Abstract
PURPOSE
To analyze long-term outcomes after treatment discontinuation of anti–programmed death-1 (anti–PD-1) therapy in a cohort of patients with melanoma with the longest follow-up yet available to our knowledge, including a majority of patients treated outside of a clinical trial. We also assessed efficacy of retreatment with anti–PD-1 therapy with or without ipilimumab in relapsing patients.
METHODS
We retrospectively analyzed all patients with nonuveal, unresectable stage III/IV melanoma treated with single-agent anti–PD-1 therapy at Memorial Sloan Kettering from 2009-2018 who had discontinued treatment and had at least 3 months of follow-up after discontinuation (n = 396). Overall survival for patients with complete response (CR) was calculated from time of CR. Time to treatment failure for patients with CR was time from CR to the next melanoma treatment or death.
RESULTS
CRs were seen in 102 of 396 patients (25.8%). The median number of months of treatment after CR was zero (range, stopped before CR to 26 months after CR). With a median follow-up of 21.1 months from time of CR in patients who did not relapse, the probability of being alive and not needing additional melanoma therapy at 3 years was 72.1%. There was no significant association between treatment duration and relapse risk. In multivariable analysis, CR was associated with M1b disease and cutaneous versus mucosal or acral primaries. Among the 78 patients (of 396) retreated after disease progression, response was seen in 5 of 34 retreated patients with single-agent anti–PD-1 therapy and 11 of 44 patients escalated to anti–PD-1 plus ipilimumab.
CONCLUSION
In our cohort, most patients discontinued treatment at the time of CR. Most CRs were durable but the probability of treatment failure was 27% at 3 years. Responses to retreatment were infrequent. The optimal duration of treatment after CR is not yet established.
INTRODUCTION
Pembrolizumab and nivolumab, two Food and Drug Administration–approved monoclonal antibodies directed at programmed cell death protein-1 (PD-1) have dramatically improved outcomes for patients with unresectable and metastatic melanoma.1-3 In clinical trials, approximately 8%-15% of patients achieve a complete response (CR) according to RECIST.2,4 These CRs are thought to be durable after treatment discontinuation. In the KEYNOTE-001 trial, an estimated 89% of patients who achieved a CR to pembrolizumab were disease free 2 years after discontinuing treatment.4,5
Given the potential risk of toxicity,6 high cost of these agents,7 and potential for durable responses, interest is growing in understanding the long-term outcomes after treatment discontinuation and determining the optimal duration of treatment after CR. To address the question of long-term outcomes after CR, we performed a retrospective analysis of all patients with melanoma who had discontinued anti–PD-1 therapy at Memorial Sloan Kettering Cancer Center. This cohort included patients treated in early clinical trials with some of the longest follow-up to date, to our knowledge. Because little information is available on long-term outcomes in CR patients treated with anti–PD-1 outside of clinical trials, we also investigated the relationship between baseline factors and CR to anti–PD-1 therapy.
As for the question of the optimal duration of treatment after CR, it would be beneficial to determine whether prolonged treatment after CR is truly necessary for optimal overall survival (OS). A majority of patients in our cohort were treated outside of clinical trials and received little anti–PD-1 therapy after CR. This allowed us to determine the estimated risk of treatment failure and the conditional probability of relapse after CR in this cohort who received limited maintenance immunotherapy after CR.
In patients who later experienced disease progression after anti–PD-1 treatment discontinuation, clinicians often consider a second course of anti–PD-1 therapy. Data are limited on the efficacy of retreatment, which complicates efforts to counsel patients. Three small series of retreated patients have been reported (n = 88; n = 45; and n = 199), but the results are highly variable given the small numbers and differences in practice patterns (eg, time receiving therapy) among institutions and trials. We report results of retreatment in, to our knowledge, the largest cohort to date.
METHODS
Patients
Eligible patients were 17 years of age or older, had a confirmed diagnosis of advanced melanoma (unresectable stage III or stage IV), and received ≥ 1 dose of single-agent anti–PD-1 therapy (nivolumab or pembrolizumab), followed by ≥ 1 scan that could be evaluated for response to therapy. Patients with uveal melanoma were excluded. All patients were treated at Memorial Sloan Kettering Cancer Center. In addition, patients must have had ≥ 3 months of follow-up after discontinuation of anti–PD-1 therapy or have a known date of death within this period. The timing of therapy discontinuation and decision to treat with a second course of anti–PD-1 therapy were at the discretion of the treating oncologist. In the minority of patients treated with on-protocol therapy, the time of discontinuation was determined by the protocol. The reason for discontinuation was recorded as documented in the clinician’s note. Retreated patients were considered eligible for inclusion if they received at least 1 dose of anti–PD-1 or anti–PD-1 with ipilimumab therapy ≥ 3 months after discontinuing the first course of anti–PD-1 therapy and had an evaluable response by scans or clinical assessment; patients who received only 1 retreatment dose within 1 week of death were excluded.
Study Design
After obtaining a waiver of consent from the institutional review board, we conducted a single-center, retrospective analysis of patients treated between May 2009 and April 2018 with single-agent anti–PD-1 therapy who had discontinued treatment of ≥ 3 months. Patients who received concurrent radiation therapy for brain metastases or a painful lesion were included if another site of disease could be assessed for response to anti–PD-1 therapy. We collected patient demographics, neutrophil-to-lymphocyte ratio (NLR), lactate dehydrogenase (LDH) level, tumor mutation burden (TMB), prior lines of treatment, duration of treatment, time off treatment, reason for discontinuation, and data about retreatment. TMB was estimated by calculating the number of nonsynonymous variants and dividing by the total sequenced exon length, consistent with prior reports.10,11 We collected efficacy data consisting of best overall response (BOR), time to treatment failure (TTF), and OS through November 9, 2018.
Best Overall Response
Tumor responses were assessed based on clinician determination of response and investigator review of imaging. CR was defined by (1) absence of radiologically apparent disease (radiologic CR); (2) biopsy that showed no viable melanoma after a radiologic response (pathologic CR); or (3) complete regression of clinically evaluable disease, even if there were no radiologically measurable target lesions. If patients had a clear antitumor response but could not be considered to have a CR by 1 of the 3 criteria, these patients were considered to have a “response < CR.” If there was no clear antitumor effect and no clear progression, patients were scored as stable. Radiologic CRs were reviewed by 1 of 2 reference radiologists and examined for concordance with RECIST version 1.1.
Statistical Analysis
BOR rates were estimated with exact binomial CIs. Associations between factors and CR were assessed with univariable and multivariable logistic regression. Factors significant at P < .05 on univariable analyses were considered for multivariable analyses. OS was estimated from the start of anti–PD-1 therapy until death. Patients alive at last follow-up were censored. TTF was estimated from the start of anti–PD-1 therapy until the next treatment or death. Patients alive with no evidence of progression and who did not receive additional treatment by last follow-up were censored. We used Kaplan-Meier methods to estimate and visualize survival and Cox proportional hazards regression to assess association with baseline factors. We also assessed outcomes from the time of best response by BOR and OS from the time of additional retreatment.
Conditional survival from 1 year after CR was assessed for complete responders. TMB and NLR were log transformed for regression analyses. We assessed the relationship between patient characteristics and additional PD-1 treatment with Fisher's exact test and Wilcoxon rank sum test, where appropriate.
Two-sided P values less than .05 were considered statistically significant. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
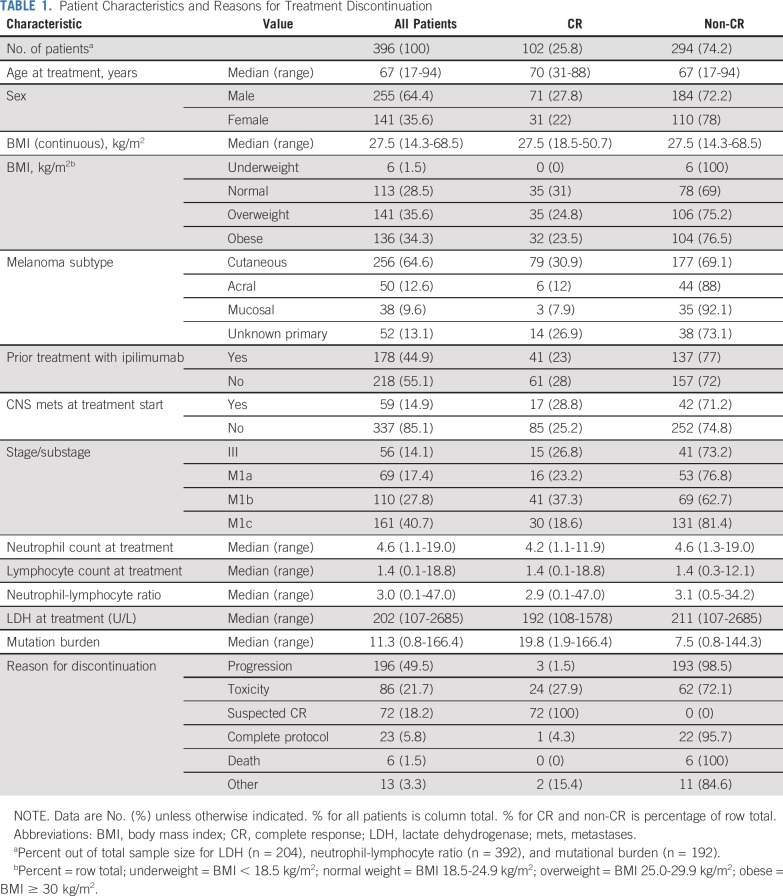
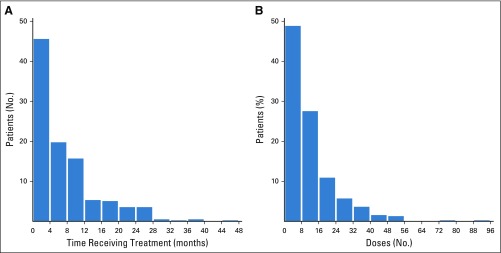
A total of 1,325 records were reviewed to identify the 396 evaluable patients included in this study. Patients were treated with either single-agent pembrolizumab (85.9% of patients) or nivolumab (14.1% of patients). Treatment was given outside of a clinical trial in 69.2% of patients. The median follow-up in survivors was 28.9 months (range, 4.9-77.9 months); 25% of patients were observed for ≥ 43.8 months. Patient characteristics are shown in Table 1. At the time of starting anti–PD-1 therapy, 45% of patients had received prior ipilimumab therapy; 15% of patients had CNS metastases. The median time receiving anti–PD-1 therapy was 4.8 months (range, < 1.0-44.2 months; Appendix Fig A1A, online only), and the median number of doses was 8 (range, 1-94 doses; Appendix Fig A1B). The most common reason overall for discontinuing therapy was progression of disease (49.5% of patients), followed by toxicity (21.7% of patients).
TABLE 1.
Patient Characteristics and Reasons for Treatment Discontinuation
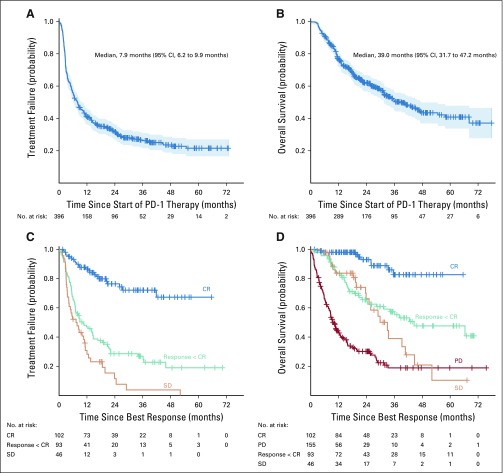
For the entire cohort, the median TTF was 7.9 months (95% CI, 6.2 to 9.9 months); at 5 years after treatment start, treatment failure-free survival was 21.5% (95% CI, 16.5% to 27%; Fig 1A). The median OS was 39 months (95% CI, 31.7 to 47.2 months); 5-year OS was 40.8% (95% CI, 33.7% to 47.8%; Fig 1B).
FIG 1.
Time to treatment failure (TTF) and overall survival (OS) data for the entire cohort and by best overall response (BOR). (A) The median overall TTF was 7.9 months; (B) the median OS was 39 months. (C) TTF from the time of BOR showed a 3-year TTF of 72% for patients with complete response (CR), 26.9% for responders with less than CR, and 3.8% for stable disease (SD). (D) Overall survival from time of BOR showed a 3-year OS of 82.7%, 57.3%, 39%, and 18.9%, respectively, for patients with CR, response less than CR, SD, and progressive disease. Tick marks indicate censored patients. Blue shaded areas in (A) and (B) represent 95% CIs.
Best Overall Response
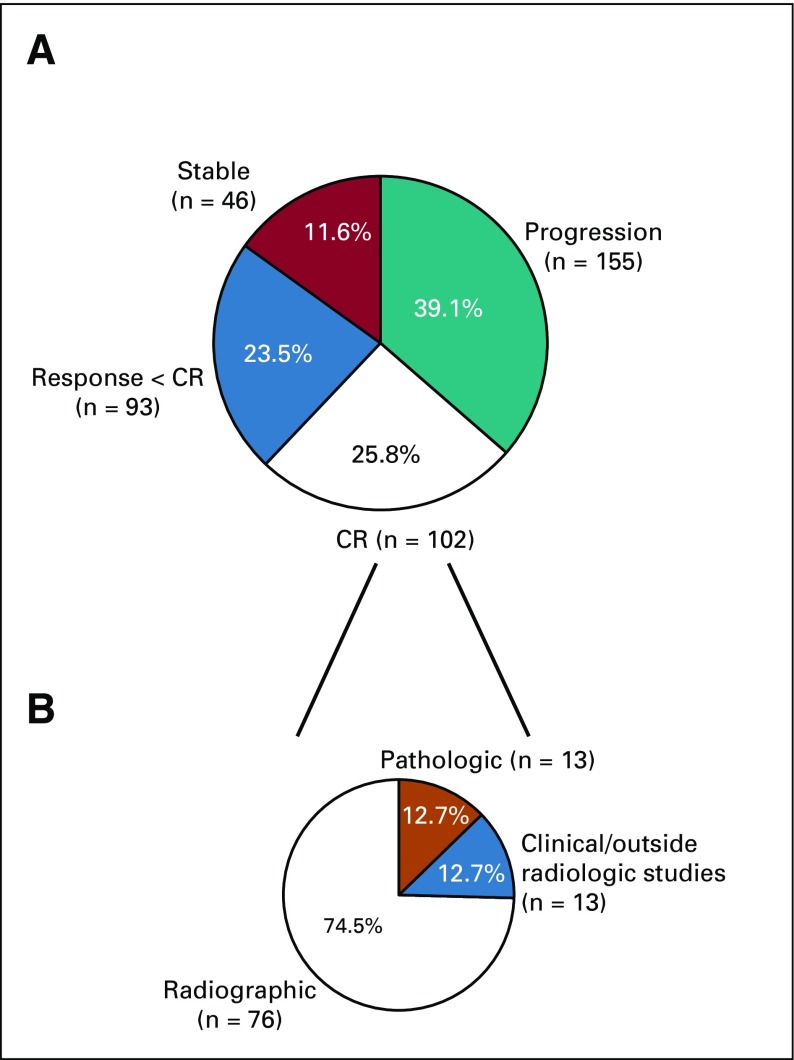
In 25.8% (95% CI, 21.5% to 30.4%) of patients, the treating oncologist considered the patient to have had a CR. An additional 23.5% (95% CI, 19.4% to 28.0%) of the patients had a significant antitumor response, with decrease in tumor size but less than CR. In the remaining patients, the BOR was stable disease (11.6%; 95% CI, 8.6% to 15.2%) or progressive disease (PD; 39.1%; 95% CI, 34.3% to 44.1%; Appendix Fig A2, online only). Figures 1C and 1D show the TTF and OS by BOR, starting from the time of BOR.
Complete Responders
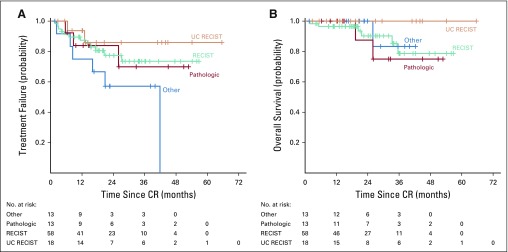
Of the 102 patients (25.8%) who were considered to have had a CR, 76 were considered a CR by the treating physician because of complete radiologic resolution of disease. These 76 patients were reviewed by reference radiologists, who confirmed CR by formal RECIST in 58 patients (76%; Fig 2A). In the other 18 patients, the clinician felt that the patient had radiographic resolution of disease but there were tumor residua that did not meet criteria for RECIST CR (UC RECIST).
FIG 2.
Time to treatment failure and overall survival of patients with complete response (CR). (A) Time to treatment failure and (B) overall survival were calculated from the time of CR. Patients were determined to have a CR by RECIST confirmed by 1 of 2 reference radiologists (RECIST cohort), a radiologic CR that could not be confirmed by the reference radiologists (UC RECIST), or major response with a negative biopsy (pathologic). The “other” group includes patients who had a CR by clinical evaluation but did not have radiologically assessable disease or patients who had a radiologic CR as assessed by an outside radiologist but for whom the outside scans were not available for review at our institution.
Of the remaining 26 CRs not based on radiologic evaluations, 13 were pathologic CRs, and 13 were considered CRs based on physical examination (not radiologically evaluable) or were radiologic CRs by scans performed at outside institutions but were not available for review (other CR). We found no difference in TTF (Fig 2A) or OS (Fig 2B) on the basis of how the CR was determined. The median duration of treatment of the CR patients was 9.4 months (range, 1.6-36.1 months); the median duration of treatment after CR was 0 months (range, −12 to 26 months; negative value indicates that treatment was stopped before CR). The reasons for treatment discontinuation were suspected CR (n = 72; 70.6%), toxicity (n = 24; 23.5%), progression of disease (n = 3; 2.9%), completing the defined treatment per protocol (n = 1; 1%), or other (n = 2; 2%; Table 1).
With a median follow-up from time of CR of 21.1 months for those treatment failure free (range, 1.6-65.6 months), neither the median TTF (Fig 1C, blue curve) nor the median OS (Fig 1D, blue curve) from time of CR had been reached. The probability of being alive and not requiring additional melanoma therapy was 72.1% (95% CI, 59.9% to 81.1%) at 3 years (Fig 1D). The estimated 3-year OS from time of CR was 82.7% (95% CI, 67.9% to 91.1%).
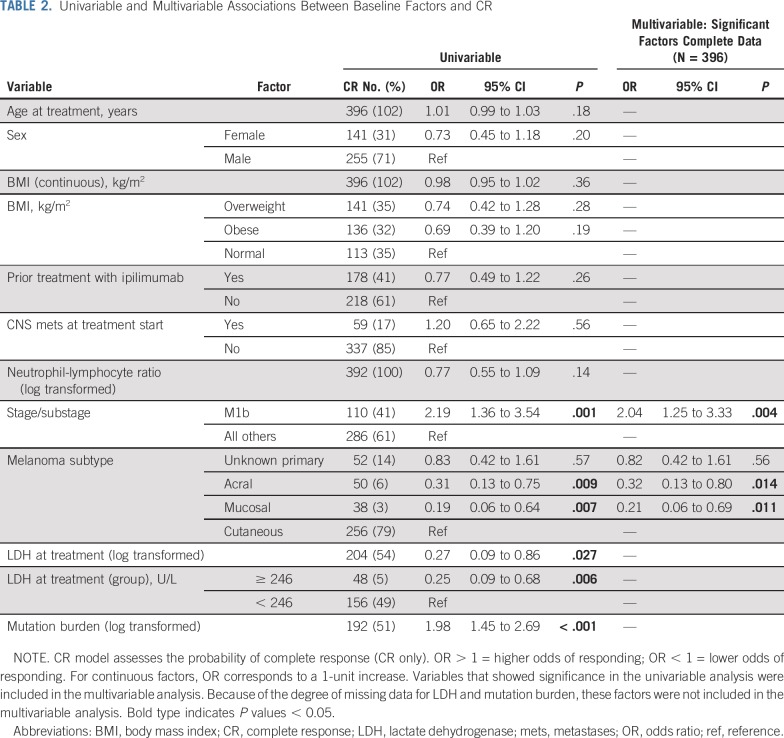
By univariable analysis (Table 2), we observed that patients with M1b disease were more likely to have a CR (odds ratio [OR], 2.19; 95% CI, 1.36 to 3.54; P = .001) compared with other stage IV substages and stage III combined. In contrast, patients with mucosal (OR, 0.19; 95% CI, 0.06 to 0.64; P = .007) or acral primaries (OR, 0.31; 95% CI, 0.13 to 0.75; P = .009) were less likely to have a CR compared to cutaneous primaries. Higher TMB (OR, 1.98, 95% CI, 1.45 to 2.69; P < 0.001) was associated with higher odds of CR and elevated LDH (≥ 246 U/L) was associated with lower odds of CR (OR, 0.25, 95% CI, 0.09 to 0.68; P = 0.006) but too many patients were missing these data to include these two variables in the multivariable model. Of note, there were no significant associations found between CR and age, sex, prior ipilimumab, presence of CNS metastases, body mass index (BMI), and NLR or with the presence of common somatic driver mutations (such as BRAF, RAS, or NF-1). On multivariable analysis, stage and site of disease remained significant (Table 2). Tumor burden was not assessed in this study, which is a limitation of our analysis.
TABLE 2.
Univariable and Multivariable Associations Between Baseline Factors and CR
Twenty-three patients who had a CR ultimately experienced treatment failure. Among patients who achieved and remained in CR for 1 year after CR was identified, the conditional probability of remaining in CR for 2 more years was 83.3% (95% CI, 73.0% to 93.6%). Twelve of the patients (60%) who experienced disease progression developed new extracranial metastases, 5 (25%) had new intracranial metastases, and 3 (15%) had progression at a previous site of disease. There was no significant association between treatment duration before CR and treatment failure after CR (hazard ratio, 0.94; P = .16).
Retreatment With Checkpoint Inhibitor Therapy
In 78 patients (19.7%) who discontinued anti–PD-1 therapy for any reason and later experienced disease progression, a second course of checkpoint inhibitor (CPI) therapy was administered, either single-agent anti–PD-1 therapy (34 patients) or combination ipilimumab + nivolumab (44 patients; Fig 3). The BOR to the first course of therapy had been a CR in 10 patients, tumor shrinkage less than CR in 18 patients, stable disease in 13 patients, and progressive disease in 37 patients. The median time between initial anti–PD-1 discontinuation and the start of retreatment was 6.3 months (range, 0.3-28.6 months).
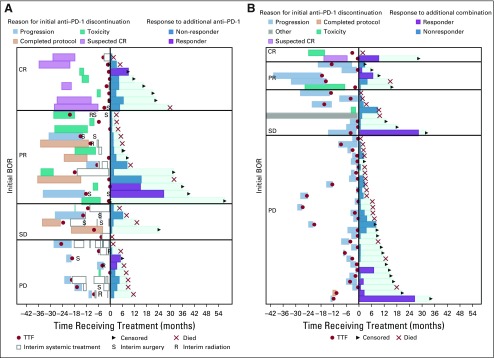
FIG 3.
Event-history plot showing outcomes of patients who were retreated with (A) single-agent anti–programmed death-1 (anti–PD-1) therapy or (B) combination anti–PD-1 with anti-CTLA4. Time zero is the start of retreatment. Patients are grouped on the basis of the best overall response to initial anti–PD-1 therapy (complete response [CR], partial response [PR], stable disease [SD], progressive disease [PD]), which is noted on the left side of the panel. The duration of the first course of anti–PD-1 is indicated by light blue, progression of disease; and the reason for discontinuation is indicated by the color (red, suspected CR; teal, toxicity; orange, the course of planned therapy; purple, toxicity; yellow, progression of disease). Red dots indicate time of progression of disease. Open bars indicate nonimmunotherapy systemic treatments. In addition, some patients received radiation therapy (R) or surgery (S). After time zero, colored bars indicate the duration of retreatment. Purple bars show patients who responded; dark blue bars are patients whose best response to retreatment was disease progression. The aqua bars indicate the duration of survival. Patients have either died (red X) or were censored (arrowhead). BOR, best overall response; TFF, time to treatment failure.
Five patients (14.7%) responded to retreatment with single-agent anti–PD-1 therapy; 2 were CRs. Eleven patients (25%) responded to retreatment with ipilimumab-nivolumab; 3 had a CR. There was no correlation between BOR to the initial course of anti–PD-1 therapy and response to retreatment. Of the retreated patients who had a CR to the initial anti–PD-1 therapy, only 2 of 10 responded to retreatment. Of the 5 patients who responded to retreatment with single-agent anti–PD-1, 3 patients initially discontinued because of disease progression, and 2 patients initially discontinued because of toxicity. Of the retreated patients, including those taking single-agent and combination therapy, 15 discontinued initial treatment because of toxicity, and 6 patients discontinued because of suspected CR.
The median duration of retreatment was 1.6 months (range, < 1.0-28.3 months). The estimated median OS for all 78 retreated patients from the start of retreatment was 9.9 months (95% CI, 6.8 to 17.9 months); the 2-year OS was 37.6% (95% CI, 25.5% to 49.7%).
DISCUSSION
In this large, single-institution cohort of patients who were treated with, and subsequently discontinued, single-agent anti–PD-1 therapy, we observed generally durable responses after treatment discontinuation. There are many factors contributing to the desire to discontinue therapy, including risk of significant late toxicity, economic burden of long-term treatment, and quality-of-life concerns. A significant minority of patients who experience a CR will ultimately relapse. In our cohort, approximately one quarter of these relapses occurred in the CNS.
KEYNOTE-001 supports the safety of treatment discontinuation; nearly 90% of patients who achieved CR continued to be disease free after a median of 3.5 years of follow-up since treatment start.4 However, data from patients in the CheckMate-153 trial, in which patients with non–small-cell lung cancer responding to anti–PD-1 therapy were randomly assigned to 1 year versus continuous nivolumab, suggest that longer treatment with nivolumab may be beneficial.12 Similar data are not yet available for melanoma, and the question of optimal duration of treatment after CR remains open.
In our patients, CRs were observed in 25.8% of patients. There was a higher rate of CR in patients with stage IV M1b disease (37.3%) than in other stage IV substages or even patients with stage III disease. This supports the general impression that patients with melanoma with M1b disease have a high response rate to CPIs. In most of our patients, CRs were determined by the treating physician, and 13 patients were classified as having a pathologic CR. Overall, only 57% of the total CRs (76% of radiologic CRs) would qualify as CR by RECIST. We believe the definition of CR is overly strict in the setting of CPI therapy and that there are many CRs to CPI therapy that do not meet the RECIST definition. RECIST response definitions were developed in the pre–CT scan era to enhance clinical trial reproducibility and were not designed to correlate with patient outcomes or to be used for patient management decisions.13,14
In our patients, the median duration of treatment to CR was 7 months, with little maintenance therapy after CR. This resulted in durable CRs; at 3 years, the probability of being alive and not requiring additional melanoma therapy was 72.1%. There was no significant association between treatment duration before CR and treatment failure. Of the 23 patients who relapsed, 87% relapsed within the first 2 years. In patients who remained in CR for 1 year, the conditional probability of remaining in CR for 2 more years was 83.3%.
When considering treatment discontinuation, patients often inquire about resuming anti–PD-1 therapy if their disease were to progress in the future. Data regarding outcomes of patients with melanoma who receive a second course of anti–PD-1 therapy are scarce and widely variable because of small numbers and institutional variation in practice patterns. From the KEYNOTE-006 trial, 8 patients with initial CR were treated with a second course of anti–PD-1 therapy, of whom 1 patient achieved a CR and 3 had a partial response (PR).8 Hamid et al5 reported 4 patients who were treated with a second course of anti–PD-1 therapy in the KEYNOTE-001 trial. All 4 patients had an initial CR followed by treatment discontinuation, but only 1 patient had tumor shrinkage in response to retreatment. Jansen et al9 recently reported on a pooled cohort from Europe and Australia, in which 19 patients were retreated with anti–PD-1 therapy after an initial BOR of CR (9 patients), PR (6 patients), or progression of disease (4 patients). Of these 19 patients, only 6 responded (32%), with 2 CR and 4 PR. Of the 9 patients who had had CR as the initial BOR, the BOR on re-treatment was PR in 2 patients and CR in 2 patients.
Overall, 15% of our patients responded to retreatment with anti–PD-1 therapy, and 25% responded to retreatment with the ipilimumab-nivolumab combination. Surprisingly, the response to retreatment did not seem to be related to the magnitude of response to the first course of anti–PD-1 therapy. The response rate was lower for retreatment than would be expected for treatment-naïve patients but is consistent with previous observations from smaller cohorts.
This markedly lower response rate in retreated patients compared with treatment-naïve patients treated with CPIs seems to be in contrast to the experience of retreating patients with melanoma with a second course of the anti–CTLA-4 antibody ipilimumab after ipilimumab progression. Objective response (PR plus CR) rates of patients retreated with ipilimumab were 16%15 and 23%16 in 2 separate reports—response rates that are similar or higher than the expected response rates for ipilimumab in treatment-naïve patients. This suggests that resistance mechanisms arising in the course of anti–PD-1 therapy may be more persistent than the resistance mechanisms that arise during anti–CTLA-4 therapy.
Several mechanisms of resistance to CPIs have been proposed, including adaptive immune resistance and phenotypic changes of residual tumor cells, such as changes in HLA class I, antigen processing or presentation, or programmed death-ligand-1 expression.17-21 The long duration of therapy and immunologic effects of anti–PD-1 antibodies may lead to prolonged selective pressure for these resistant clones so that recurrences that arise are likely to express these resistant phenotypes.
There are important distinctions between our patient cohort and other previously published cohorts. Most of our patients (69.2%) were treated outside of a clinical trial with a mixture of prior ipilimumab and CNS involvement. As discussed, we used a more liberal definition of CR than in formal clinical trials. A final distinction is that our CR patients received little maintenance therapy after achieving a CR; the median number of months of treatment after CR was zero compared with the KEYNOTE-001 trial, in which the median duration of maintenance therapy was 7 months. The risks and benefits of maintenance therapy in patients with melanoma who achieve a CR remain unquantified and is an important topic for future study.
Response rates to retreatment with CPI therapy after relapse from single-agent anti–PD-1 were disappointingly low; future studies should seek to determine alternative strategies to overcome resistance. These data would also predict that patients who progress after receiving adjuvant anti–PD-1 therapy will have a lower response rate to CPI therapy given for recurrent disease.
Appendix
FIG A1.
Histogram of (A) months on treatment and (B) No. of anti—programmed death-1 (PD-1) doses given to patients in this analysis.
FIG A2.
Distribution of (A) best overall responses and (B) basis of complete responses (CRs). Of the 76 radiologic CRs, 58 were confirmed by reference radiologists to meet formal RECIST for CR.
PRIOR PRESENTATION
Preliminary analyses were presented in abstract form at the Society for Melanoma Research International Congress, Manchester, England, October 24-27, 2018; and the 2019 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
A.B.W. was supported by a Young Investigator Award from the Conquer Cancer Foundation of ASCO. The investigators are supported by Cancer Center Support Grant No. P30 CA08748 from the National Institutes of Health/National Cancer Institute and the Parker Institute for Cancer Immunotherapy.
See accompanying Editorial on page 1645
AUTHOR CONTRIBUTIONS
Conception and design: Allison Betof Warner, Debra A. Goldman, Jedd D. Wolchok, Paul B. Chapman
Administrative support: Allison Betof Warner
Provision of study materials or patients: Allison Betof Warner, Parisa Momtaz, Jedd D. Wolchok
Collection and assembly of data: Allison Betof Warner, Jessica S. Palmer, Alexander N. Shoushtari, Debra A. Goldman, Katherine S. Panageas, Sara A. Hayes, Raazi Bajwa, Paul B. Chapman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Allison Betof Warner
Honoraria: LG Chem Life Sciences Innovation Center
Research Funding: Leap Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/966362/summary
Alexander N. Shoushtari
Consulting or Advisory Role: Castle Biosciences, Immunocore, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Immunocore, Xcovery, AstraZeneca (Inst), Polaris
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Debra A. Goldman
Stock and Other Ownership Interests: Johnson & Johnson
Katherine S. Panageas
Stock and Other Ownership Interests: AstraZeneca, Catalyst Biotech, Dynavax Tech, Pfizer, Sunesis Pharmaceuticals, Viking Therapeutics
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I), Kleo Pharmaceuticals (I)
Consulting or Advisory Role: AstraZeneca, Moderna Therapeutics, Merck, Immunocore
Research Funding: Bristol-Myers Squibb (Inst)
Other Relationship: Clinical Care Options, Potomac Center for Medical Education
Jedd D. Wolchok
Stock and Other Ownership Interests: Potenza Therapeutics, Tizona Therapeutics, Serametrix, Adaptive Biotechnologies, Trieza Therapeutics, ImVaq, Beigene, Elucida Oncology, Ascentage Pharma, Linneaus, Kleo Pharmaceuticals
Honoraria: American Association for Cancer Research, Institut Jules Bordet Instituut, Esanex
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, Polynoma, Polaris, Genentech, Beigene, Sellas Life Sciences, Lilly, Potenza Therapeutics, Tizona Therapeutics, Amgen, Chugai Pharma, Adaptive Biotechnologies, Ascentage Pharma, Ono Pharmaceutical, Bayer, PsiOxus Therapeutics, Kleo Pharmaceuticals, F-Star Biotechnology, Surface Oncology, Advaxis, Apricity Therapeutics, Array BioPharma, PureTech, Serametrix, Syndax, Neon Therapeutics, Celgene, PsiOxus Therapeutics, Trieza Therapeutics, Astellas Pharma
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), MedImmune (Inst), Leap Therapeutics (Inst), Incyte (Inst), Merck Sharp & Dohme (Inst), F-Star Therapeutics
Patents, Royalties, Other Intellectual Property: I am a co-inventor on an issued patent for DNA vaccines for treatment of cancer in companion animals. I am a co-inventor on a patent for use of oncolytic Newcastle Disease virus. I am a co-inventor and receive royalties for a blood test for monitoring myeloid derived suppressor cells. I am co-inventor and receive royalties for a patent for immune modulating antibodies. I am a co-inventor on a patent for CAR+ T cells targeting differentiation antigens as means to treat cancer. I am a co-inventor on a patent for anti-CD40 agonist MAb fused to monophosphoryl lipid A (MPL) for cancer therapy. Alphavirus replicon particles expressing TRP2. Engineered vaccinia viruses for cancer immunotherapy
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Chugai Pharma, Roche, Janssen, Amgen, Ono Pharmaceutical, Astellas Pharma, Celgene Therapeutics, Potenza Pharmaceuticals, Tizona Therapeutics, MedImmune, Novartis
Michael A. Postow
Honoraria: Bristol-Myers Squibb, Merck
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma, Newlink Genetics, Incyte, Merck, Aduro Biotech
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Array BioPharma (Inst), Infinity Pharmaceuticals (Inst), Rgenix (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Paul B. Chapman
Stock and Other Ownership Interests: Rgenix
Consulting or Advisory Role: Genentech, Takeda, Cell Medica, Merck, Immunocore, AstraZeneca
Research Funding: Pfizer, NCI (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 7.Tartari F, Santoni M, Burattini L, et al. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat Rev. 2016;48:20–24. doi: 10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (IPI)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36(supp; abstr 95031) [Google Scholar]
- 9.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann Oncol. 2019;30:1154–1161. doi: 10.1093/annonc/mdz110. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. doi: 10.1038/nm.4333. Zehir A, Benayed R, Shah RH, et al: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [Erratum: Nat Med 23:1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153) J Thorac Oncol. 2019;14:1628–1639. doi: 10.1016/j.jtho.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14. Osgood C, Mulkey F, Mishra-Kalyani PS, et al: FDA analysis of depth of response (DpR) and survival across 10 randomized controlled trials in patients with previously untreated unresectable or metastatic melanoma (UMM) by therapy type. J Clin Oncol 37, 2019 (suppl; abstr 9508) [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebbé C, Weber JS, Maio M, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol. 2014;25:2277–2284. doi: 10.1093/annonc/mdu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Urso CM, Wang ZG, Cao Y, et al. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Invest. 1991;87:284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restifo NP, Marincola FM, Kawakami Y, et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sucker A, Zhao F, Real B, et al. Genetic evolution of T-cell resistance in the course of melanoma progression. Clin Cancer Res. 2014;20:6593–6604. doi: 10.1158/1078-0432.CCR-14-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]