compare the level or severity of physician- and patient-reported symptomatic AEs.
However, it is much more straightforward to compare the total reported symptomatic AEs regardless of severity. For example, any fecal incontinence experienced by the patient should be reported as at least a grade 1 fecal incontinence by the clinician. Our data show that this is not occurring (Fig 2A). Clinicians reported that only 3.0% of patients had any-grade fecal incontinence, compared with 52.5% of patients reporting fecal incontinence of any frequency and 51.7% of patients reporting at least some interference with daily activities. There are multiple explanations for this discrepancy. Clinicians may not ask about a specific symptom when assessing toxicity, especially when they do not expect patients to have the specific symptom. This may explain the dramatic difference in reporting rates for fecal incontinence. Also, clinicians frequently manage severe symptoms, which may skew their perceptions of both symptom presence and severity. Additionally, as stated previously, patients must be able to communicate their symptoms to clinicians, which adds another barrier to accurate clinician assessment of symptomatic AEs. For some patients, discussing symptoms that they perceive to be embarrassing can be quite difficult, especially if the clinician does not introduce the symptom by asking about it. This discrepancy in the total reported symptomatic AEs highlights the importance of using PRO measures to gain a better, more accurate assessment of symptomatic AEs.
There are multiple reasons why accurate assessment of symptomatic AEs is important. First, PROs might provide valuable information with regard to overall survival (OS). There have been several large pooled studies showing that incorporating health-related QOL scales alongside clinician AE reporting improved the accuracy of predicting OS.15,16 Another pooled analysis from 14 European Organisation for Research and Treatment of Cancer trials demonstrated that patients’ self-assessment of symptoms and clinician-reported toxicity both made a positive and additive contribution to the predictive accuracy of survival models.6 A single-institution study showed that patient-reported AEs are independent predictors for survival.17
Second, it is important to give patients who are deciding about a potential treatment an understanding of the symptomatic AEs from the perspective of their peers, in addition to the perspective of treating physicians. Treatment options for patients with cancer have increased in recent years, each with its own AEs for drugs or interventions. Patients are faced with making difficult treatment decisions and need the best available AE data to assist them in this process. Knowing that 47% of patients who receive pelvic RT experience fecal incontinence, as opposed to the physician-reported rate of 1% in our study, may have a significant impact on a patient’s decision to undergo pelvic external-beam RT. Including patients in shared decision making requires they have access to information that is relevant to them, which should include data from their peers on symptomatic AEs.18-21
There are several potential limitations to our analysis. An interval time point comparison between patient and clinician ratings was not feasible, because the clinician-reported CTCAE AEs were assessed on a continuous basis and reported at specific time intervals. Therefore, if a patient were hospitalized after a reporting time and the AE resolved before the next reporting period, the AE still had to be reported. This is in contrast to patient-reported toxicity in that an AE experienced between reporting periods may not have been caught if it was outside the recall window, which is 7 days for the PRO-CTCAE. Also, there are challenges in attempting to directly compare patient- and clinician-reported symptomatic AEs. Patient compliance in completing the PRO-CTCAE questionnaire, although generally high, was not 100%, and it was only approximately 50% at the 3-year time point. Additionally, there may be confounders that were not adjusted for in this analysis. Because both the CTCAE and PRO-CTCAE report toxicity, confounders that contribute to more severe toxicity outcomes would affect both measures, but the effects may not be equal. In light of these limitations, it is important to note that the results of our analysis should not lead us to dismiss the utility of physician-reported AEs or exclusively use patient-reported AEs. Rather, this analysis suggests that patient-reported AEs are complementary to physician-reported CTCAE data.
In conclusion, our findings strongly support the use of PROs as part of symptomatic AE assessment in oncology clinical trials. Patients are the most qualified to report on their symptoms, and it is clear from the data that clinicians underreport symptoms. In trials for which the primary end point is a symptomatic AE, strong consideration should be given to using PROs as the primary toxicity outcome measure.
SUPPORT
Supported by Grants No. U10CA21661, U10CA37422, CA81647, U24CA180803, U10CA180868, U10CA180822, and UG1CA189867 from the National Cancer Institute.
AUTHOR CONTRIBUTIONS
Conception and design: Stephanie L. Pugh, Ann H. Klopp, Karen M. Gil, Lari Wenzel, Shannon N. Westin, William Small Jr, Deborah W. Bruner, Lisa A. Kachnic
Financial support: Deborah W. Bruner
Administrative support: Deborah W. Bruner
Provision of study material or patients: William Small Jr, Spencer Thompson, Amy Chang, Vijayananda Kundapur, Dasarahally S. Mohan, Catherine L. Ferguson, Deborah W. Bruner
Collection and assembly of data: Stephanie L. Pugh, Ann H. Klopp, William Small Jr, Spencer Thompson, Desiree E. Doncals, Brian P. Yaremko, Amy Chang, Vijayananda Kundapur, Dasarahally S. Mohan, Yong Bae Kim, Catherine L. Ferguson, Deborah W. Bruner
Data analysis and interpretation: Anamaria R. Yeung, Ann H. Klopp, Karen M. Gil, Lari Wenzel, Shannon N. Westin, David K. Gaffney, Guilherme H.C. Cantuaria, Dasarahally S. Mohan, Michael L. Haas, Snehal Deshmukh, Deborah W. Bruner, Lisa A. Kachnic
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephanie L. Pugh
Research Funding: Pfizer/Astellas Pharma (Inst), Millennium Pharmaceuticals (Inst)
Lari Wenzel
Consulting or Advisory Role: Array BioPharma
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Casdin Capital, Medscape, Clovis Oncology, Watermark Research Partners, Gerson Lehrman Group, Vaniam Group, Tesaro, Merck, Pfizer, Bioascend, Curio Sciences, Novartis, OncLive, Circulogene Theranostics, Targeted Oncology, GlaxoSmithKline
Research Funding: AstraZeneca, Novartis, Karyopharm Therapeutics (I), Celgene (I), Bayer, Tesaro, Kite Pharma (I), Cotinga Pharmaceuticals, Clovis Oncology, Roche/Genentech, ArQule, GOG Foundation (Inst)
William Small Jr
Honoraria: Carl Zeiss Meditec
Consulting or Advisory Role: Varian Medical Systems, Merck
Research Funding: Carl Zeiss Meditec
Travel, Accommodations, Expenses: Carl Zeiss Meditec
Spencer Thompson
Travel, Accommodations, Expenses: Mevion Medical Systems
Desiree E. Doncals
Research Funding: NRG Oncology, DCISionRT
Dasarahally S. Mohan
Employment: Permanente Medical Group
Yong Bae Kim
Honoraria: Accuray Incorporate
Research Funding: Accuray Incorporate
Travel, Accommodations, Expenses: Accuray Incorporate
Lisa A. Kachnic
Consulting or Advisory Role: INSYS Therapeautics, TRM Oncology
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121:2422–2430. doi: 10.1002/cncr.29362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin MS, Lubeck DP, Henning JM, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: Results of the CaPSURE database. J Urol. 1998;159:1988–1992. doi: 10.1016/S0022-5347(01)63222-1. [DOI] [PubMed] [Google Scholar]
- 3.Grossman SA, Sheidler VR, Swedeen K, et al. Correlation of patient and caregiver ratings of cancer pain. J Pain Symptom Manage. 1991;6:53–57. doi: 10.1016/0885-3924(91)90518-9. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey—The Fatigue Coalition. Semin Hematol. 1997;34(suppl 2):4–12. [PubMed] [Google Scholar]
- 5.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103:1851–1858. doi: 10.1093/jnci/djr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health: NCI PRO-CTCAE Items–English: Item Library Version 1.0. https://healthcaredelivery.cancer.gov/pro-ctcae/pro-ctcae_english.pdf.
- 8. US Department of Health and Human Services, National Cancer Institute, National Institutes of Health: Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
- 9. Klopp AH, Yeung AR, Deshmukh S, et al: A phase III randomized trial comparing patient reported toxicity and quality of life (QOL) during pelvic IMRT as compared to conventional RT. https://www.astro.org/uploadedFiles/_MAIN_SITE/News_and_Publications/News_and_Media_Center/Press_Kits/2016/Annual_Meeting/Content_Pieces/Klopp_slides.pdf. [Google Scholar]
- 10.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1:1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3:1043–1050. doi: 10.1001/jamaoncol.2016.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Falchook AD, Green R, Knowles ME, et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2016;142:517–523. doi: 10.1001/jamaoto.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalski JM, Yan Y, Watkins-Bruner D, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 16.Qi Y, Schild SE, Mandrekar SJ, et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol. 2009;4:1075–1082. doi: 10.1097/JTO.0b013e3181ae27f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinten C, Martinelli F, Coens C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer. 2014;120:302–311. doi: 10.1002/cncr.28382. [DOI] [PubMed] [Google Scholar]
- 18.Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 19.Trask PC, Hsu MA, McQuellon R. Other paradigms: Health-related quality of life as a measure in cancer treatment: Its importance and relevance. Cancer J. 2009;15:435–440. doi: 10.1097/PPO.0b013e3181b9c5b9. [DOI] [PubMed] [Google Scholar]
- 20.Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: The European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25:5082–5086. doi: 10.1200/JCO.2007.11.3183. [DOI] [PubMed] [Google Scholar]
- 21.Bruner DW. Outcomes research in cancer symptom management trials: The Radiation Therapy Oncology Group (RTOG) conceptual model. J Natl Cancer Inst Monogr. 2007;2007:12–15. doi: 10.1093/jncimonographs/lgm004. [DOI] [PubMed] [Google Scholar]