Abstract
Alcoholism synergizes the development of the hepatocellular carcinoma (HCC) in patients infected with hepatitis B or C virus (HBV or HCV). Tumor-initiating stem-like cells (TICs) are refractory to therapy and have expression of stemness transcription factors. Leaky-gut-derived endotoxin stimulates TLR4-NANOG pathway that skews asymmetric cell division and that metabolically reprograms hepatocytes/liver progenitor cells, leading to self-renewal. TICs isolated from mouse HCC models or human HCCs are tumorigenic and have p53 degradation via phosphorylation of the protective protein NUMB and its dissociation from p53 by the oncofetal protein TBC1D15. Furthermore, dysregulation of lncRNA promotes genesis of TICs, leading to HCC development. This review describes roles of cell fate decision, metabolic reprogramming and lncRNA for TIC genesis and liver oncogenesis. This project was supported by NIH grants 1R01AA018857-01, 5R21AA025470, P50AA11999 (Animal Core, Morphology Core, and Pilot Project Program), R24AA012885 (Non-Parenchymal Liver Cell Core) and pilot project funding (5P30DK048522-13).
Keywords: Alcohol, HCC, cancer stem cells, tumor-initiating stem-like cells (TICs), lncRNA, NUMB
Introduction
Alcoholic liver disease (ALD), obesity and viral hepatitis (HBV and HCV) are associated with development of hepatocellular carcinoma (HCC) (Okuda, 2000) (He et al., 2008; Okuda et al., 2002). HCV/HBV infection, ALD, and non-alcoholic fatty liver disease (NAFLD) synergizes liver oncogenesis through leaky-gut derived endotoxinemia, oxidant stress, organelle stress, and metabolic dysregulation.
Tumor-initiating stem cell-like cells (TICs) or so-called cancer stem cells (CSCs) are rare, highly malignant cells that are present in diverse tumor types and refractory to chemotherapy, radiation therapy and surgical resection. There is still controversy as to what defines a tumor-initiating cell in HCC. In studies for blood cancer, Dr. Morrison’s group successfully isolated cancer stem cells., Many other studies showed multipotent tumor-initiating stem-like cells, but not pluripotent cancer stem cells, HCV NS5A Tg mice fed alcohol western diet are specific models by which TICs are defined in the studies described and potential caveats to these model systems. Findings are listed, with deeper analysis and scrutiny in our published papers. Normal stem cells and TICs share three major characteristics, (i) self-renewal, (ii) clonality, and (iii) plasticity (Machida et al., 2009; Rountree et al., 2008). A multi-kinase inhibitor Sorafenib is the commonly used and FDA-approved monotherapy agent for the treatment of HCC; however, resistance to sorafenib eventually occurs in patients (Villanueva et al., 2008). Forty percent of HCC has clonality that originates from hepatocytes/progenitor cells (Alison, 2005; Roskams, 2006; Tang et al., 2008; Zender et al., 2006). TICs are chemo-resistant (Rountree et al., 2008), survive during an initial therapy, clonally expand, leading to tumor recurrence (Liu et al., 2010; Lobo et al., 2007; Visvader, 2011)
Co-morbidity of life-style factors for disease burdens (alcoholism and obesity) with viral hepatitis (HBV and HCV) synergizes HCC development
Alcohol and obesity are leading life-style factors for disease burdens around the world and synergistically elevate HCC incidence with infection of hepatitis viruses. More than 70 million people worldwide are infected with HCV (Okuda, 2000; Okuda et al., 2002; Yao and Terrault, 2001). Coexistence of alcohol abuse or obesity synergizes HCV risk of developing HCC by additional 8-fold, culminating to an overall 45-55-fold increase in the risk as compared to normal subjects (Yuan et al., 2004). Therefore, co-morbidity of lifestyle factors with viral hepatitis is an urgent unmet need for fundamental understanding mechanisms and discovering new therapeutic targets. Obesity and alcoholism increase gut permeability leading to endotoxemia, which in turn activates Toll-like receptor 4 (TLR4) in the liver with production of cytokines and an inflammatory response, leading to subsequent development of obesity/alcohol-related liver disease (Hritz et al., 2008). Circulating endotoxin binds CD14-TLR4 complex, activates hepatocytes/hepatoblasts and induces the stem cell marker NANOG. This process generates TLR4/NANOG-dependent, chemo-resistant tumor-initiating stem-like cells (TICs; CD133+), which can induce HCC in mice (Chen et al., 2013b). Tumors contain double-positive cells for NANOG and CD133 or CD49f (24). Liver-specific expression of the HCV NS5A protein in mice fed alcohol for 12 months develop liver tumors in a TLR4-dependent manner (Chen et al., 2013b). Treatment with sorafenib made TICs more susceptible to tumor growth retardation, with a decrease in tumor size by ~55% when combined with knockdown of NANOG-inducible proto-oncogenes (including YAP1, which induces antioxidant gene programs) (Chen et al., 2013b). However, the underlying mechanism of chemo-resistance and self-renewal of TICs remains incompletely understood.
TLR4-NANOG pathway metabolically reprograms drug resistance TICs via suppression of mitochondrial OXPHOS and activation of FAO
Toll-like receptor 4 (TLR4) is a putative proto-oncogene involved in the genesis/maintenance of TICs and liver tumor in HCV transgenic models. TLR4 silencing reduces heightened expression of stemness genes and cell proliferation (Chen et al., 2013a). CD133+/CD49f+ cells are TLR4/NANOG-dependent TICs. Hepatoblastic HCC subtype with poor prognosis has a gene expression profile with markers of hepatic oval cells (Andersen et al., 2010; Cai et al., 2012; Lee et al., 2006; Yamashita et al., 2009). HCC often recurs after chemotherapy due presumably to the presence of chemo-resistant TICs (Reya et al., 2001).
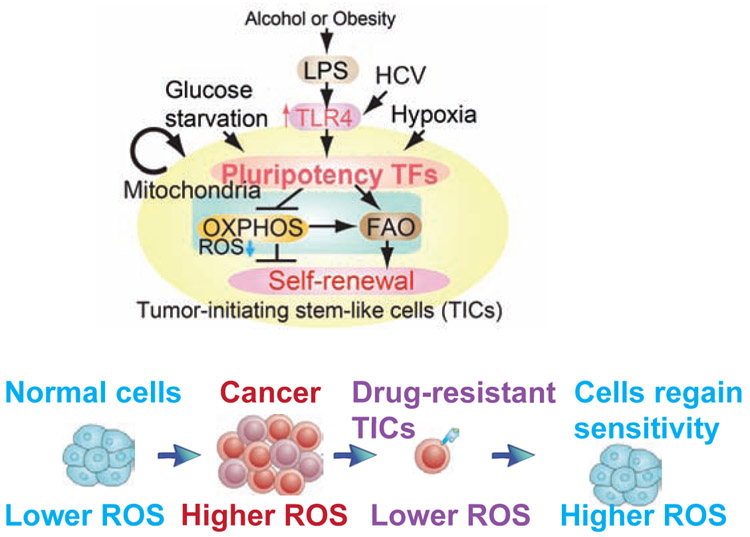
Endotoxin ligand-TLR4-CD14 complex formation triggers E2F1 phosphorylation to transactivate NANOG (Chen et al., 2013b). Complementary NANOG ChIP-seq and metabolomics studies of TICs demonstrated that NANOG induced by TLR4 suppressed mitochondrial OXPHOS and activated fatty acid oxidation (FAO), thus inhibiting OCR and ROS production. NANOG ChIP-seq identified genes associated with NANOG-dependent mitochondrial metabolic pathways to maintain TICs. The causal roles of NANOG in mitochondrial metabolic reprogramming occurred through the inhibition of oxidative phosphorylation (OXPHOS) with decreased production of mitochondrial ROS and activation of FAO, which was required for self-renewal and drug resistance (Chen et al., 2013b). Restoration of OXPHOS activity and inhibition of FAO rendered TICs susceptible to a standard care chemotherapy drug, sorafenib (Chen et al., 2013b). NANOG-dependent downstream effect on mitochondrial function contributed to the chemotherapy resistance (Chen et al., 2013b). These metabolic reprogramming promoted self-renewal/oncogenesis, and explained how NANOG activation inhibited therapy-mediated apoptosis by quenching ROS production. Restoration of OXPHOS or decreased FAO reduces tumorigenic capacity of TICs and increases susceptibility to chemotherapy (Chen et al., 2013b) (Fig. 1). The metabolic bases of altered cell functions and cell fate in TICs define potentially new approaches for chemo-sensitization and elimination of TICs for more efficacious HCC therapies.
Fig. 1.
Environmental factors (alcohol exposure and obesity) and virus infection (HBV and HCV) generates TIC through metabolic reprogramming and resistance to therapy-mediated ROS burst (Top) Endotoxinemia leads to TLR4-NANOG activation and promotes self-renewal. (Bottom) Drug resistant TICs acquires resistance to therapy-induced ROS burst and survive via reprogramming of metabolism.
As TICs rely on active FAO for their maintenance and function, FAO inhibitor suppresses self-renewal of TICs (Samudio et al., 2010). The FAO gene silencing reduces TIC phenotype while overexpression of FAO genes restores the original TIC phenotype. Thus FAO metabolically switches the fate of stem cells (Ito et al., 2012). Potential mechanisms by which elevation of FAO maintains self-renewal ability include: (i) shunting of long-chain FA away from lipid and cell membrane synthesis; (ii) downregulation of ROS through production of NADPH to avoid loss of TICs; and (iii) reduction of metabolic resistance to chemotherapy. By these criteria, NANOG function could be construed to serve as a gatekeeper for FAO activity. Activation of fatty acid oxidation (FAO) metabolically switches the fate of stem cells and TICs. Therefore, to develop better therapeutics, the underlying molecular mechanisms regulating obesity/alcohol/HCV-induced hepatocarcinogenesis should be elucidated.
NANOG maintains chemotherapy resistance of TICs involving not only the direct activation of self-renewal via stemness genes, but also the subsequent metabolic reprogramming in these cells leading to amplification of TIC oncogenic activity and their overall survival. Our data showed that NANOG reprogramming of mitochondrial metabolism was indeed responsible for human TIC oncogenicity and chemo-resistance. These studies have led to a paradigm shift in our understanding the underlying basis of alcohol/HCV-associated cancer, thus facilitating future development of new personalized treatment strategies targeted towards NANOG+ TICs arising from obesity, alcohol, or HCV-related HCC. The studies provide insights into the mechanisms of NANOG-mediated generation of TICs, tumorigenesis and chemo-resistance due to metabolic reprograming of mitochondrial functions.
HCV-mediated mechanisms also promote genesis of TICs to synergize oncogenesis through multiple direct oncogenic functions.
HCV proteins (nucleocapside Core and NS5A) are linked to transformation through overproduction of reactive oxygen species which may cause mitochondrial or nuclear DNA damage (Korenaga et al., 2005; Moriya et al., 2001; Okuda et al., 2002). The Core protein inhibits microsomal triglyceride transfer protein activity and VLDL (very-low-density lipoprotein) secretion (Perlemuter et al., 2002), which contributes the genesis of fatty liver. The core also induces insulin resistance in mice and cell lines, and this effect may be mediated by degradation of insulin receptor substrates (IRS) 1 and 2 via up regulation of SOCS3 (Kawaguchi et al., 2004) in a manner dependent on PA28γ 73, or via IRS serine phosphorylation (Banerjee et al., 2008). These mechanisms promote oncogenesis.
TICs have the heightened expression and activation of a pluripotency-associated transcription factor network
TICs maintain expression and activation of a pluripotency (stemness)-associated transcription factor (TF) network (Jaenisch and Young, 2008), such as Wnt/β-catenin, Notch, Hedgehog/SMO, and Oct3/4 (Beachy et al., 2004; Chambers and Smith, 2004; Valk-Lingbeek et al., 2004). TICs also have dysregulated signaling and gene expression, including PI3K/AKT (Ma et al., 2008), signal transducer and activator of transcription 3 (STAT3) (Wurmbach et al., 2007; Yeoh et al., 2007), and hedgehog (Sicklick et al., 2006a; Sicklick et al., 2006b) while defective tumor suppressor transforming growth factor-beta (TGF-β) pathway is also implicated (Kitisin et al., 2007; Nguyen et al., 2007).
Inactivation of cell fate determinant NUMB skews asymmetric cell division to generate TICs, leading to oncogenesis via p53 degradation, NUMB inactivation and stemness activation
Self-renewing divisions maintained stem cell populations since one daughter cell commits to a particular fate while the other retains the multipotent characteristics of its parent. Disruption of p53 reprograms cells to the pluripotent state (Aparicio and Eaves, 2009; Kawamura et al., 2009; Marion et al., 2009) while p53 represses the expression of pluripotency-associated transcription factors in proliferating stem cells (Li et al., 2013), interacts with the polarity determinant NUMB protein to maintain asymmetry and cell polarity. A tumor suppressor NUMB is asymmetrically distributed in dividing stem cells and is segregated into the daughter cell which undergoes differentiation. The association with NUMB stabilizes p53 (Bric et al., 2009; March et al., 2011) and protects deregulated expansion of TICs. Interaction between NUMB and p53 maintain the homeostasis of both stem cells and differentiated cells. NUMB-associated oncoproteins MDM2 E3 ubiquitin ligase destabilize the NUMB-p53 complex and promote proteolysis of p53 (Amson et al., 2012). We identified that phosphorylation of NUMB destabilize p53 and promotes self-renewal of TICs by pluripotency-associated transcription factor NANOG dependent manner. Phosphorylated NUMB is dissociated with binding partners and lose asymmetric functions, NANOG phosphorylates NUMB via aPKCζ, through the direct induction of Aurora A kinase (AURKA) and the repression of an aPKCζ inhibitor, LGL-2. Phosphorylation of NUMB by aPKCζ destabilizes the NUMB-p53 interaction, p53 proteolysis and to deregulate self-renewal in TICs. Furthermore, non-phosphorylatable mutant of NUMB expression reduced HCC incidence in HVC NS5A transgenic mice fed alcohol western diet (unpublished observation). These results indicate that NUMB phosphorylation is therapeutic targets for HCC treatment.
Dysregulated LncRNAs generates TICs to promote HCC development
Non-coding RNAs [LncRNA, microRNA (miRNA) and etc.] are dysregulated in liver carcinogenesis via regulation of gene expression. The roles of LncRNAs are very broad both in physiology and in cancer. Several LncRNAs regulate cancer biology, including MALAT and etc. LncRNAs have pleiotropic effects on miRNAs, mRNAs, and proteins and alter miRNA and mRNA expression and stability, affect protein expression, degradation, structure, or interactions with transcriptional regulators. Therefore, novel lncRNAs serve as biomarkers to achieve precision therapy for HCC (Lim et al., 2019). The function of the 98% of non-coding sequences in the human genome is elusive (Wong et al., 2018). Many microRNAs function as 'oncomiRs' to repress tumor-suppressor genes or, serve as a tumor suppressor to counteract oncogenes (Wong et al., 2018). LncRNAs regulate gene expression and other molecular functions through interacting with DNA, RNA and proteins (Wong et al., 2018). LncRNAs regulate liver carcinogenesis of diverse etiologies, liver TIC formation, epigenetic reprogramming and metastasis. LncRNAs serve as biomarkers and therapeutic targets of human HCCs (Wong et al., 2018). LncRNAs guide epigenetic activators/repressors (i.e, HOTAIR, HOTTIP, H19, ANRIL, UCA1, TUG1), block transcription factor binding sites (i.e, lncRNA-DILC), sequester miRs (i.e, DANCR, ICR, PCNA-AS1) or proteins (i.e, MALAT1, lnc-PRAL, GASS, lncBRM), serve as protein scaffold (i.e, lnc-β-Catm, MEG3, HULC, lncRNA-MVIH) (Wong et al., 2018). Therefore, more thorough investigation on lncRNA’s roles in cancer biology are required.
LncRNAs expression (LINC01419, AK021443, UCA1 and WRAP53) are increased in HCCs compared to those of non-cancerous tissues (dysplasia) while lncRNA AF070632 is decreased in advanced HCC samples compared with early HCC. These lncRNAs are associated with Child-Pugh score. Therefore, these association suggests roles of lncRNA contribution to liver oncogenesis. LINC01419 and AK021443 were mostly involved in cell cycle progression, whereas AF070632 regulates cofactor binding, oxidation-reduction and carboxylic acid catabolic process (Zhang et al., 2015). Two lncRNAs, including urothelial carcinoma associated-1 (UCA1) and WD repeat containing, antisense to TP53 (WRAP53) are upregulated in serum. UCA1 and WRAP53 (+) HCC patients had a decreased recurrence-free survival (RFS) and increased cumulative hazards. WRAP53 was an independent prognostic factor of RFS (Kamel et al., 2016). Some of these lncRNAs were dysregulated predominantly in one specific hepatitis virus-related HCC, including PCAT-29 in HBV-related HCC, aHIF and PAR5 in HCV-related HCC, and Y3 in HDV-related HCC. DBH-AS1, hDREH and hPVT1 were differentially expressed in HCC of different viral etiology (Zhang et al., 2016). Unknown mechanism of alcohol-mediated oncogenesis through lncRNA pathways warrants further investigation to elucidate mechanisms of TIC genesis through dysregulation of lncRNAs.
Conclusions and discussions
TICs were identified in HCC sections of alcoholic patients by immunostaining and isolated from such patients to validate induction of TLR4-dependent stemness genes and transformation. Nearly 40% of HCCs are derived from clonal expansion of TICs, indicating that targeting TICs are more strategic ways to eliminate HCC and inhibit recurrence issues. NANOG-mediated p53 degradation disengages from the protective NUMB protein via TBC1D15 interaction. NANOG-AURKA-aPKCζ pathway post-translationally modifies NUMB in TICs self-renewal and tumorigenesis. As the NANOG-NUMB-p53 signaling regulates TICs self-renewal and liver tumorigenesis, targeting NUMB-phosphorylation is a therapeutic strategy. However, further in depth in vivo and clinical studies are warranted to verify this suggestion. These studies are now exploring potential mechanistic connections to metabolic programming known to occur in cancer cells and TICs in promoting and maintaining “stem cell fate” via molecular, genetic, and epigenetic mechanisms via dysregulation of lncRNA.
Table 1.
LncRNAs linked to HCC.
| lncRNA | Classification | Size (kb) | Tissues | Expression | Function | Reference |
|---|---|---|---|---|---|---|
| BC017743 | Unknown | 2.3 | Liver | Up | Tumor suppressor region | (Zhang et al., 2016) |
| BC043430 | Unknown | 1.9 | Liver | Up | Tumor suppressor region | (Zhang et al., 2016) |
| LINC01152 | Unknown | 3.1 | Liver | Down | Unknown | (Zhang et al., 2016) |
| aHIF | Unknown | 1.0 | Liver | Down | Poor prognostic outcomes | (Zhang et al., 2016) |
| PWAR5 (PAR5) | Unknown | ~3.6 | Liver | Down | Poor prognostic outcomes | (Zhang et al., 2016) |
| AF070632 | Unknown | ~1.9 | Liver | Down | LncRNA-protein interaction,supress angiogenesis,potential biomarker and therapeutic target | (Zhang et al., 2015) |
| AK021443 | Unknown | ~1.6 | Liver | Up | Cell cycle regulation, Proliferation | (Zhang et al., 2015) |
| LINC01419 | Unknown | ~5.1 | Liver | Up | Cell cycle regulation, Proliferation | (Zhang et al., 2015) |
| UCA1 | Unknown | ~7.3 | Liver, bladder, gastric, ovary, esophag us | Up | LncRNA-miRNA interaction, Proliferation | (Kamel et al., 2016) |
| WRAP53 | Antisense | ~1.8 | Liver | Up | Unknown, Biomarker | (Kamel et al., 2016) |
| Linc-p21 | Unknown | Liver | Up | Unknown, Biomarker | (Lim et al., 2019) | |
| H19 | Unknown | Liver | Up | Unknown, Biomarker | (Lim et al., 2019) | |
| LET | Unknown | Liver | Up | Unknown, Biomarker | (Lim et al., 2019) | |
| HULK | Unknown | Liver | Up | IGF2BP1 regulation, Biomarker | (Lim et al., 2019) | |
| HOTAIR | Unknown | Liver | Up | Guide of epigenetic repressors | (Wong et al., 2018) | |
| HOTTIP | Unknown | Liver | Up | Guide of epigenetic activators | (Wong et al., 2018) |
Highlights.
Co-morbidity of life-style factors with viral hepatitis synergizes HCC development
TLR4-NANOG pathway metabolically reprograms drug resistance TICs
HCV-mediated mechanisms promote genesis of TICs to synergize oncogenesis
Inactivation of NUMB skews asymmetric cell division to generate TICs
Dysregulated LncRNAs generates TICs to promote HCC development
ACKNOWLEDGMENTS
This project was supported by NIH grants 1R01AA018857-01, 5R21AA025470, P50AA11999 (Animal Core, Morphology Core, and Pilot Project Program), R24AA012885 (Non-Parenchymal Liver Cell Core) and pilot project funding (5P30DK048522-13). This research is also supported by a Research Scholar Grant, RSG-12-177-01-MPC and pilot funding (IRG-58-007-48) from American Cancer Society. Microscopy was performed by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases (P30 DK048522). Liver tissues were obtained from The Liver Tissue Cell Distribution System (LTCDS) in University of Minnesota. We also acknowledge the Animal and Morphology Core facilities of the NIAAA-supported Southern California Research Center for ALPD and Cirrhosis (P50 AA011999) for providing a mouse model of intragastric ethanol infusion and morphological analyses of liver tissues.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alison MR 2005. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev 1:253–260. [DOI] [PubMed] [Google Scholar]
- Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D, Colaluca I, Viale G, Rodrigues-Ferreira S, Wynendaele J, Chaloin O, Hoebeke J, Marine JC, Di Fiore PP, and Telerman A. 2012. Reciprocal repression between P53 and TCTP. Nat Med 18:91–99. [DOI] [PubMed] [Google Scholar]
- Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, and Thorgeirsson SS. 2010. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology 51:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio S, and Eaves CJ. 2009. p53: a new kingpin in the stem cell arena. Cell 138:1060–1062. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, and Ray R. 2008. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol 82:2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, and Berman DM. 2004. Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331. [DOI] [PubMed] [Google Scholar]
- Bric A, Miething C, Bialucha CU, Scuoppo C, Zender L, Krasnitz A, Xuan Z, Zuber J, Wigler M, Hicks J, McCombie RW, Hemann MT, Hannon GJ, Powers S, and Lowe SW. 2009. Functional identification of tumor-suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell 16:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, Qian G, Zhao Q, Li Y, Gao L, Cong W, Zhu M, Yan Z, Shi L, Wu D, Wei L, Shen F, and Wu M. 2012. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology 56:1804–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, and Smith A. 2004. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23:7150–7160. [DOI] [PubMed] [Google Scholar]
- Chen CL, Tsukamoto H, Liu JC, Kashiwabara C, Feldman D, French SW, Sher L, Hyeongnam JJ, and Machida K. 2013a. TLR4-NANOG pathway in tumor-initiating cells inhibits TGF-β-mediated tumor suppression. Journal of Clinical Investigation In press [Google Scholar]
- Chen CL, Tsukamoto H, Liu JC, Kashiwabara C, Feldman D, Sher L, Dooley S, French SW, Mishra L, Petrovic L, Jeong JH, and Machida K. 2013b. Reciprocal regulation by TLR4 and TGF-beta in tumor-initiating stem-like cells. J Clin Invest 123:2832–2849. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- He N, Park K, Zhang Y, Huang J, Lu S, and Wang L. 2008. Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology 134:793–802. [DOI] [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, and Szabo G. 2008. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, and Pandolfi PP. 2012. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med 18:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, and Young R. 2008. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132:567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel MM, Matboli M, Sallam M, Montasser IF, Saad AS, and El-Tawdi AH. 2016. Investigation of long noncoding RNAs expression profile as potential serum biomarkers in patients with hepatocellular carcinoma. Transl Res 168:134–145. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, and Sata M. 2004. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol 165:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, and Izpisua Belmonte JC. 2009. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, Katuri V, Kallakury B, Pishvaian M, Albanese C, Mendelson J, Zasloff M, Rashid A, Fishbein T, Evans SR, Sidawy A, Reddy EP, Mishra B, Johnson LB, Shetty K, and Mishra L. 2007. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene 26:7103–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, and Weinman SA. 2005. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem 280:37481–37488. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, and Thorgeirsson SS. 2006. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 12:410–416. [DOI] [PubMed] [Google Scholar]
- Li Y, Feng H, Gu H, Lewis DW, Yuan Y, Zhang L, Yu H, Zhang P, Cheng H, Miao W, Yuan W, Cheng SY, Gollin SM, and Cheng T. 2013. The p53-PUMA axis suppresses iPSC generation. Nat Commun 4:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LJ, Wong SYS, Huang F, Lim S, Chong SS, Ooi LL, Kon OL, and Lee CG. 2019. Roles and Regulation of Long Noncoding RNAs in Hepatocellular Carcinoma. Cancer Res 79:5131–5139. [DOI] [PubMed] [Google Scholar]
- Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, and Clarke MF. 2010. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A 107:18115–18120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, and Clarke MF. 2007. The biology of cancer stem cells. Annu Rev Cell Dev Biol 23:675–699. [DOI] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW, and Guan XY. 2008. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 27:1749–1758. [DOI] [PubMed] [Google Scholar]
- Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, Seki E, Deshaies R, Miyake K, and Lai MM. 2009. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A 106:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March HN, Rust AG, Wright NA, ten Hoeve J, de Ridder J, Eldridge M, van der Weyden L, Berns A, Gadiot J, Uren A, Kemp R, Arends MJ, Wessels LF, Winton DJ, and Adams DJ. 2011. Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis. Nat Genet 43:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, and Blasco MA. 2009. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, Imai K, Todoroki T, Kimura S, and Koike K. 2001. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res 61:4365–4370. [PubMed] [Google Scholar]
- Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, Knight B, Yeoh GC, Fausto N, and Parks WT. 2007. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology 45:31–41. [DOI] [PubMed] [Google Scholar]
- Okuda K 2000. Hepatocellular carcinoma. J Hepatol 32:225–237. [DOI] [PubMed] [Google Scholar]
- Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, and Weinman SA. 2002. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 122:366–375. [DOI] [PubMed] [Google Scholar]
- Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chretien Y, Koike K, Pessayre D, Chapman J, Barba G, and Brechot C. 2002. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. Faseb J 16:185–194. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, and Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature 414:105–111. [DOI] [PubMed] [Google Scholar]
- Roskams T 2006. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 25:3818–3822. [DOI] [PubMed] [Google Scholar]
- Rountree CB, Senadheera S, Mato JM, Crooks GM, and Lu SC. 2008. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology 47:1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, and Andreeff M. 2010. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. The Journal of clinical investigation 120:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, and Diehl AM. 2006a. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 27:748–757. [DOI] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, Reid LM, and Diehl AM. 2006b. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol 290:G859–870. [DOI] [PubMed] [Google Scholar]
- Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, Jessup JM, Shetty K, Zasloff M, Mishra B, Reddy EP, Johnson L, and Mishra L. 2008. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci U S A 105:2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk-Lingbeek ME, Bruggeman SW, and van Lohuizen M. 2004. Stem cells and cancer; the polycomb connection. Cell 118:409–418. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van Laarhoven S, Fiel MI, Di Feo A, Hoshida Y, Yea S, Toffanin S, Ramos A, Martignetti JA, Mazzaferro V, Bruix J, Waxman S, Schwartz M, Meyerson M, Friedman SL, and Llovet JM. 2008. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 135:1972–1983, 1983 e1971-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE 2011. Cells of origin in cancer. Nature 469:314–322. [DOI] [PubMed] [Google Scholar]
- Wong CM, Tsang FH, and Ng IO. 2018. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol 15:137–151. [DOI] [PubMed] [Google Scholar]
- Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, Bottinger E, Friedman S, Waxman S, and Llovet JM. 2007. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 45:938–947. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, and Wang XW. 2009. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136:1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, and Terrault N. 2001. Hepatitis C and hepatocellular carcinoma. Curr Treat Options Oncol 2:473–483. [DOI] [PubMed] [Google Scholar]
- Yeoh GC, Ernst M, Rose-John S, Akhurst B, Payne C, Long S, Alexander W, Croker B, Grail D, and Matthews VB. 2007. Opposing roles of gp130-mediated STAT-3 and ERK-1/2 signaling in liver progenitor cell migration and proliferation. Hepatology 45:486–494. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Govindarajan S, Arakawa K, and Yu MC. 2004. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer 101:1009–1017. [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, and Lowe SW. 2006. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125:1253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu C, Zhao Y, Li M, Wu L, Yang X, Wan X, Wang A, Zhang MQ, Sang X, and Zhao H. 2015. Long non-coding RNA expression profiles of hepatitis C virus-related dysplasia and hepatocellular carcinoma. Oncotarget 6:43770–43778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Matsuura K, Kleiner DE, Zamboni F, Alter HJ, and Farci P. 2016. Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. J Transl Med 14:328. [DOI] [PMC free article] [PubMed] [Google Scholar]