Abstract
PURPOSE
Surgery is the primary therapy for localized chondrosarcoma; for locally advanced and/or metastatic disease, no known effective systemic therapy exists. Mutations in the isocitrate dehydrogenase 1/2 (IDH1/2) enzymes occur in up to 65% of chondrosarcomas, resulting in accumulation of the oncometabolite D-2-hydroxyglutarate (2-HG). Ivosidenib (AG-120) is a selective inhibitor of mutant IDH1 approved in the United States for specific cases of acute myeloid leukemia. We report outcomes of patients with advanced chondrosarcoma in an ongoing study exploring ivosidenib treatment.
PATIENTS AND METHODS
This phase I multicenter open-label dose-escalation and expansion study of ivosidenib monotherapy enrolled patients with mutant IDH1 advanced solid tumors, including chondrosarcoma. Ivosidenib was administered orally (100 mg twice daily to 1,200 mg once daily) in continuous 28-day cycles. Responses were assessed every other cycle using RECIST (version 1.1).
RESULTS
Twenty-one patients (escalation, n = 12; expansion, n = 9) with advanced chondrosarcoma received ivosidenib (women, n = 8; median age, 55 years; range, 30-88 years; 11 had received prior systemic therapy). Treatment-emergent adverse events (AEs) were mostly grade 1 or 2. Twelve patients experienced grade ≥ 3 AEs; only one event was judged treatment related (hypophosphatemia, n = 1). Plasma 2-HG levels decreased substantially in all patients (range, 14%-94.2%), to levels seen in healthy individuals. Median progression-free survival (PFS) was 5.6 months (95% CI, 1.9 to 7.4 months); the PFS rate at 6 months was 39.5%. Eleven (52%) of 21 patients experienced stable disease.
CONCLUSION
In patients with chondrosarcoma, ivosidenib showed minimal toxicity, substantial 2-HG reduction, and durable disease control. Future studies of ivosidenib monotherapy or rational combination approaches should be considered in patients with advanced mutant IDH1 chondrosarcoma.
INTRODUCTION
Chondrosarcomas are rare primary malignancies of bone. Approximately 85% are of the conventional subtype and comprise a matrix of hyaline and/or myxoid cartilage; the remaining 15% include dedifferentiated, mesenchymal, and clear cell subtypes that have distinct clinicopathologic features.1,2 Conventional chondrosarcomas are categorized as central, peripheral, or periosteal according to anatomic location, and 90% are low or intermediate histologic grade at presentation.3 Current treatment options are limited, with surgery being the mainstay for most patients with localized disease.1 Chemotherapy is ineffective in conventional chondrosarcoma, and although chemotherapy options exist for dedifferentiated and mesenchymal subtypes, they provide minimal benefit.1 Therefore, new therapies are urgently needed for unresectable, metastatic, and refractory disease.
Isocitrate dehydrogenase (IDH) is a metabolic enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing α-ketoglutarate (α-KG). Gain-of-function mutations in IDH1 and IDH2 have been described in several malignancies, including glioma,4,5 acute myeloid leukemia (AML),6-8 and cholangiocarcinoma.9 IDH1/2 mutations are also documented in cartilaginous neoplasms, including up to 65% of conventional chondrosarcomas and up to 57% of dedifferentiated chondrosarcomas, as well as sporadic central cartilaginous tumors, periosteal chondromas, and enchondromas (a manifestation of the Ollier and Maffucci syndromes, which progress to chondrosarcoma in approximately 40% of cases).2,10-14
IDH mutations result in the neomorphic enzyme activity of reduction of α-KG to the oncometabolite D-2-hydroxyglutarate (2-HG).15 Elevated 2-HG levels have been reported in cells from mutant IDH glioma,15 chondrosarcoma,16 cholangiocarcinoma,9 and AML.6 The accumulation of 2-HG contributes to malignancy via epigenetic pathways, although the precise mechanism remains unclear. 2-HG competitively inhibits α-KG–dependent dioxygenases, such as the methylcytosine dioxygenase TET2, and histone lysine demethylases; these enzymes regulate DNA and histone methylation, respectively, and control gene expression epigenetically.17-22 The induced hypermethylated phenotype has been reported in leukemias, gliomas, and enchondromas.11,17,18,23
In a preclinical study, mutant IDH1 mice showed impaired chondrocyte differentiation and developed enchondromas.24 Furthermore, mutant IDH1 inhibition in chondrosarcoma cell lines decreased 2-HG production in a dose-dependent manner and inhibited tumor cell growth.16 These data provide a biologic rationale for mutant IDH1 inhibition as a clinical strategy in mutant IDH1 chondrosarcoma.
Recently, agents targeting mutant IDH125 and IDH226 have received US Food and Drug Administration (FDA) approval for adults with specific cases of AML with a susceptible IDH mutation as detected by an FDA-approved test. The mutant IDH1 inhibitor ivosidenib is currently under investigation in an ongoing phase I clinical study in patients with mutant IDH1 advanced solid tumors; here we report results for the chondrosarcoma cohort in this study.
PATIENTS AND METHODS
Study Design and Treatment
We performed a phase I multicenter open-label dose-escalation and expansion study of ivosidenib monotherapy in patients with advanced solid tumors with an IDH1 mutation, including cholangiocarcinoma, chondrosarcoma, and glioma. The primary objectives were to assess safety and tolerability and to determine the maximum tolerated dose and recommended phase II dose of ivosidenib.
Ivosidenib was administered orally in continuous 28-day cycles. For dose escalation, a standard 3 + 3 design was used, and doses tested were 100 mg twice daily and 300, 400, 500, 600, 800, 900, and 1,200 mg once daily. Patients were subsequently enrolled into four expansion cohorts: chondrosarcoma, cholangiocarcinoma, glioma, and other solid tumors not otherwise eligible for the other tumor-specific cohorts. On the basis of safety, tolerability, and pharmacokinetic/pharmacodynamic data from the dose-escalation phase, the 500 mg once daily dose was selected for all expansion cohorts.
Here, we report data from patients with chondrosarcoma enrolled in the dose-escalation and expansion cohorts. Inclusion criteria for this cohort in the escalation phase included known mutant IDH1 advanced chondrosarcoma that had recurred or progressed during or not responded to standard therapy (or for which the investigator felt there was no standard therapy) and measurable disease by RECIST (version 1.1)27; in the expansion phase, criteria were mutant IDH1 chondrosarcoma that was either locally advanced or metastatic and not amenable to complete surgical excision and an Eastern Cooperative Oncology Group performance status of 0 to 1. Key exclusion criteria included a heart rate–corrected QT interval ≥ 450 ms or other factors that would increase the risk of QT prolongation or arrhythmic events and use of medications known to prolong the QT interval. Mutant IDH status for enrollment was determined by participating sites and confirmed retrospectively by centralized tissue testing.
The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation for Good Clinical Practice. The protocol, amendments, and informed consent form were approved by the institutional review board or independent ethics committee of each study site before study start. Informed consent was obtained from each participant or a legally authorized representative.
Pharmacokinetic and Pharmacodynamic Assessments
Serial blood samples were drawn before and after dosing at multiple time points in both the escalation and expansion phases to determine circulating plasma concentrations of ivosidenib and 2-HG. Additional details are provided in the Data Supplement and the published pharmacokinetic/pharmacodynamic report.28
Levels of 2-HG were also measured in tumor biopsy samples performed when feasible at baseline, cycle 3 day 1, cycle 7 day 1 (if the patient had stable disease [SD] or a partial response [PR]), at any time disease progression (PD) was suspected, and/or at the end of treatment. 2-HG was extracted from tissue and evaluated using liquid chromatography and mass spectrometry (Data Supplement).
Study Assessments
Adverse events (AEs) were assessed at every visit and reported per the Common Terminology Criteria for Adverse Events (version 4.03; Data Supplement provides additional details). Prolongation of QT interval on ECG was specified as an AE of special interest because it is a known effect associated with ivosidenib.25
Clinical activity of ivosidenib was evaluated with serial radiographic evaluations to determine response to treatment based on RECIST (version 1.1).27 Computed tomography (CT) or magnetic resonance imaging (MRI) was conducted at screening and approximately every 56 days (8 weeks) during study treatment (independent of dose delays and/or dose interruptions) and/or at any time when PD was suspected. Radiographic assessment was also conducted at the end-of-treatment visit for patients who discontinued the study for reasons other than PD. Responses were classified as complete response (CR), PR, SD, or PD. End points of clinical activity included progression-free survival (PFS) and best overall response.
Exploratory assessments included confirmation of baseline mutant IDH1 status and identification of cooccurring mutations. Archival formalin-fixed paraffin-embedded samples were collected for analysis by next-generation sequencing using the FoundationOne panel (Foundation Medicine, Cambridge, MA),29 which includes 361 genes. Fresh-frozen tumor samples were also collected at baseline and analyzed by next-generation sequencing using the ACE Extended Cancer Panel (Personalis, Menlo Park, CA), which includes 1,642 genes.
Statistical Analysis
The safety and full analysis sets for chondrosarcoma both comprised all patients who had received at least one ivosidenib dose (dose-escalation and expansion phases combined). The data cutoff date for the analyses reported here was January 16, 2019. Time-to-event end points were estimated using the Kaplan-Meier method. Descriptive statistics were used for other clinical, pharmacokinetic, and pharmacodynamic parameters.
RESULTS
Patients
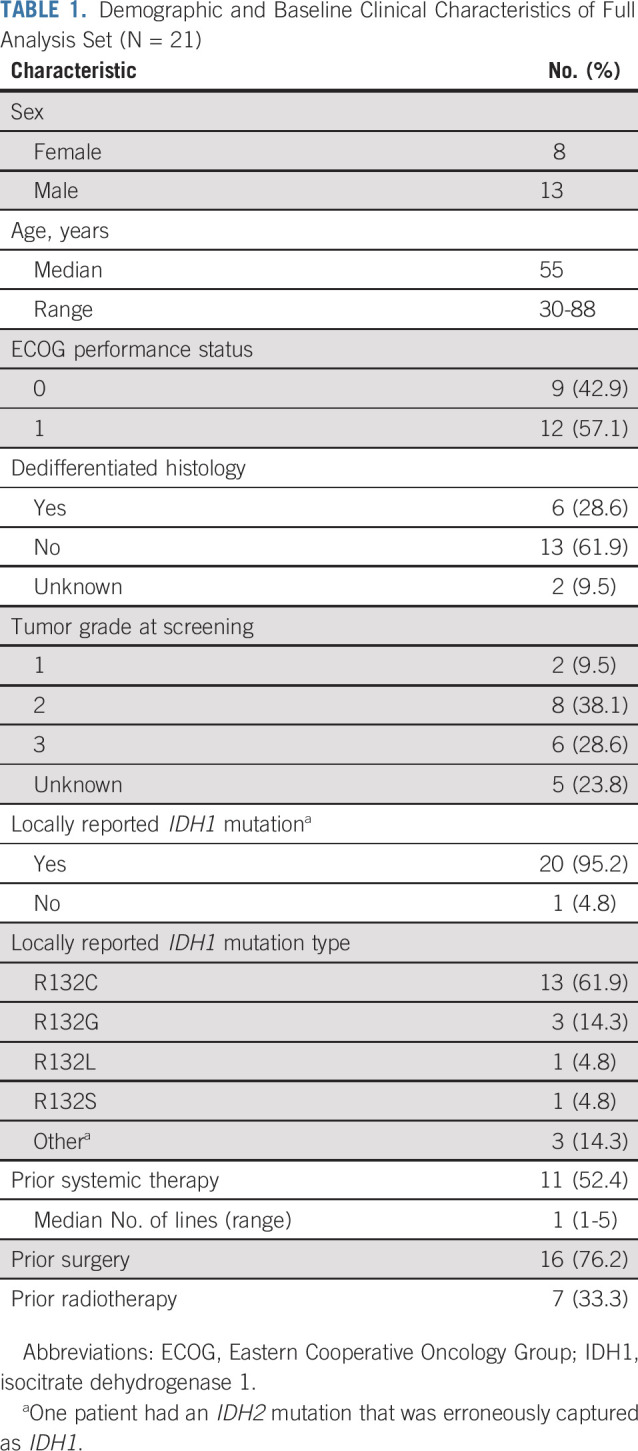
This trial was initiated in March 2014, with a total of 168 patients with mutant IDH1 advanced solid tumors receiving study treatment, of whom 21 had chondrosarcoma (dose escalation, n = 12; expansion, n = 9 [closed because of slow accrual]). Enrollment is complete, and the study is ongoing. Doses received in patients with chondrosarcoma were 100 mg twice daily (n = 1), 400 mg once daily (n = 4), 500 mg once daily (n = 11), 800 mg once daily (n = 3), and 1,200 mg once daily (n = 2). Of the 21 patients, four (19%) were still receiving treatment at the time of data cutoff (ivosidenib doses of 500, 800, 1,200, and 1,200 mg, respectively). Reasons for treatment discontinuation were PD (n = 10; 47.6%), AEs (n = 2; 9.5%), death (n = 2; 9.5%), withdrawal by patient (n = 1; 4.8%), clinical progression (n = 1; 4.8%), and other (transitioned to single-patient investigational new drug application, n = 1; 4.8%). Median age was 55 years, and most patients (n = 14) had grade 2 or 3 chondrosarcoma at screening (grade 1 chondrosarcoma, n = 2); six had dedifferentiated histology. Eleven patients (52%) had received prior systemic therapy (Table 1 lists demographic characteristics).
TABLE 1.
Demographic and Baseline Clinical Characteristics of Full Analysis Set (N = 21)
Pharmacokinetics and Pharmacodynamics
In patients with chondrosarcoma, ivosidenib was rapidly absorbed, with a half-life of 72 to 297 hours after a single dose (day −3), supporting a daily dosing regimen. Steady state was achieved within 14 days of dosing. Plasma exposure increased less than proportionally to dose with increasing doses.
Maximal 2-HG inhibition in plasma was achieved during the first 28-day cycle in patients receiving 500 mg once daily, with no additional inhibition observed at higher doses, in all tumor types. Plasma 2-HG was inhibited in all patients with chondrosarcoma, with consistent and substantial inhibition (median, 56.7%; range, 14%-94.2%) after multiple doses of ivosidenib, to levels consistent with those seen in healthy volunteers (Data Supplement). Similar plasma 2-HG inhibition was also observed in patients with cholangiocarcinoma.28 In two patients with chondrosarcoma who underwent tumor biopsies during the study, substantial reduction in 2-HG was also observed in tumor compared with baseline (85.4% and 98.6% inhibition, respectively). Additional details for all solid tumor types assessed in this study are provided in the full pharmacokinetic and pharmacodynamic report.28
Safety
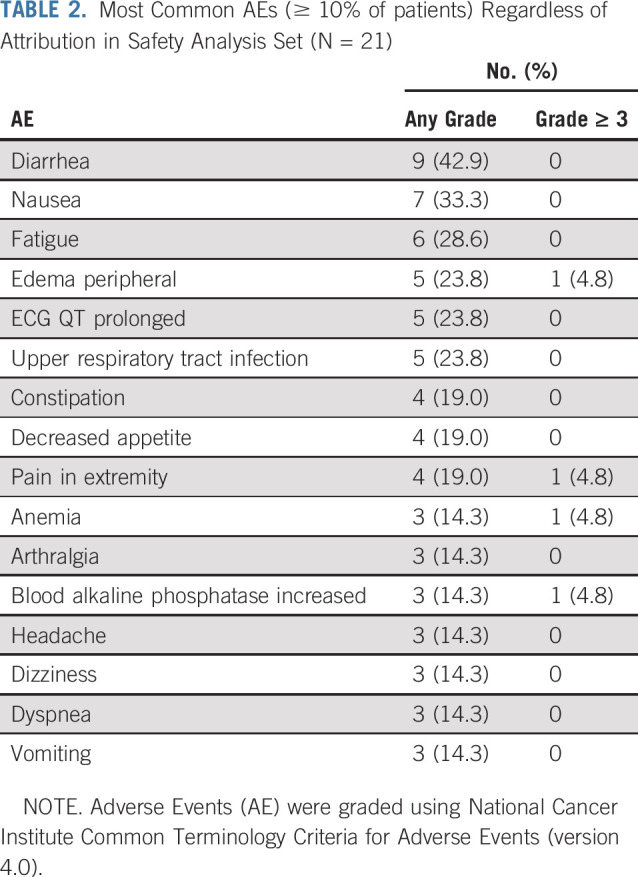
The maximum tolerated dose was not established in this study for the entire patient population (including all solid tumors), because there were no dose-limiting toxicities. On the basis of findings from the pharmacokinetic and pharmacodynamic analyses, including less than dose proportional increases in exposure beyond 500 mg and maximum suppression of plasma 2-HG at 500 mg, the 500 mg once-daily dose level was selected for the expansion phase.28 Here we report safety results from the chondrosarcoma cohort only; the safety profile in this cohort was generally consistent with that observed in the cholangiocarcinoma and glioma cohorts in this study, with fatigue, diarrhea, and nausea among the most common AEs.30,31 The most common treatment-emergent AEs in the chondrosarcoma cohort (Table 2) were mostly grade 1 or 2. Of the grade ≥ 3 AEs in 12 patients (57.1%; Data Supplement), only one was judged to be treatment related by the investigator (hypophosphatemia, n = 1). One patient had an AE leading to permanent drug discontinuation (hydronephrosis at 500 mg), which was not considered treatment related, and two had AEs leading to drug hold (anemia at 400 mg and confusion at 500 mg, respectively). There were no AEs leading to dose reductions. Grade 1 or 2 ECG QT prolongation occurred in five patients (23.8%); there were no grade ≥ 3 events, and no dose modifications were required for QT prolongation. There were two deaths during treatment resulting from serious AEs: acute respiratory failure at 400 mg and respiratory failure at 500 mg, respectively, both considered unrelated to study treatment by the investigator.
TABLE 2.
Most Common AEs (≥ 10% of patients) Regardless of Attribution in Safety Analysis Set (N = 21)
Clinical Efficacy
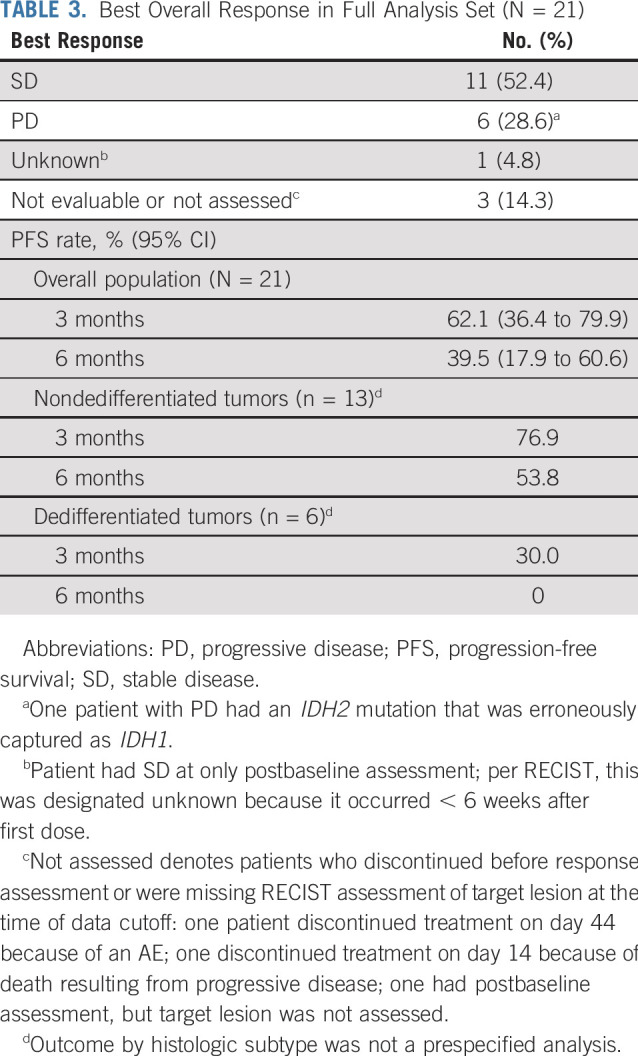
All 21 patients with chondrosarcoma were included for efficacy evaluation; 11 (52%) experienced SD as the best overall response by RECIST, six (29%) had PD, one (5%) had a response of unknown, and three (14%) were not assessed. There were no CRs or PRs. As of the data cutoff, median PFS for all patients (n = 21) was 5.6 months (95% CI, 1.9 to 7.4 months), with 3- and 6-month PFS rates of 62.1% (95% CI, 36.4% to 79.9%) and 39.5% (95% CI, 17.9% to 60.6%), respectively (Table 3; Data Supplement). Although the number of patients with dedifferentiated histology was small (n = 6), their outcomes were poor, with only 30% of patients progression free at 3 months and none by 6 months. In comparison, the 3-, 6-, and 12-month PFS rates for patients with nondedifferentiated tumors (n = 13) were 76.9%, 53.8%, and 30.8%, although outcome by histologic subtype was not a prespecified analysis. Six patients (28.6%) experienced PD as the best overall response, one of whom had an IDH2 mutation (originally erroneously reported as mutant IDH1 positive in the local pathology report, which was later corrected).
TABLE 3.
Best Overall Response in Full Analysis Set (N = 21)
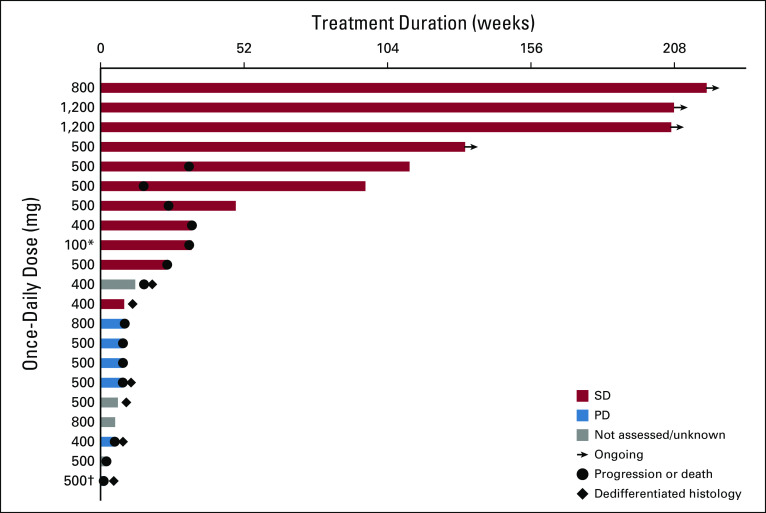
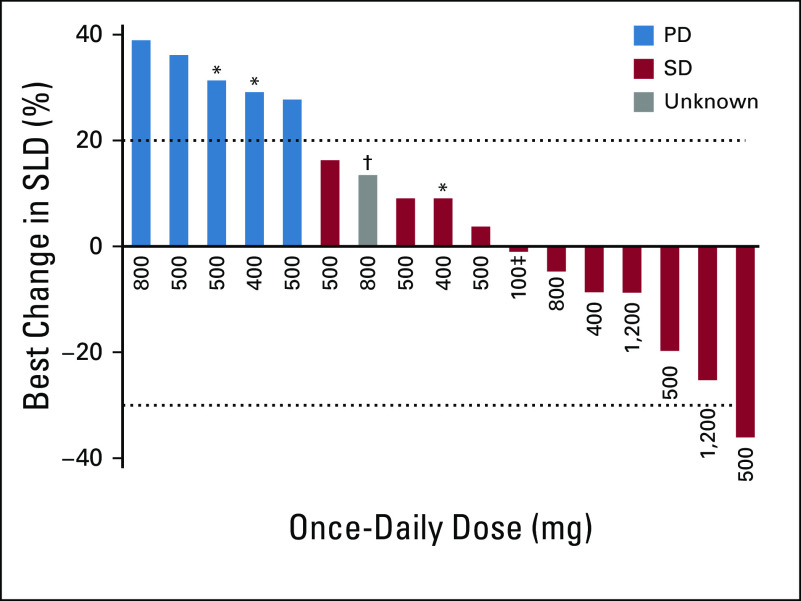
Duration of therapy is shown in Figure 1. Four patients (19%) have continued therapy for ≥ 2.5 years with a best response of SD. Of these, one patient previously had radiation therapy and therapy with the hedgehog pathway inhibitor saridegib for 1 year until PD in October 2011, followed by resection in November 2011. Further PD was detected in May 2014, followed by resection in January 2015 after progression of remaining sites; ivosidenib then commenced in late January 2015. Another patient had previously received saridegib therapy (4 months) followed by pazopanib (15 months to October 2013) until PD; a scan in September 2014 revealed metastasis, and ivosidenib commenced in September 2014. One patient diagnosed in October 2014 had documented PD in 2015 and commenced ivosidenib in July 2016. Several patients with SD experienced a reduction in tumor size (Fig 2). An example is shown in the Data Supplement, and according to the treating investigator, this patient reported resolution of pain, was able to discontinue analgesics, and had a > 60% decrease in T2 hyperintense tumor volume by MRI; the patient received therapy for > 3 years. Additionally, the Data Supplement illustrates improvement in fluorodeoxyglucose-avid disease after four cycles of ivosidenib in a patient with metastatic chondrosarcoma who continued the study drug for 343 days.
FIG 1.
Duration of treatment and best overall response (full analysis set; patients classified according to assigned dose level). Patients could continue to receive therapy beyond disease progression (PD) if they were experiencing clinical benefit in the investigator’s opinion. (*) Administered twice daily. (†) One patient with PD had an IDH2 mutation that was erroneously captured as IDH1. SD, stable disease.
FIG 2.
Best percentage change in the sum of the longest diameter (SLD) of the target lesions (full analysis set; patients classified according to assigned dose level). Dashed lines represent RECIST thresholds for progressive disease (PD; 20% increase) and partial response (30% decrease). Postbaseline assessments were not available for three patients; also excluded is the patient with an IDH2 mutation that was erroneously captured as IDH1. SD, stable disease. (*) Patients with dedifferentiated disease. (†) Patient had one postbaseline assessment of target lesion; per RECIST, this was designated unknown because it occurred < 6 weeks after first dose. (‡) Administered twice daily.
Translational Analyses
Baseline cooccurring mutation profiling was conducted for 13 patients with available tumor tissue. Figure 3 shows the known or likely oncogenic mutations that were detected at baseline, with patients ordered by PFS. In this limited data set, patients with shorter PFS were more likely to have dedifferentiated histology and mutations in TP53 or receptor tyrosine kinase pathway genes at baseline, and patients without cooccurring mutations as detected by these panels had longer PFS. One patient who was known to have a locally positive IDH1 R132H mutation did not have central confirmation.
FIG 3.
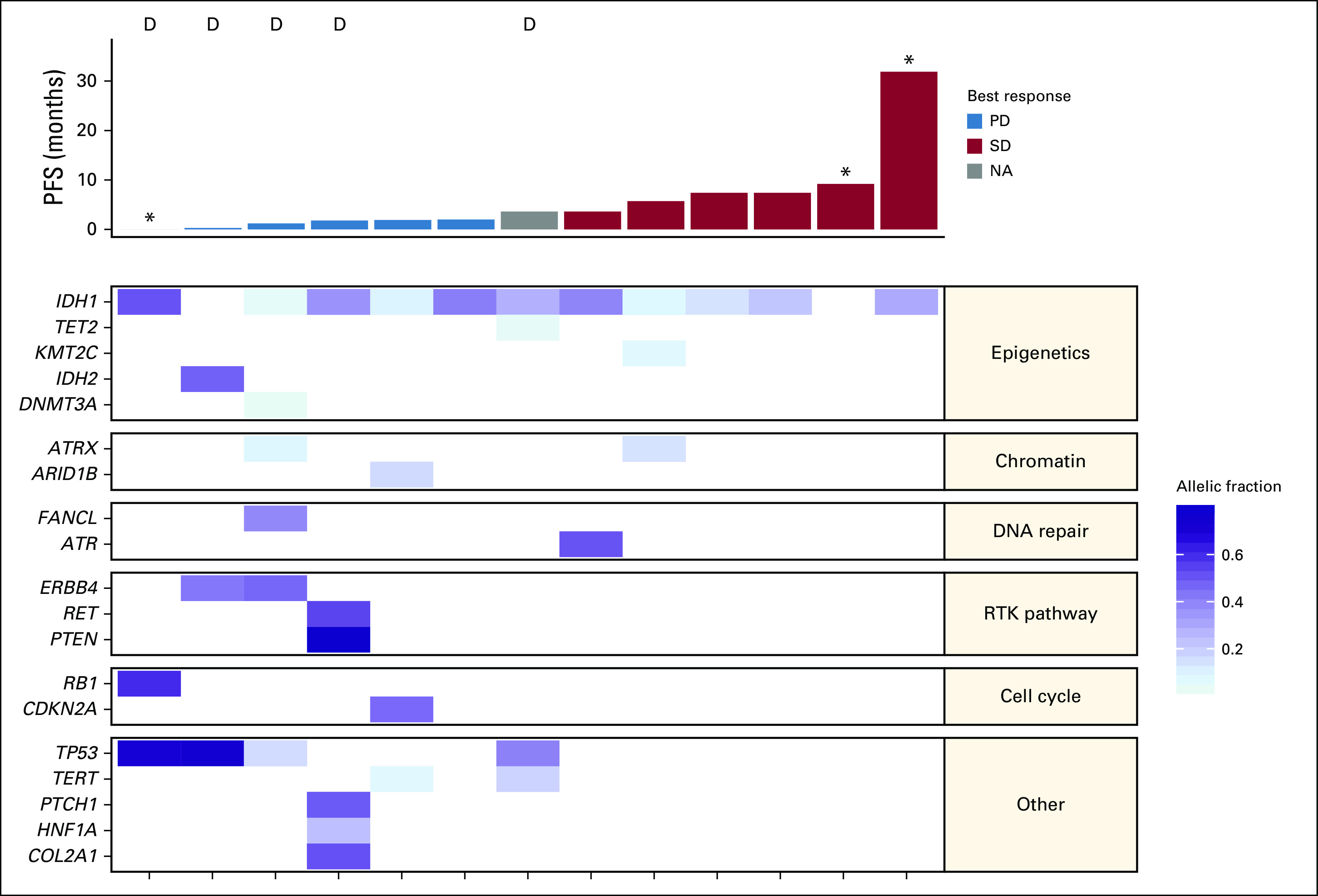
Cooccurring mutations (n = 13). The heatmap shows the allelic fraction of known or likely oncogenic mutations that were detected at baseline, with patients ordered by progression-free survival (PFS). Only genes present in both FoundationOne and Personalis panels are included. D, dedifferentiated histology at baseline; NA, not assessed; PD, progressive disease; RTK, receptor tyrosine kinase; SD, stable disease. (*) Denotes censoring.
DISCUSSION
A majority of patients with chondrosarcoma have subtypes that are resistant to standard chemotherapy. Therefore, novel therapies are urgently needed for patients with metastatic or locally advanced chondrosarcoma. The observation that IDH mutations are commonly detected in chondrosarcomas offers hope that targeted mutant IDH inhibitors, such as ivosidenib, may provide a novel treatment strategy and improve outcomes for these patients.
In this phase I study, ivosidenib was well tolerated in a chondrosarcoma population. A majority of AEs were consistent with those seen in other mutant IDH solid tumors in this study.30,31 There was a low frequency of grade ≥ 3 treatment-related AEs. The AE of interest of ECG QT prolongation was reported in five patients (23.8%; no grade ≥ 3); guidelines for the management of such events were provided to all investigators. Ivosidenib resulted in significant reductions in plasma and tumor 2-HG, indicating an on-target pharmacodynamic effect.
Ivosidenib also demonstrated clinical activity, with an SD rate of 52%, median PFS of 5.6 months, and 6-month PFS rate of 39.5%. Moreover, the higher PFS rates in patients without dedifferentiated histology suggest that ivosidenib may be more effective in conventional chondrosarcoma. Dedifferentiated chondrosarcomas are clinically aggressive, with previous literature reporting that patients with these tumors have worse outcomes.32-34 The additional molecular aberrations seen in dedifferentiated tumors may explain the lack of response. However, several of the patients with conventional chondrosarcoma had evidence of progression after prior interventions and went on to experience prolonged disease control and clinical benefit with ivosidenib. Diverse treatment regimens have been studied in patients with advanced chondrosarcoma. The hedgehog pathway, which is important for regulating chondrocyte proliferation and differentiation, is deregulated in chondrosarcoma. Hedgehog pathway inhibitors (eg, GDC-0449 and saridegib) have shown mixed results, with one GDC-0449 study reporting a median PFS of approximately 3.5 months in advanced chondrosarcoma,35 whereas a subsequent global phase II randomized placebo-controlled trial of saridegib in chondrosarcoma showed no improvement in PFS, and the trial was ultimately stopped after a planned futility analysis.36 In a study comparing outcomes of first-line systemic treatments for unresectable conventional chondrosarcoma, mean PFS included: doxorubicin, 2.5 months (n = 2); doxorubicin with either cisplatin or ifosfamide, 3.6 months (n = 10); gemcitabine and docetaxel, 2 months (n = 3); pazopanib with or without trametinib, 3.7 months (n = 7); dasatanib, 2.2 months (n = 4); vorinostat and hydroxychloroquine, 5 months (n = 4); and antihormonal therapy, 6.7 months (n = 7).37 In our study, four patients treated with ivosidenib received the study drug for ≥ 2.5 years without PD, despite experiencing an overall shorter treatment duration with prior therapy. This signal of disease control is exceptional for patients with previously treated advanced chondrosarcoma. One could posit that these favorable outcomes with ivosidenib, seen in selected patients with mutant IDH1 tumors, occurred because mutant IDH1 tumors are clinically indolent compared with wild-type IDH1 tumors. Currently available literature is mixed in this regard, suggesting both worse and favorable outcomes among mutant IDH chondrosarcomas based on retrospective reports.38,39
Our study also highlights the limitation of using RECIST to meaningfully assess tumor response in patients with chondrosarcoma. Specifically, durable progression-free intervals were seen in several patients treated with ivosidenib despite lack of response on RECIST. Interestingly, there were other imaging features noted in some cases, including reduction in MRI signal enhancement, increased calcifications on CT scans, and reduction in fluorodeoxyglucose avidity over the course of therapy. Similar limitations with RECIST have been reported in clinical trials for patients with other types of sarcoma.40 There is therefore a need to develop additional methods to quantify treatment benefit in these tumor types.
Although the results of this phase I trial are encouraging and provide hope for patients with chondrosarcoma, this analysis focused primarily on tolerability in a limited number of patients with chondrosarcoma. Overall survival was also not captured in this patient cohort, and therefore, it was not possible to draw conclusions on whether there is a survival benefit with ivosidenib. Patient samples were also limited for translational studies, and therefore, longitudinal mutational analysis could not be performed to determine the emergence of resistant clones during treatment.
In conclusion, ivosidenib showed durable disease control in patients with mutant IDH1 conventional chondrosarcoma. In addition to minor tumor regression by RECIST, four patients received treatment for ≥ 2.5 years without PD, and substantial reductions in 2-HG were reported in the plasma and available tumor biopsies. Importantly, ivosidenib was well tolerated, with no dose-limiting toxicities and few grade ≥ 3 treatment-related AEs. The recommended phase II dose was determined to be 500 mg once daily. The development of ivosidenib has the potential to offer therapy to patients with mutant IDH1 chondrosarcoma who have no treatment options.
ACKNOWLEDGMENT
We thank Helen Varley, PhD, CMPP (Excel Medical Affairs, Horsham, United Kingdom), for providing medical writing assistance, supported by Agios Pharmaceuticals; Julia Auer (Agios Pharmaceuticals) for scientific and operational contributions; and Ty Subhawong (Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL) for contribution to the radiographic findings.
PRIOR PRESENTATION
Presented in abstract and poster formats at the 21st Annual Meeting of the Connective Tissue Oncology Society, Lisbon, Portugal, November 9-12 2016.
SUPPORT
Supported by Agios Pharmaceuticals and in part by National Cancer Institute Grants No. P30 CA008748 (W.D.T.) and 1 P30 CA 240139-01 (J.C.T.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: William D. Tap, Howard Burris, Liewen Jiang, Bin Wu, Bin Fan, Sam Agresta, Shuchi S. Pandya, Jonathan C. Trent
Provision of study material or patients: William D. Tap, Victor M. Villalobos, Gregory M. Cote, Filip Janku, Olivier Mir, Sam Agresta, Jonathan C. Trent
Collection and assembly of data: William D. Tap, Victor M. Villalobos, Gregory M. Cote, Howard Burris, Filip Janku, Olivier Mir, Murali Beeram, Andrew J. Wagner, Liewen Jiang, Bin Wu, Camelia Gliser, Shuchi S. Pandya, Jonathan C. Trent
Data analysis and interpretation: William D. Tap, Victor M. Villalobos, Gregory M. Cote, Howard Burris, Filip Janku, Andrew J. Wagner, Liewen Jiang, Bin Wu, Sung Choe, Katharine Yen, Camelia Gliser, Bin Fan, Jonathan C. Trent
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study of the Mutant IDH1 Inhibitor Ivosidenib: Safety and Clinical Activity in Patients With Advanced Chondrosarcoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
William D. Tap
Leadership: Certis Oncology Solutions, Atropos Pharmaceuticals
Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos Pharmaceuticals
Consulting or Advisory Role: EMD Serono, Eli Lilly, Daiichi Sankyo, Eisai, Blueprint Medicines, Agios Pharmaceuticals, GlaxoSmithKline, NanoCarrier, Deciphera
Research Funding: Novartis, Eli Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines, Immune Design, BioAtla, Deciphera
Patents, Royalties, Other Intellectual Property: Companion diagnostic for CDK4 inhibitors 14/854,329
Victor M. Villalobos
Consulting or Advisory Role: Janssen, Eli Lilly, Novartis, AbbVie, Ignyta, Agios Pharmaceuticals, Epizyme, Blueprint Medicines, Springworks Therapeutics, NanoCarrier, Daiichi Sankyo
Travel, Accommodations, Expenses: Eli Lilly, Janssen, Xencor, GenMab, Epizyme
Gregory M. Cote
Consulting or Advisory Role: Agios Pharmaceuticals, PharmaMar, Epizyme
Research Funding: Macrogenics (Inst), Boston Biomedical (Inst), PharmaMar (Inst), Epizyme (Inst), Agios Pharmaceuticals (Inst), Eisai (Inst), Merck (Inst), Plexxikon (Inst), CBA (Inst), Bavarian Nordic, Bayer (Inst), Springworks Therapeutics (Inst)
Howard Burris
Employment: HCA Healthcare/Sarah Cannon
Leadership: HCA Healthcare/Sarah Cannon
Stock and Other Ownership Interests: HCA Healthcare/Sarah Cannon
Consulting or Advisory Role: AstraZeneca (Inst), FORMA Therapeutics (Inst), Celgene (Inst), Incyte (Inst)
Research Funding: Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst), Macrogenics (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Seattle Genetics (Inst), Merck (Inst), Agios Pharmaceuticals (Inst), Jounce Therapeutics (Inst), Moderna Therapeutics (Inst), CytomX Therapeutics (Inst), GlaxoSmithKline (Inst), Verastem (Inst), Tesaro (Inst), Millennium Pharmaceuticals (Inst), BioMed Valley Discoveries (Inst), TG Therapeutics (Inst), Vertex (Inst), eFFECTOR Therapeutics (Inst), Janssen (Inst), Gilead Sciences (Inst), BioAlta (Inst), CicloMed (Inst), Harpoon Therapeutics (Inst), Arch (Inst), Arvinas (Inst), Revolution Medicines (Inst), Array BioPharma (Inst), Bayer (Inst), BIND Therapeutics (Inst), Kymab (Inst), miRNA Therapeutics (Inst), Pfizer (Inst)
Expert Testimony: Novartis (Inst)
Uncompensated Relationships: Daiichi Sankyo (Inst), Pfizer (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/201030/summary
Filip Janku
Stock and Other Ownership Interests: Trovagene
Consulting or Advisory Role: Deciphera, Trovagene, Novartis, Sequenom, Foundation Medicine, Guardant Health, Immunome, Synlogic, Valeant/Dendreon, IFM Therapeutics, Sotio, PureTech
Research Funding: Novartis (Inst), BioMed Valley Discoveries (Inst), Roche (Inst), Agios Pharmaceuticals (Inst), Astellas Pharma (Inst), Deciphera (Inst), Plexxikon (Inst), Piqur (Inst), Fujifilm (Inst), Symphogen (Inst), Bristol-Myers Squibb (Inst), Asana Biosciences (Inst), Astex Pharmaceuticals (Inst)
Other Relationship: Bio-Rad
Olivier Mir
Stock and Other Ownership Interests: Transgene, Amplitude Surgical, Ipsen
Honoraria: Roche
Consulting or Advisory Role: Eli Lilly, Pfizer, Roche, Lundbeck, Janssen
Speakers’ Bureau: Eli Lilly, Roche, Pfizer
Travel, Accommodations, Expenses: Roche, Pfizer
Murali Beeram
Honoraria: Genentech (Inst), Johnson & Johnson (Inst)
Consulting or Advisory Role: Novartis (I)
Speakers’ Bureau: Genentech (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
Research Funding: Eli Lilly (Inst), Zymeworks (Inst), Mersana (Inst)
Travel, Accommodations, Expenses: Genentech, Merck
Andrew J. Wagner
Honoraria: Novartis
Consulting or Advisory Role: Eli Lilly, Five Prime Therapeutics, Loxo, Daiichi Sankyo, Deciphera
Research Funding: Eli Lilly (Inst), Plexxikon (Inst), Daiichi Sankyo (Inst), Karyopharm Therapeutics (Inst), AADi (Inst)
Liewen Jiang
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios Pharmaceuticals
Bin Wu
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Agios Pharmaceuticals
Sung Choe
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Patents derived from my work at Agios Pharmaceuticals
Travel, Accommodations, Expenses: Agios Pharmaceuticals
Katharine Yen
Employment: Agios Pharmaceuticals
Leadership: Auron Therapeutics
Stock and Other Ownership Interests: Agios Pharmaceuticals, Auron Therapeutics
Consulting or Advisory Role: Agios Pharmaceuticals
Research Funding: Auron Therapeutics
Patents, Royalties, Other Intellectual Property: Patents around IDH-mutant inhibitors and methods of treatment
Travel, Accommodations, Expenses: Agios Pharmaceuticals, Auron Therapeutics
Camelia Gliser
Employment: Agios Pharmaceuticals, KSQ Therapeutics (I)
Stock and Other Ownership Interests: Agios Pharmaceuticals, Agios Pharmaceuticals (I), KSQ Therapeutics (I)
Bin Fan
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios Pharmaceuticals
Travel, Accommodations, Expenses: Agios Pharmaceuticals
Sam Agresta
Leadership: Infinity
Stock and Other Ownership Interests: Infinity
Shuchi S. Pandya
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios Pharmaceuticals
Research Funding: Agios Pharmaceuticals
Travel, Accommodations, Expenses: Agios Pharmaceuticals
Jonathan C. Trent
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Novartis, Eli Lilly, Janssen, Blueprint Medicines, Deciphera, Daiichi Sankyo, Epizyme
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Bone Cancer—Version 1.2020. https://www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PMC free article] [PubMed]
- 2.Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 3.Jamil N, Howie S, Salter DM. Therapeutic molecular targets in human chondrosarcoma. Int J Exp Pathol. 2010;91:387–393. doi: 10.1111/j.1365-2613.2010.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amary MF, Damato S, Halai D, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43:1262–1265. doi: 10.1038/ng.994. [DOI] [PubMed] [Google Scholar]
- 11.Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43:1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai M, Nobusawa S, Ikota H, et al. Frequent IDH1/2 mutations in intracranial chondrosarcoma: A possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol. 2012;29:201–206. doi: 10.1007/s10014-012-0085-1. [DOI] [PubMed] [Google Scholar]
- 13.Tallegas M, Miquelestorena-Standley É, Labit-Bouvier C, et al. IDH mutation status in a series of 88 head and neck chondrosarcomas: Different profile between tumors of the skull base and tumors involving the facial skeleton and the laryngotracheal tract. Hum Pathol. 2019;84:183–191. doi: 10.1016/j.humpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Verdegaal SH, Bovée JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: An international multicenter study of 161 patients. Oncologist. 2011;16:1771–1779. doi: 10.1634/theoncologist.2011-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Paz AC, Wilky BA, et al. Treatment with a small molecule mutant IDH1 inhibitor suppresses tumorigenic activity and decreases production of the oncometabolite 2-hydroxyglutarate in human chondrosarcoma cells. PLoS One. 2015;10:e0133813. doi: 10.1371/journal.pone.0133813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterfall JJ, Killian JK, Meltzer PS. The role of mutation of metabolism-related genes in genomic hypermethylation. Biochem Biophys Res Commun. 2014;455:16–23. doi: 10.1016/j.bbrc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Cairns RA, Harris I, McCracken S, et al. Cancer cell metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:299–311. doi: 10.1101/sqb.2011.76.012856. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1038/nature13441. Saha SK, Parachoniak CA, Ghanta KS, et al: Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 513:110-114, 2014 [Erratum: Nature 528:152, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata M, Sasaki M, Cairns RA, et al. Mutant IDH is sufficient to initiate enchondromatosis in mice. Proc Natl Acad Sci USA. 2015;112:2829–2834. doi: 10.1073/pnas.1424400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agios Pharmaceuticals: TIBSOVO (ivosidenib tablets) prescribing information. https://www.tibsovopro.com/pdf/prescribinginformation.pdf.
- 26. Celgene: IDHIFA (enasidenib) tablets for oral use (prescribing information). https://media.celgene.com/content/uploads/idhifa-pi.pdf.
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Fan B, Mellinghoff IK, Wen PY, et al: Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs 38:433–444.2020 [Google Scholar]
- 29.He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004–3014. doi: 10.1182/blood-2015-08-664649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mellinghoff IK, Touat M, Maher EA, et al: AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: Updated results from the phase 1 non-enhancing glioma population. Neuro-oncol 19:vi10-vi11, 2017 (suppl 6; abstr ACTR-46) [Google Scholar]
- 31.Lowery MA, Burris HA, III, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:711–720. doi: 10.1016/S2468-1253(19)30189-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Mavrogenis AF, Angelini A, Drago G, et al. Survival analysis of patients with chondrosarcomas of the pelvis. J Surg Oncol. 2013;108:19–27. doi: 10.1002/jso.23351. [DOI] [PubMed] [Google Scholar]
- 34.Strotman PK, Reif TJ, Kliethermes SA, et al. Dedifferentiated chondrosarcoma: A survival analysis of 159 cases from the SEER database (2001-2011) J Surg Oncol. 2017;116:252–257. doi: 10.1002/jso.24650. [DOI] [PubMed] [Google Scholar]
- 35.Italiano A, Le Cesne A, Bellera C, et al. GDC-0449 in patients with advanced chondrosarcomas: A French Sarcoma Group/US and French National Cancer Institute single-arm phase II collaborative study. Ann Oncol. 2013;24:2922–2926. doi: 10.1093/annonc/mdt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Infinity Pharmaceuticals: Infinity stops phase 2 trials of saridegib in chondrosarcoma and myelofibrosis. https://www.businesswire.com/news/home/20120618005411/en/Infinity-Stops-Phase-2-Trials-Saridegib-Chondrosarcoma.
- 37.van Maldegem A, Conley AP, Rutkowski P, et al. Outcome of first-line systemic treatment for unresectable conventional, dedifferentiated, mesenchymal, and clear cell chondrosarcoma. Oncologist. 2019;24:110–116. doi: 10.1634/theoncologist.2017-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugowska I, Teterycz P, Mikula M, et al. IDH1/2 mutations predict shorter survival in chondrosarcoma. J Cancer. 2018;9:998–1005. doi: 10.7150/jca.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu GG, Nafa K, Agaram N, et al. Genomic profiling identifies association of IDH1/IDH2 mutation with longer relapse-free and metastasis-free survival in high-grade chondrosarcoma. Clin Cancer Res. 2020;26:419–427. doi: 10.1158/1078-0432.CCR-18-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]