Abstract
PURPOSE
The previously published single institution randomized prospective trial failed to show superiority in the 5-year biochemical and/or clinical disease failure (BCDF) rate with moderate hypofractionated intensity-modulated radiation therapy (H-IMRT) versus conventionally fractionated IMRT (C-IMRT). We now present 10-year disease outcomes using updated risk groups and definitions of biochemical failure.
METHODS
Men with protocol-defined intermediate- and high-risk prostate adenocarcinoma were randomly assigned to receive C-IMRT (76 Gy in 38 fractions) or H-IMRT (70.2 Gy in 26 fractions). Men with high-risk disease were all prescribed 24 months of androgen deprivation therapy (ADT) and had lymph node irradiation. Men with intermediate risk were prescribed 4 months of ADT at the discretion of the treating physician. The primary endpoint was cumulative incidence of BCDF. We compared disease outcomes and overall mortality by treatment arm, with sensitivity analyses for National Comprehensive Cancer Network (NCCN) risk group adjustment.
RESULTS
Overall, 303 assessable men were randomly assigned to C-IMRT or H-IMRT. The median follow-up was 122.9 months. Per updated NCCN risk classification, there were 28 patients (9.2%) with low-risk, 189 (62.4%) with intermediate-risk, and 86 (28.4%) with high-risk prostate cancer. The arms were equally balanced for clinicopathologic factors, except that there were more black patients in the C-IMRT arm (17.8% v 7.3%; P = .02). There was no difference in ADT use (P = .56). The 10-year cumulative incidence of BCDF was 25.9% in the C-IMRT arm and was 30.6% in the H-IMRT arm (hazard ratio, 1.31; 95% CI, 0.82 to 2.11). The two arms also had similar cumulative 10-year rates of biochemical failure, prostate cancer–specific mortality, and overall mortality; however, the 10-year cumulative incidence of distant metastases was higher in the H-IMRT arm (rate difference, 7.8%; 95% CI, 0.7% to 15.1%).
CONCLUSION
H-IMRT failed to demonstrate superiority compared with C-IMRT in long-term disease outcomes.
INTRODUCTION
Prostate cancer is the most common solid malignancy in men, with more than 1.4 million new occurrences diagnosed per year worldwide.1 External beam radiation therapy is one treatment option for localized prostate cancer and can be used alone or with systemic therapy, such as androgen deprivation therapy (ADT). Improvements in image guidance and the adaptation of intensity-modulated radiation therapy (IMRT) have increased confidence in the precision of targeting of the prostate gland and avoidance of the surrounding normal tissue. This has led to successful escalation of radiation doses and increased interest in hypofractionation for prostate cancer.
Moderate hypofractionation, loosely defined as doses per fraction between 2.1 and 3.4 Gy, has several theoretical potential advantages: increased disease control, decreased normal tissue toxicity, patient convenience, reduced treatment cost, and improvement in medical resource utilization. Randomized trials of moderate hypofractionation have reported promising early outcomes.2-5 As a result, the American Society for Radiation Oncology (ASTRO), American Society of Clinical Oncology, and American Urological Association have published guidelines supporting the use of moderate hypofractionation.6 Retrospective efficacy and safety for prostate hypofractionation exists.7 However, the natural history of prostate cancer is long,8 and long-term data from randomized trials are lacking, especially for high-risk prostate cancers.9,10 This may cause hesitation in the adoption of moderate hypofractionation.11 In this study, we present long-term disease outcomes of a phase III prospective, randomized trial comparing conventionally fractionated IMRT (C-IMRT) with moderate hypofractionated IMRT (H-IMRT).
METHODS
Protocol Design
Men with localized prostate cancer were randomly assigned to C-IMRT (76 Gy in 38 fractions at 2 Gy per fraction) or H-IMRT (70.2 Gy in 26 fractions at 2.7 Gy per fraction). The H-IMRT arm was hypothesized to have an equivalent dose in 2 Gy per fractions (EQD2) of 84.4 Gy, assuming an α/β ratio of 1.5. The study was designed to demonstrate improved biochemical and/or clinical disease failure (BCDF) among men treated with H-IMRT compared with C-IMRT. It was powered to find a 15% difference in the cumulative incidence of BCDF at 4 years. The anticipated BCDF improvement in the H-IMRT arm was derived from the Fox Chase Cancer Center prostate database. The use of ADT was considered during the calculation. A sample size of 300 randomly assigned patients would achieve 90% power to detect a hazard ratio (HR) of 0.46 with an α of .05 using a two-sided log-rank test. The current article is the long-term update of the previously reported Fox Chase Cancer Center trial; this update was not included in the initial study design and was not included as part of the initial study design.
Patient Selection
Men with protocol-defined intermediate- or high-risk prostate adenocarcinoma at a single institution were eligible for the trial. The protocol defined intermediate-risk prostate cancer as a Gleason score of 7, a pretreatment initial prostate-specific antigen (iPSA) of 10-20 ng/mL, or ≥3 biopsy cores with Gleason scores ≥ 5 and without any high-risk features. Protocol-defined high-risk prostate cancer was a Gleason score of 8-10, a Gleason score of 7 in ≥ 4 cores, clinical T3 classification, or an iPSA > 20 ng/mL. Patients with intermediate-risk disease were prescribed 4 months of ADT at the discretion of the treating physician. All patients with high-risk disease were prescribed ADT for 2 years. Risk groups were reclassified by the National Comprehensive Cancer Network (NCCN) prostate cancer risk groups for this analysis.12
Patients were ineligible if they had history of ADT for > 4 months before random assignment, an iPSA of > 80 ng/mL, prior pelvic radiotherapy, prior radical prostatectomy, or prior malignancy other than nonmetastatic skin cancers or early-stage small lymphocytic lymphoma within the last 5 years.
Radiation Treatment Planning and Delivery
The simulation and treatment planning techniques were described in detail previously.13 Patients were simulated with a moderately full bladder in the supine position. Computed tomography and magnetic resonance imaging (MRI) simulation were performed, unless an MRI was contraindicated, and fused. Ultrasound image guidance was used for interfraction prostate position corrections.
The first clinical target volume (CTV1) included the prostate and proximal seminal vesicles. CTV2 contained the distal seminal vesicles, and CTV3 contained the pelvic lymph nodes (periprostatic, periseminal vesicle, external iliac, obturator, and internal iliac lymph nodes). CTV2 and CTV3 were contoured only for patients who met the original classification for high-risk prostate cancer. Planning target volumes (PTVs) for C-IMRT were created by adding an 8-mm margin in all dimensions except posteriorly (there, a 5-mm margin). For H-IMRT, PTVs were created by adding 7 mm in all dimensions except posteriorly (there, 3 mm). The PTV margins differences were planned so that the 90% isodose line for H-IMRT plans would include the same volume as the 100% isodose line in C-IMRT plans. The PTV margins for H-IMRT were smaller to reduce the potential increased risk of complications from hypofractionation.
The prescription dose in the C-IMRT arm was 76 Gy to PTV1 and 56 Gy to PTV2 and PTV3 in 38 fractions. The prescription dose in the H-IMRT arm was 70.2 Gy to PTV1 and 50-52 Gy (most received 50 Gy) to PTV2 and PTV3 in 26 fractions. For C-IMRT, the rectal constraints were V65Gy (volume receiving at least 65 Gy) ≤ 17% and V40Gy ≤ 35%, and the bladder constraints were V65Gy ≤ 25% and V40Gy ≤ 50%. For H-IMRT, the bladder constraints were V50Gy ≤ 17% and V31Gy ≤ 35%, and the rectal constraints were V50Gy ≤ 25% and V31Gy ≤ 50%. There were no protocol violations in the target or organs at risk dose constraints.
Statistics
The primary endpoint was the cumulative incidence of BCDF, measured from the start of radiation to earliest failure or start of salvage therapy. Although the study was originally designed using the ASTRO definition of biochemical failure (BF), we report BF using the now more widely accepted Phoenix definition (nadir PSA plus 2 ng/mL). Clinical failure included local failure (local progression or prostate biopsy proven disease with increasing PSA) or regional/distant metastasis (radiographic or pathologic), and those without BCDF were censored at date of the most recent PSA measurement. Patients with no contact for more than 3 years were considered lost to follow-up. For recurrence and mortality outcomes, patients who were alive or lost to follow-up without the event were censored as of their status date. Risk groups were based on NCCN-defined categories. We assessed differences in characteristics by treatment arm with χ2, t, or Wilcoxon rank sum tests, as appropriate.
For nonmortality outcomes, we estimated cumulative incidence distributions and considered death as a result of any cause a competing risk. For prostate cancer–specific mortality (PCSM), death as a result of other or unknown cause was treated as a competing risk. For each outcome, we used bootstrap methods with 10,000 resamples to estimate percentile-based 95% CIs for cumulative incidence by arm and for the difference between the arms. The cumulative incidence of overall mortality, rather than overall survival, was reported for ease of comparison with the other outcomes.
For the BCDF, BF, metastatic, and PCSM outcomes, study arms were compared using Gray’s test, and overall mortality distributions used the log-rank test. Unadjusted subdistribution HRs were used to estimate the relative differences by study arm (H-IMRT v C-IMRT) for each outcome using competing risk regression except for overall mortality, for which Cox proportional hazards regression was used. HRs were assessed using Wald χ2 tests. The study arms were balanced on all characteristics except ethnicity, so we also reported ethnicity-adjusted HRs.
In a sensitivity analysis of differences in BCDF by arm, we adjusted for known risk factors, including age, NCCN risk group, and actual hormone duration for BCDF. Analyses were performed in the intention-to-treat arms using two-sided 5% significance levels for hypothesis tests calculated with SAS statistical software (version 9.4, SAS Institute, Cary, NC) except for bootstrap analyses and cumulative incidence plots, for which Stata (version 15, StataCorp, College Station, TX) was used.
RESULTS
Patients
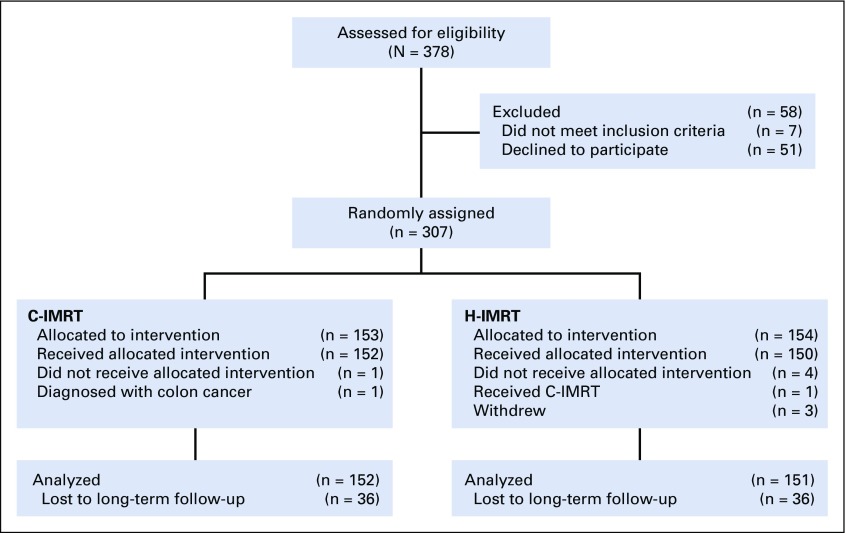
Between June 2002 and May 2006, 303 men were randomly assigned to the C-IMRT arm (n = 152) or the H-IMRT arm (n = 151), and data were analyzed (Fig 1). The two treatment groups were balanced on age, Gleason score, clinical T classification, iPSA, ADT usage, and NCCN prostate risk group (Table 1). There was a higher proportion of black men in the C-IMRT arm (17.8 v 7.3%; P = .02). The median and mean follow-up times for the whole cohort were 122.9 months and 107.7 months, respectively (range, 7-181 months). The median follow-up was similar between the two treatment groups (P = .19). Of those still alive, the median follow-up period was 130.9 months (range, 39-171 months) for C-IMRT and 128.5 months (range, 25-182 months) for H-IMRT.
FIG 1.
CONSORT diagram. C-IMRT, conventionally fractionated intensity-modulated radiation therapy; H-IMRT, moderate hypofractionated intensity-modulated radiation therapy.
TABLE 1.
Patient and Disease Characteristics by Treatment Arm
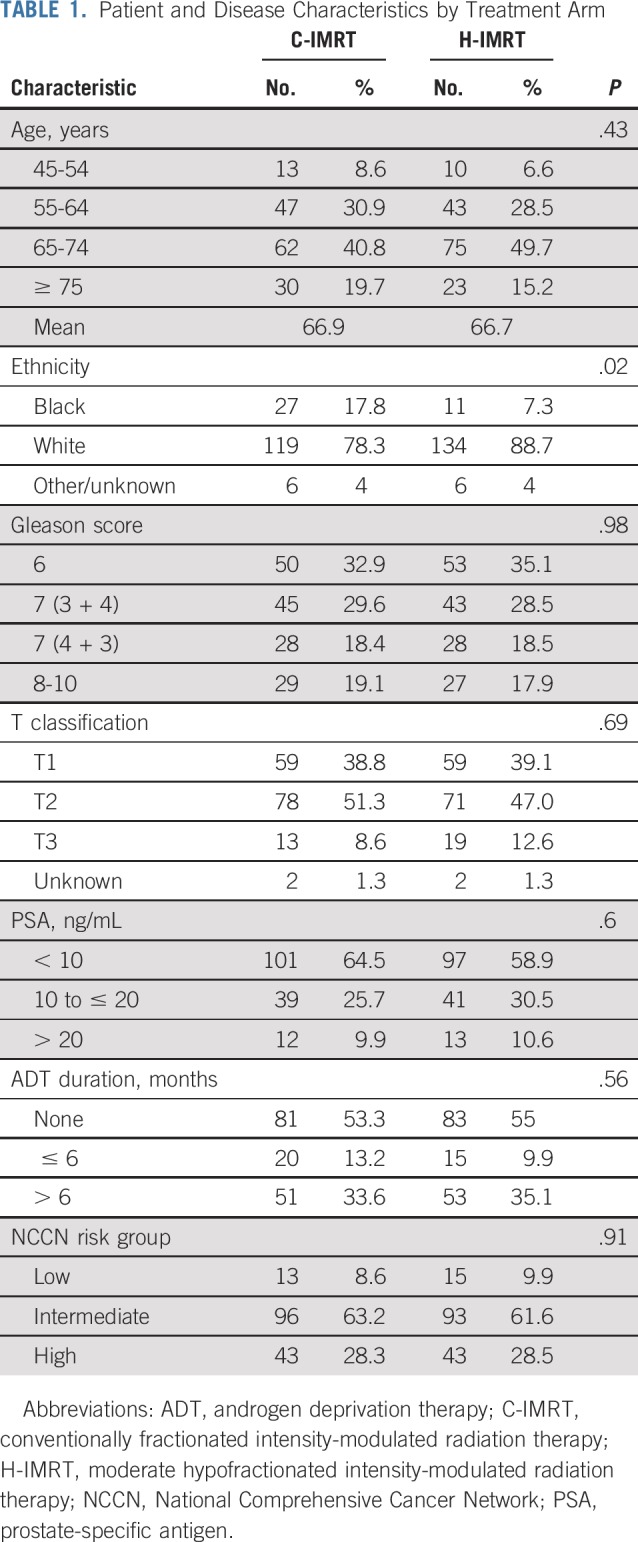
ADT Usage
The median short- and long-term ADT durations were 4.2 and 24.2 months, respectively. There was no difference in the median ADT use between the two treatment groups (P = .56). A total of 35 (17.6%) of men with protocol-defined intermediate risk were to get short-term ADT: 19.8% and 15.3% in the C-IMRT and H-IMRT arms, respectively. The median lengths of ADT in the men with NCCN-defined intermediate-risk prostate cancer were 4.1 and 5.3 months in the C-IMRT and H-IMRT arms, respectively (P = .34). All of the men with protocol-defined high-risk disease received ADT, and 79 (76%) of 104 patients had at least 20 months of ADT. The median length of ADT in the men with high-risk prostate cancer was 24.2 months in both treatment arms.
Outcomes
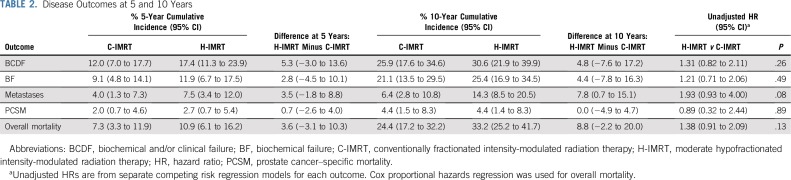
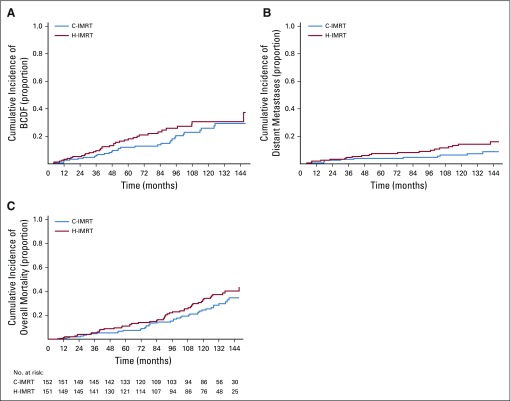
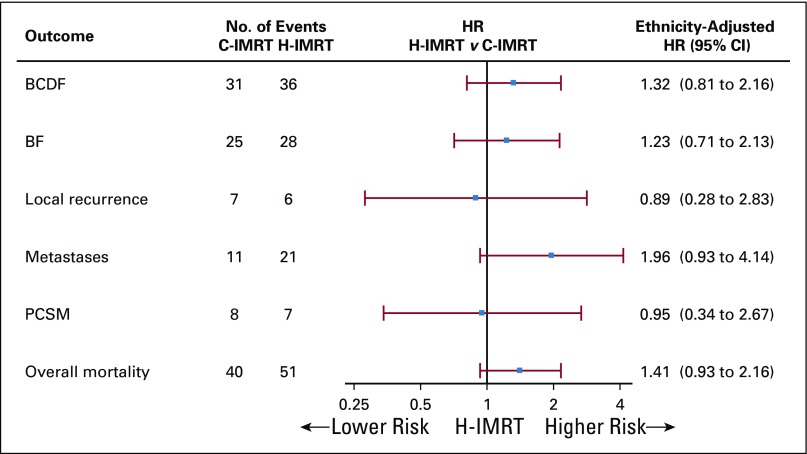
The 10-year cumulative incidence of BCDF for the cohort was 28.3%; it was 25.9% in the C-IMRT arm (95% CI, 17.6% to 34.4%) and was 30.6% in the H-IMRT arm (95% CI, 21.9% to 39.9%; P = .25 by Gray’s test; Table 2; Fig 2A). The 10-year cumulative incidence of BF was 21.1% in the C-IMRT group (95% CI, 13.5% to 29.5%) and was 25.4% in the H-IMRT group (95% CI, 16.9% to 34.5%; P = .26). The 10-year local recurrence rates were low and similar between the two arms: 4.7% and 4%, respectively (P = .82). Metastasis development was seen in 17 patients with high risk (19.8%), 14 patients with intermediate risk (7.4%), and one patient with low risk (3.6%). The 10-year cumulative incidence of distant metastasis was 14.3% (95% CI, 8.5% to 20.5%) in the H-IMRT arm versus 6.4% (95% CI, 2.8%to 10.8%) in the C-IMRT arm (P = .08; Fig 2B). The rate difference of 7.8% (95% CI, 0.7% to 15.1%) was statistically significant. Distant metastasis occurred before or at the time of BCDF in 81.8% of patients in the C-IMRT arm and in 66.7% of patients in the H-IMRT arm (P = .44). The 10-year cumulative incidence of all-cause mortality was 24.4% (95% CI, 17.2% to 32.2%) in the C-IMRT–treated men and was 33.2% (95% CI, 25.2% to 41.7%) in the H-IMRT–treated men (P = .12; Fig 2C). The 10-year PCSM was 4.4% for each arm (95% CI,1.5% to 8.3% for C-IMRT and 1.4% to 8.3% for H-IMRT). However, the cause of death was not known in 31.9% of patients. Ethnicity-adjusted disease outcomes are displayed in Figure 3.
TABLE 2.
Disease Outcomes at 5 and 10 Years
FIG 2.
Cumulative incidence over time of (A) biochemical and/or clinical failure (BCDF); (B) distant metastases; and (C) overall mortality. C-IMRT, conventionally fractionated intensity-modulated radiation therapy; H-IMRT, moderate hypofractionated intensity-modulated radiation therapy.
FIG 3.
Forest plot of outcomes. BCDF, biochemical and/or clinical failure; BF, biochemical failure; C-IMRT, conventionally fractionated intensity-modulated radiation therapy; H-IMRT, moderate hypofractionated intensity-modulated radiation therapy; HR, hazard ratio; PCSM, prostate cancer–specific mortality.
In a sensitivity analysis for BCDF and treatment, additional adjustment for age, ADT usage, and NCCN risk group had little impact on the relative difference by treatment arm (H-IMRT v C-IMRT: HR, 1.36; 95% CI, 0.83 to 2.25; Table 3).
TABLE 3.
Multivariable Analysis for BCDF

DISCUSSION
To our knowledge, this is the first randomized trial of conventional fractionation versus moderate hypofractionation to report results with a median follow-up of more than 10 years. In addition, the trial used contemporary recommendations for ADT length, treatment techniques (daily image guidance, IMRT, computed tomography/MRI simulation), and dose-escalated radiation treatment in the control arm. The trial also was unique in its enrollment of patients with high-risk prostate cancer and treatment of regional lymph nodes for these men. This analysis showed no statistically significant difference in the 10-year rates of BCDF, local recurrence, PCSM, and overall mortality between the two treatment groups of men with mostly NCCN-defined intermediate- and high-risk prostate cancer. Patient-reported acute and late toxicity were published previously and showed that acute toxicity and long-term quality-of-life outcomes were similar between the moderate hypofractionation and conventionally fractionated arms.13,14
These 1ong-term results are consistent with our previous 5-year findings.15 The reason for a continued lack of BCDF benefit despite the dose intensification can be postulated. Although it is widely accepted that the α/β ratio of prostate cancer is approximately 1.5 Gy, several studies report higher α/β ratio values, even exceeding 4 Gy.16-18 If the α/β ratio for prostate is 4 Gy, the EQD2 of the H-IMRT arm would decrease from 84.4 Gy to 78.4 Gy. This would result in a dose escalation of only approximately 2 Gy compared with the C-IMRT arm. It is also possible that there is minimal benefit to dose escalation above 76 Gy.19 There are retrospective analyses that suggest benefit with radiation doses ≥ 80 Gy,20-22 which are supported by the brachytherapy boost studies that show improved outcomes for high-risk and intermediate-risk prostate cancer.23,24 However, all randomized, dose-escalation trials of conventionally fractionated radiation have compared doses ≤ 70.2 Gy against doses ≤ 80 Gy.25-30 It is possible that the dose response curve flattens around 76 Gy and that there is a lack of continuous increase in disease control benefit above this dose. Our results are in line with the Dutch HYPRO trial,4 the only other randomized moderate hypofractionation trial with EQD2 dose escalation (EQD2, 90.4 Gy) comparable to this trial. They found similar 5-year recurrence-free survival in their intermediate- and high-risk prostate cancer cohorts despite a 12.4-Gy EQD2 increase with hypofractionation.
There was a trend toward a higher rate of 10-year cumulative incidence of distant metastasis with H-IMRT that was statistically significant when the difference between the two groups was compared at 10 years. One possible explanation for these findings is the reduced margins in the H-IMRT arm. However, the margins for H-IMRT were designed to have the 90% (EQD2, 76 Gy) isodose line similar to the 100% (76 Gy) isodose line in the C-IMRT arm, so the reduced margins theoretically should not have led to undertreatment of microscopic disease that proceeded to metastasize. One would expect to see significant differences in other disease outcome parameters in that situation as well. Contemporary treatment techniques were used, and excellent compliance to ADT was seen in both groups. The increase in metastasis did not translate into a higher PCSM rate in the H-IMRT group despite the long-term follow-up.
One of the unique aspects of this trial is the treatment of pelvic lymph nodes in high-risk disease. The role of lymph node radiation in modern dose-escalated radiation therapy is a controversial topic that is beyond the scope of this paper and is currently being investigated.31 No current multi-institutional randomized trials evaluating moderate hypofractionation in patients with high-risk disease include treatment of lymph nodes.3,4 There was reasonable fear of increased morbidity with the additional treated volume in hypofractionated regimens. This is addressed in our trial design, which used smaller PTV margins and strict normal tissue dose criteria in the H-IMRT arm. Current long-term disease control results and previously reported long-term toxicity show the feasibility of treatment of pelvic lymph nodes with a moderate hypofractionation regimen.
This trial and the Italian trial by Arcangeli et al10 are, to our knowledge, the only two randomized trials comparing moderate hypofractionation versus conventional fractionation that report long-term outcomes in high-risk and intermediate-risk prostate cancer. The Italian hypofractionation trial (80Gy in 40 fractions v 62 Gy in 20 fractions [EQD2, 81.5 Gy]) enrolled 168 men with high-risk prostate cancer, as defined at the time of enrollment.32 The updated analysis did not reorganize the patients into more modern risk categories, so the percent of patients with disease that would now be considered intermediate risk is unclear. They found an association between hypofractionation and improved mean prostate cancer–specific survival and mean freedom from BF despite the modest increase in EQD2 dose.10 The difference in the disease control outcomes compared with the results of our trial can be attributed to the different fractionation regimen, different patient population, different treatment techniques, or shorter duration of ADT (9 months). The evidence that long-term ADT provides a survival benefit compared with short-term ADT in high-risk patients did not exist at the time of trial design.33,34 It is possible that the use of long-term ADT in our high-risk cohort negated a possible similar benefit of dose escalation with hypofractionation.
The MD Anderson group also recently published the 8.5-year follow-up of their randomized trial of moderate hypofractionation (75.6 Gy in 42 fractions [EQD2, 71.28 Gy] v 72 Gy in 30 fractions [EQD2, 80.23 Gy]) of 206 men with low- and intermediate-risk prostate cancer.9 Lower rates of failure (defined as biochemical failure or initiation of salvage therapy) were seen in the hypofractionation arm in the subgroup of patients who did not receive ADT and in the subgroup of patients who had an iPSA ≤ 10 ng/mL. The failure rate between the two treatment arms started to separate after 5 years, supporting the necessity of long-term follow-up in prostate cancer trials. The conventional fractionation arm had an EQD2 dose that was substantially lower than in our trial, which may have driven the benefit in the hypofractionation arm not seen in our trial. In the subset analysis of patients who received ADT, the failure rate was identical between the two fractionation regimens. This also suggests that ADT usage negated some of the potential advantages of dose escalation.
In this long-term analysis of a randomized study comparing C-IMRT versus H-IMRT, the 10-year BCDF, BF, overall mortality, PCSM, and local control rates were similar between the two treatment arms. There was a significant increase in the difference in the 10-year cumulative incidence of metastases with H-IMRT without an increase in PCSM. This study differs from other moderate hypofractionation randomized trials in multiple ways: its relatively large cohort size, the longest median follow-up to date, the contemporary dose-escalated control arm, the significant portion of patients with high-risk disease, the contemporary recommendations for ADT length, the contemporary treatment techniques, and the included lymph node radiation for patients with high-risk disease.
PRIOR PRESENTATION
Presented in part at the 60th Annual Meeting of the American Society for Radiation Oncology (ASTRO), San Antonio, TX, October 21 - 24, 2018, and at the Best of ASTRO Meeting, San Francisco, CA, November 30 - December 1, 2018.
SUPPORT
Supported by Grant No. P30 CA006927 from the National Cancer Institute, National Institutes of Health, and in part by a grant from Varian Medical Systems.
CLINICAL TRIAL INFORMATION
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or Varian Medical Systems.
AUTHOR CONTRIBUTIONS
Conception and design: Vladimir Avkshtol, Brooke Leachman, Robert G. Uzzo, Charlie Ma, Alan Pollack, Eric M. Horwitz
Collection and assembly of data: Vladimir Avkshtol, Richard E. Greenberg, Robert A. Price Jr, Brooke Leachman, Robert G. Uzzo, Mark L. Sobczak, Alan Pollack, Eric M. Horwitz
Provision of study material or patients: Robert G. Uzzo, Mark L. Sobczak, Alan Pollack, Eric M. Horwitz
Data analysis and interpretation: Vladimir Avkshtol, Karen J. Ruth, Eric A. Ross, Mark A. Hallman, Richard E. Greenberg, Brooke Leachman, Robert G. Uzzo, Charlie Ma, David Chen, Daniel M. Geynisman, Mark L. Sobczak, Eddie Zhang, Jessica K. Wong, Alan Pollack, Eric M. Horwitz
Administrative support: Alan Pollack, Eric M. Horwitz
Financial support: Alan Pollack, Eric M. Horwitz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ten-Year Update of a Randomized, Prospective Trial of Conventional Fractionated Versus Moderate Hypofractionated Radiation Therapy for Localized Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric A. Ross
Patents, Royalties, Other Intellectual Property: Method for Screening Muscle Invasive Bladder Cancer Patients for Neoadjuvant Chemotherapy Responsiveness (Inst)
Mark A. Hallman
Consulting or Advisory Role: New Century Health
Brooke Leachman
Employment: Proscia (I)
Robert G. Uzzo
Consulting or Advisory Role: Pfizer, Genentech, Roche, Urogen Pharma
Speakers” Bureau: Janssen Oncology
Research Funding: Novartis
David Chen
Stock and Other Ownership Interests: Pfizer, Pfizer (I)
Daniel M. Geynisman
Consulting or Advisory Role: Pfizer, Exelixis, AstraZeneca, Seattle Genetics, Astellas, Eisai
Research Funding: Genentech (Inst), Merck (Inst), Calithera Biosciences (Inst), Astellas Pharma (Inst)
Mark L. Sobczak
Consulting or Advisory Role: New Century Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 3.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061–1069. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: An ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol. 2018;36:3411–3430. doi: 10.1200/JCO.18.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Gheida I, Reddy CA, Kotecha R, et al. Ten-year outcomes of moderately hypofractionated (70 Gy in 28 fractions) intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2019;104:325–333. doi: 10.1016/j.ijrobp.2019.01.091. [DOI] [PubMed] [Google Scholar]
- 8.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36:2943–2949. doi: 10.1200/JCO.2018.77.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: Final results of a phase III randomized trial. J Clin Oncol. 2017;35:1891–1897. doi: 10.1200/JCO.2016.70.4189. [DOI] [PubMed] [Google Scholar]
- 11.Stokes WA, Kavanagh BD, Raben D, et al. Implementation of hypofractionated prostate radiation therapy in the United States: A National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270–278. doi: 10.1016/j.prro.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Prostate cancer NCCN guidelines (version 4.2018).
- 13.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaikh T, Li T, Handorf EA, et al. Long-term patient-reported outcomes from a phase 3 randomized prospective trial of conventional versus hypofractionated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2017;97:722–731. doi: 10.1016/j.ijrobp.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 17.Valdagni R, Italia C, Montanaro P, et al. Is the alpha-beta ratio of prostate cancer really low? A prospective, non-randomized trial comparing standard and hyperfractionated conformal radiation therapy. Radiother Oncol. 2005;75:74–82. doi: 10.1016/j.radonc.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Nahum AE, Movsas B, Horwitz EM, et al. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: Implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57:391–401. doi: 10.1016/s0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 19.Zaorsky NG, Palmer JD, Hurwitz MD, et al. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol. 2015;115:295–300. doi: 10.1016/j.radonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Eade TN, Hanlon AL, Horwitz EM, et al. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cahlon O, Zelefsky MJ, Shippy A, et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity-modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Kishan AU, Shaikh T, Wang PC, et al. Clinical outcomes for patients with Gleason score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: A multi-institutional comparative analysis. Eur Urol. 2017;71:766–773. doi: 10.1016/j.eururo.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4:e180039. doi: 10.1001/jamaoncol.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 27.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heemsbergen WD, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: Impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110:104–109. doi: 10.1016/j.radonc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 31.Radiation Therapy Oncology Group Androgen deprivation therapy and high dose radiotherapy with or without whole-pelvic radiotherapy in unfavorable intermediate or favorable high risk prostate cancer: A phase III randomized trial. https://clinicaltrials.gov/ct2/show/NCT01368588
- 32.Arcangeli G, Saracino B, Gomellini S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:11–18. doi: 10.1016/j.ijrobp.2009.07.1691. [DOI] [PubMed] [Google Scholar]
- 33.Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320–327. doi: 10.1016/S1470-2045(15)70045-8. [DOI] [PubMed] [Google Scholar]
- 34.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]