Abstract
Background
Real‐world data of the CM regimen [cyclophosphamide (CTX) plus methotrexate (MTX)] in metronomic pattern for advanced breast cancer is limited to small‐sample or retrospective studies. This study was aimed to determine the effectiveness and safety of CM regimen in treating advanced breast cancer and to identify which patients are most likely to benefit from metronomic CM regimen.
Methods
Patients with advanced breast cancer who received the metronomic CM regimen at least once between January 2009 and February 2019 in Sun Yat‐sen University Cancer Center were included. Clinicopathological characteristics were collected. Overall survival (OS) and progression‐free survival (PFS) were assessed using Kaplan‐Meier estimates. Characteristics between patients with PFS < 6 months and ≥6 months were compared using the Chi‐square test. Univariate and multivariate Cox regression model was used to estimate the prognostic factors for PFS and OS.
Results
A total of 186 patients were included. The median age and follow‐up were 49 years and 13.3 months, respectively. Over 50% of the patients were estrogen receptor/progesterone receptor‐positive, and 60.8% had been heavily treated (≥3 lines). The objective response rate was 3.8%, the disease control rate at 12 weeks was 41.4%, and the clinical benefit rate at 24 weeks was 31.2% (58/186). The median PFS was 4.0 months [95% confidence interval (CI): 3.6‐4.7 months], the median duration of clinical benefit was 9.5 months (95% CI: 8.2‐10.8 months), and the median OS was 26.8 months (95% CI: 20.9‐37.7 months). Multivariate analysis for PFS revealed the CM regimen as maintenance therapy and no liver metastasis as favorable prognostic factors. Furthermore, patients without liver metastasis were more likely to have a PFS over 6 months than those with liver involvement (P = 0.022). Liver, lymph node, and brain metastases were unfavorable prognostic factors for OS. The CM regimen was well‐tolerated without newly reported adverse events.
Conclusions
The CM regimen was effective in selected patients. In clinical practice, it would be better used as maintenance therapy and in patients without liver metastasis. Further follow‐up investigation should be performed to examine its effect when used in combination with other treatments and determine predictive biomarkers.
Keywords: metronomic chemotherapy, advanced breast cancer, cyclophosphamide, methotrexate, real‐world
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CBR
clinical benefit rate
- CI
confidence interval
- CM
cyclophosphamide plus methotrexate
- CR
complete response
- CTX
cyclophosphamide
- DCB
duration of clinical benefit
- DCR
disease control rate
- DFI
disease‐free interval
- ER
estrogen receptor
- Her2
human epidermal growth factor receptor 2
- HR
hazard ratio
- mCHT
metronomic chemotherapy
- MTX
methotrexate
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PFS
progression‐free survival
- PR
progesterone receptor
- SD
stable disease
- VEGF
vascular endothelial growth factor
1. BACKGROUND
The term metronomic chemotherapy (mCHT) was first coined by Douglas Hanahan in 2000 [1] and refers to frequent administration of chemotherapy at a low, minimally toxic dose without prolonged drug‐free breaks [2]. For the past few decades, evidence has shown that, unlike conventional chemotherapy, mCHT has peculiar mechanisms of inhibiting tumor growth, including antiangiogenic effects, immuno‐mediated effects, anti‐proliferative effects, and endocrine effects on breast cancer [2, 3, 4, 5]. Currently, mCHT is an important alternative for advanced cancer patients, mainly in non‐small cell lung cancer [3], breast cancer [6], and hepatocellular carcinoma [7]. Both the Advanced Breast Cancer (ABC) panel [8] and Pan‐European expert meeting [9] considered metronomic regimens, such as vinorelbine plus capecitabine and the CM regimen [cyclophosphamide (CTX) plus methotrexate (MTX)], as sufficient to be recommended for some patients with advanced breast cancer in clinical practice.
Clinical trials of mCHT for breast cancer have been conducted in neoadjuvant, adjuvant, and metastatic settings [10]. In neoadjuvant setting, phase II clinical trials with limited samples showed that mCHT with or without endocrine therapy achieved some activity in patients who were intolerant to standard chemotherapy [11, 12, 13]. Studies investigating efficacy of mCHT in adjuvant setting have not shown its superiority as maintenance therapy in high‐risk, early‐stage, triple‐negative breast cancer patients after standard adjuvant chemotherapy [14]. Studies on mCHT in metastatic setting have shown durable effect and low toxicity. The objective response rate (ORR) was 18%‐62%, with a time‐to‐progression (TTP) of 3‐21 months [10]. Oral administration of CTX 50 mg per day combined with dual anti‐human epidermal growth factor receptor 2 (Her2) therapy also achieved a progression‐free survival (PFS) of over 12 months in patients who were not suitable for standard chemotherapy [15]. Since many trials in metastatic setting have shown mCHT as a well‐tolerated treatment strategy, the expert panel in the “Metronomic chemotherapy for advanced breast cancer” study group agreed that mCHT can be a treatment option for metastatic disease in selected patients [6]. Vinorelbine, capecitabine, CTX, and MTX are most commonly used agents in clinical practice, and their metronomic dosages have been assessed in several studies [16, 17, 18, 19]. Further, it is easy to modify their dosages when adverse events occur.
The VICTOR‐6 study [20] aiming at summarizing the data of mCHT in Italy has provided the real‐world effectiveness of vinorelbine, capecitabine, CTX, and MTX in treating advanced breast cancer. However, the percentage of patients treated with CTX or MTX in this study composed less than 25% of the study cohort. Furthermore, other real‐world data of mCHT with the CM regimen is limited to small‐size retrospective studies [21, 22]. In our center, we have administered metronomic CM regimen for metastatic disease since 2009. Herein, in this study, we investigated the effectiveness and safety of metronomic CM regimen in patients with advanced breast cancer, and identified patients that may benefit from metronomic CM regimen.
2. PATIENTS AND METHODS
2.1. Patient selection
Patients who received metronomic CM regimen (CTX 50 mg once a day and MTX 2.5 mg twice a day on days 1 and 2 every week) at least once between January 2009 and February 2019 at Sun Yat‐sen University Cancer Center were included. Inclusion criteria were as follow: 1) female patients with pathological diagnosis of advanced breast cancer and without other primary malignancy; 2) both CTX and MTX were administered in metronomic doses, with dose escalation or discontinuation of one drug allowed for poor tolerability; 3) patients with at least one clinical evaluation after administration of the CM regimen, including physical examination, ultrasound examination, computed tomography, and magnetic resonance imaging according to physician's discretion. Patients who received the CM regimen as maintenance therapy were also included. We defined the CM regimen as maintenance therapy when it was given to patients who had achieved complete response (CR), partial response, or stable disease (SD) from the previous treatment; otherwise, the CM regimen was defined as salvage therapy. Clinicopathological characteristics, including age, menopausal status, estrogen receptor (ER) status, progesterone receptor (PR) status, Her2 status, previous therapeutic lines and regimens, metastatic sites, disease‐free interval (DFI), ORR, disease control rate (DCR), clinical benefit rate (CBR), PFS, duration of clinical benefit (DCB), overall survival (OS), and reported adverse events (AEs), were collected retrospectively from electronic records in our center. DFI was defined as the time from curative surgery to the date of clinical evidence of recurrence or metastasis. ORR was defined as the percentage of patients with complete response or partial response according to Response Evaluation Criteria in Solid Tumor (RECIST 1.1) [23]. CBR was defined as the percentage of patients with CR, partial response, or SD ≥ 6 months according to RECIST 1.1. PFS was defined as the time from initial administration of the CM regimen to the time of disease progression or death of any cause. DCB was defined as the time from the initial administration of the CM regimen to the time of disease progression or death in patients who achieved CR, partial response, or SD after CM therapy for more than 24 weeks. OS was defined as the time from initial administration of CM regimen to death by any cause.
2.2. Follow‐up
We tracked down patients’ medical history, which included but not limited to latest outpatient visiting date, hospitalization date, and laboratory examination date in our center. Telephone follow‐up was done to access the latest health status of patients, especially those who did not visit a doctor for more than 6 months. The last follow‐up was updated on May 5th, 2019.
2.3. Statistical analysis
Clinicopathological characteristics between two groups (patients with a PFS ≥ 6 months and < 6 months) and ORR and CBR among different groups were compared using the Chi‐square test. The OS, DCB, and PFS were assessed using Kaplan‐Meier estimates and the log‐rank test. The Cox proportional hazards model with 95% confidence interval (CI) was used for the univariate analysis to verify the factors significantly related to PFS and OS. Variables with a P value less than 0.1 in the univariate analysis were included into multivariate Cox model to identify independent prognostic factors. Results were considered significant if P was less than 0.05. All analyses were performed using SPSS software for Windows (version 19; IBM SPSS, Somers, NY, USA).
3. RESULTS
3.1. Clinicopathological characteristics
A total of 186 patients were included (Table 1). The median age of all patients was 49 (range, 28‐76) years. More than 60% of the patients were ER/PR‐positive, and the Her2‐positive rate was 23.7%. Seventy‐five (40.3%) patients received the CM regimen as maintenance therapy, and 111 (59.7%) received it as salvage therapy. The median number of therapy lines for metastatic diseases was 3 (range, 0‐13). In the context of treatment combination, 38 of the 102 patients with luminal B Her2‐negative subtype received the CM regimen in combination with endocrine therapy, and 19 of the 44 patients with Her2‐positive disease received the CM regimen plus targeted therapy. Six patients received the CM regimen with other therapy (including radiotherapy or local surgery for metastatic sites), and 101 (54.3%) received the CM regimen alone. Of the 52 patients received the CM regimen in combination with endocrine therapy, 8 [6 of them were ER/PR‐positive (< 10% positive staining) in the primary tumor and ER/PR‐negative in the metastatic sites] did not receive prior endocrine therapy for metastatic diseases, 17 (32.6%) had one line and 27 (51.9%) had at least 2 lines of endocrine therapy for metastatic diseases. Of the 27 patients received the CM regimen plus targeted therapy, 23 (85.2%) received prior targeted therapy with trastuzumab; 6 (22.2%) had disease progression after treatment with lapatinb, pertuzumab, and pyrotinib.
TABLE 1.
Baseline clinicopathological characteristics of 186 patients with advanced breast cancer before treatment with the CM regimen
| Variable | Number of patients [cases (%)] |
|---|---|
| Total | 186 |
| Menopausal status | |
| Premenopausal | 70 (37.6) |
| Postmenopausal | 116 (62.4) |
| Visceral metastasis | |
| Yes | 122 (65.6) |
| No | 64 (34.4) |
| Metastatic site | |
| Bone | 75 (40.3) |
| Lung | 59 (31.7) |
| Liver | 53 (28.5) |
| Lymph nodes | 77 (41.4) |
| Brain | 21 (11.3) |
| Others | 20 (10.8) |
| ER status | |
| Positive | 115 (61.8) |
| Negative | 71 (38.2) |
| PR status | |
| Positive | 106 (57.0) |
| Negative | 80 (43.0) |
| Her2 status | |
| Positive | 44 (23.7) |
| Negative | 142 (76.3) |
| Molecular subtype | |
| Luminal B Her2‐negative | 102 (54.8) |
| Luminal B Her2‐positive | 19 (10.2) |
| ER/PR‐negative Her2‐positive | 25 (13.4) |
| Triple‐negative | 40 (21.5) |
| Prior (neo)adjuvant therapy for primary disease | |
| None | 23 (12.4) |
| Chemotherapy | 157 (84.4) |
| Endocrine therapy | 99 (53.2) |
| Radiotherapy | 79 (42.5) |
| Targeted therapy | 12 (6.5) |
| Disease status | |
| De novo stage IV | 31 (16.7) |
| Recurrence | 155 (83.3) |
| DFI < 5 years | 124 (66.7) |
| DFI ≥ 5 years | 31 (16.7) |
| Prior therapy for metastatic disease | |
| None | 7 (3.8) |
| Endocrine therapy (± anti‐Her2 therapy) | 13 (7.0) |
| Chemotherapy (± anti‐Her2 therapy) | 53 (28.5) |
| Endocrine therapy/chemotherapy (±anti‐Her2 therapy) | 113 (60.8) |
| Lines of prior therapy for metastatic disease | |
| 0 | 7 (3.8) |
| 1 | 28 (15.1) |
| 2 | 38 (20.4) |
| ≥3 | 113 (60.8) |
| Disease status prior using the CM regimen | |
| PD | 111 (59.7) |
| SD | 51 (27.4) |
| CR/partial response | 24 (12.9) |
| Therapy combined with the CM regimen | |
| None | 101 (54.3) |
| Endocrine therapy | 52 (27.9) |
| Exemestane | 17 (9.1) |
| NS‐AI | 18 (9.7) |
| Fulvestrant | 5 (2.7) |
| SERM | 11 (5.9) |
| Bicalutamide | 1 (0.5) |
| Targeted therapy | 27 (14.5) |
| Trastuzumab | 11 (5.9) |
| Lapatinib | 8 (4.3) |
| Pyrotinib | 1 (0.5) |
| Trastuzumab + lapatinib | 4 (2.2) |
| Apatinib | 3 (1.6) |
| Others (radiotherapy or surgery) | 6 (3.2) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor receptor 2; DFI, disease‐free interval; CM, cyclophosphamide and metrotrexate; PD, progressive disease; SD, stable disease; CR, complete response; NS‐AI, non‐steroidal aromatase inhibitor; SERM, selective estrogen receptor modulator.
3.2. Response rate
The ORR of all patients was 3.8% (7/186), the DCR was 41.4% (77/186) at 12 weeks and CBR was 31.2% (58/186) at 24 weeks (Table 2). Despite the ORR in patients receiving the CM regimen in combination with targeted therapy being higher (7.4%, 2/27), response rates among different combination therapies were not significantly different (P = 0.952). The DCR of patients received the CM regimen as maintenance therapy (57.3%, 43/75) was significantly higher than that of patients receiving the CM regimen as salvage therapy (38.7%, 43/111; P = 0.013). Furthermore, the DCR of patients with liver metastasis (30.2%, 16/53) was significantly lower than that of patients without liver metastasis (52.6%, 70/133; P = 0.018).
TABLE 2.
Response rates of patients with advanced breast cancer after different treatment protocols
| Treatment | Total (cases) | ORR [cases (%)] | SD (≥12 weeks) [cases (%)] | DCR (≥12 weeks) [cases (%)] | CBR (≥24 weeks) [cases (%)] |
|---|---|---|---|---|---|
| Overall | 186 | 7 (3.8) | 70 (37.6) | 77 (41.4) | 58 (31.2) |
| CM regimen alone | 101 | 3 (3.0) | 41 (40.6) | 44 (43.6) | 38 (37.6) |
| CM + endocrine therapy | 52 | 2 (3.8) | 18 (34.6) | 20 (38.5) | 12 (23.1) |
| CM + targeted therapy | 27 | 2 (7.4) | 9 (33.3) | 11 (40.7) | 6 (22.2) |
| CM + other therapy | 6 | 0 (0) | 2 (33.3) | 2 (33.3) | 2 (33.3) |
Abbreviations: ORR, overall response rate; SD, stable disease; DCR, disease control rate; CBR, clinical benefit rate; CM, cyclophosphamide and methotrexate.
3.3. PFS and OS
The median PFS and OS of all patients were 4.0 months (95% CI: 3.6‐4.7 months) and 26.8 months (95% CI: 20.9‐37.7 months), respectively (Table 3, Figure 1A&B). Both the median PFS and OS of patients without liver metastasis were longer than those of patients with liver metastasis (P = 0.022 and P = 0.043 respectively; Figure 1C&D). Patients receiving the CM regimen as maintenance therapy had a longer median PFS than those receiving the CM regimen as salvage therapy (P = 0.002; Figure 1E). However, the median OS was not significantly different (P = 0.230; Figure 1F). The median PFS in patients receiving treatment combinations ranged from 3.7 to 4.4 months and were not significantly different (P = 0.218). The median DCB of all patients was 9.5 months (95% CI: 8.2‐10.8 months). The median DCB of patients receiving the CM regimen as maintenance therapy was significantly longer than that of patients receiving the CM regimen as salvage therapy (P = 0.021). The median DCB of patients without liver metastasis was longer than that of patients with liver metastasis (P = 0.597), but without significant difference.
TABLE 3.
Survival of patients with advanced breast cancer after different treatment protocols
| Variable | PFS [months, median (95% CI)] | P value | OS [months, median (95% CI)] | P value | DCB [months, median (95% CI)] | P value |
|---|---|---|---|---|---|---|
| Overall | 4.0 (3.6‐4.7) | 26.8 (20.9‐37.7) | 9.5 (8.2‐10.8) | |||
| Visceral metastasis | 0.274 | 0.840 | 0.089 | |||
| Yes | 4.1 (3.5‐4.8) | 23.9 (16.2‐31.5) | 9.5 (7.6‐11.5) | |||
| No | 3.3 (2.4‐4.1) | 22.2 (17.5‐27.0) | 7.3 (2.0‐12.5) | |||
| Liver metastasis | 0.022 | 0.043 | 0.597 | |||
| Yes | 3.8 (3.0‐4.5) | 20.8 (12.9‐28.8) | 9.1 (7.9‐10.2) | |||
| No | 4.4 (3.4‐5.4) | 29.1 (19.5‐38.7) | 9.8 (7.5‐12.2) | |||
| Molecular subtype | 0.295 | 0.378 | 0.071 | |||
| Luminal B Her2‐negative | 4.1 (3.6‐4.7) | 26.8 (3.1‐50.6) | 7.9 (5.6‐10.0) | |||
| Luminal B Her2‐positive | 3.5 (2.4‐4.6) | 26.1 (11.4‐40.9) | 14.0 (1.2‐28.5) | |||
| ER/PR‐negative Her2‐positive | 4.1 (3.2‐5.1) | 36.9 (22.2‐51.5) | 10.3 (4.6‐16.0) | |||
| Triple‐negative | 4.0 (2.0‐5.9) | 22.1 (10.1‐34.2) | 12.9 (6.4‐19.4) | |||
| Treatment | 0.218 | 0.791 | 0.329 | |||
| CM alone | 4.4 (3.8‐7.1) | 26.1 (14.3‐40.0) | 9.1 (3.7‐14.5) | |||
| CM + endocrine therapy | 3.9 (3.0‐4.6) | 36.8 (10.3‐63.2) | 8.1 (4.7‐11.5) | |||
| CM + targeted therapy | 3.7 (3.0‐5.1) | 22.1 (19.3‐25.0) | 9.5 (8.0‐11.0) | |||
| CM therapy pattern | 0.002 | 0.230 | 0.021 | |||
| As maintenance therapy | 4.8 (2.7‐6.9) | 30.3 (21.3‐39.3) | 12.2 (7.8‐16.7) | |||
| As salvage therapy | 3.8 (3.2‐4.3) | 20.9 (11.6‐30.1) | 7.9 (6.1‐9.6) |
Abbreviations: PFS, progression‐free survival; OS, overall survival; CI, confidence interval; DCB, duration of clinical benefit; CM, cyclophosphamide and methotrexate.
FIGURE 1.
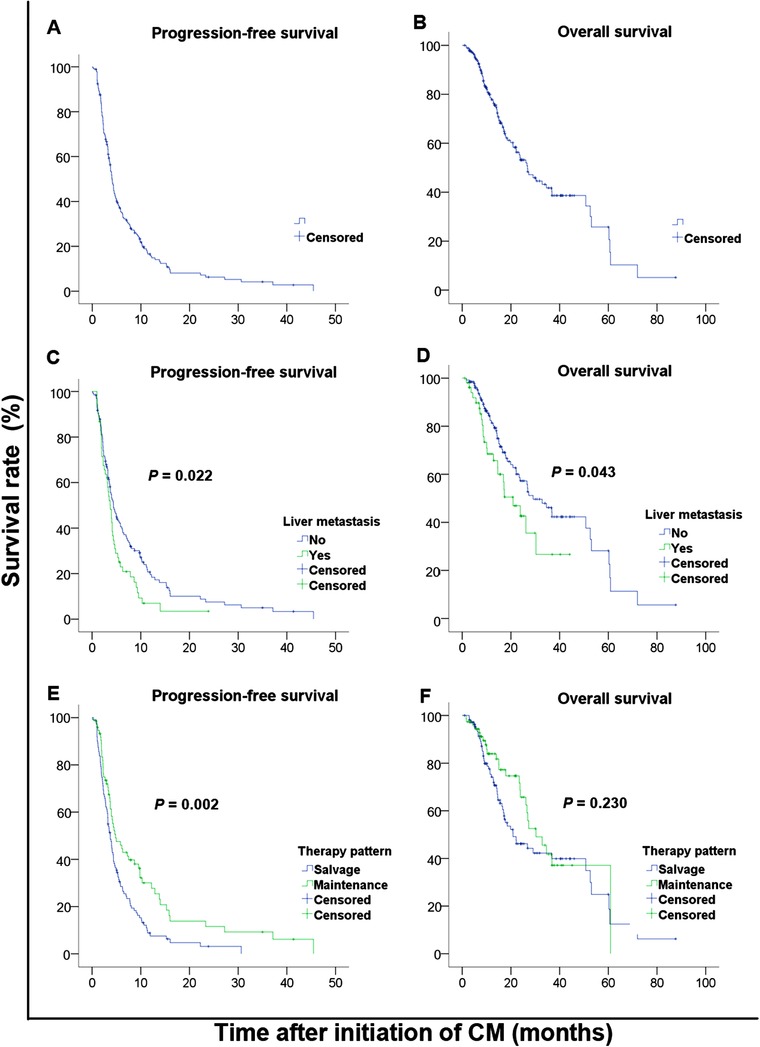
Kaplan‐Meier PFS and OS curves of patients with advanced breast cancer stratified by liver metastasis and CM administration. A&B, PFS and OS curves of all patients; C&D, PFS and OS curves of patients with or without liver metastasis; E&F PFS and OS curves of patients receiving the CM regimen as maintenance therapy or as salvage therapy. Abbreviations: PFS, progression‐free survival; OS, overall survival; CM, cyclophosphamide and metrotrexate
The median PFS and OS among patients with different molecular subtypes did not show significant difference (P = 0.295 and P = 0.378 respectively; Table 3, Figure 2A&B). For patients who received the CM regimen alone, there were no differences in PFS and OS among patients with different molecular subtypes (P = 0.053 and P = 0.237 respectively; Figure 2C&D). The CM regimen in combination with endocrine therapy was not superior to the CM regimen alone in either PFS or OS in patients with luminal B Her2‐negative subtype (P = 0.940 and P = 0.796 respectively; Figure 2E&F).
FIGURE 2.
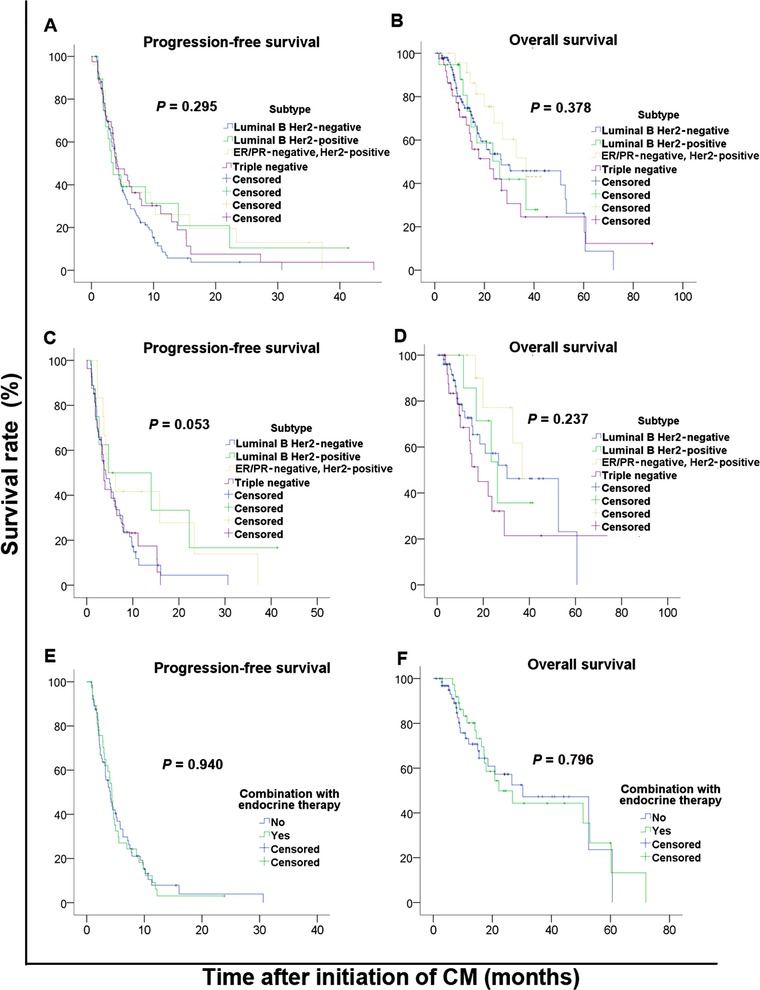
Kaplan‐Meier PFS and OS curves of patients with advanced breast cancer stratified by molecular subtype and treatment protocol. A&B, PFS and OS curves of patients with different molecular subtypes; C&D, PFS and OS curves of patients with different molecular subtypes who received the CM regimen alone; E&F, PFS and OS curves of patients with lumninal B Her2‐negative subtype who received the CM regimen alone or in combination with endocrine therapy. Abbreviations: PFS, progression‐free survival; OS, overall survival; CM, cyclophosphamide and metrotrexate; ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor receptor 2
3.4. Prognostic factors for PFS and OS
Cox regression analysis was performed to identify patients who may benefit from metronomic CM therapy. Liver metastasis and the CM regimen as maintenance therapy were prognostic factors for PFS (Table 4). Patients with liver metastasis had a 54.8% higher risk of disease progression than those without [hazard ratio (HR) = 1.43, 95% CI =: 1.00‐2.04, P = 0.046]. Patients received the CM regimen as maintenance therapy had a lower risk of disease progression than those received it as salvage therapy (HR = 0.60, 95% CI = 0.42‐0.84, P = 0.003). No other variables were significantly associated with PFS.
TABLE 4.
Univariate and multivariate analyses for PFS and OS of the patients with advanced breast cancer after different treatment protocols
| PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Variable | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| Age | 0.99 | 0.97‐1.01 | 0.253 | 1.00 | 0.98‐1.01 | 0.618 | ||||||
| Menopausal status (post vs. pre) | 0.89 | 0.64‐1.23 | 0.469 | 0.89 | 0.64‐1.22 | 0.469 | ||||||
| ER (positive vs. negative) | 0.96 | 0.72‐1.28 | 0.782 | 0.87 | 0.58‐1.30 | 0.491 | ||||||
| PR (positive vs. negative) | 1.01 | 0.76‐1.33 | 0.951 | 0.85 | 0.57‐1.26 | 0.420 | ||||||
| Her2 (positive vs. negative) | 0.88 | 0.60‐1.28 | 0.505 | 0.88 | 0.60‐1.28 | 0.505 | ||||||
| Visceral metastasis (yes vs. no) | 0.82 | 0.56‐1.20 | 0.308 | 0.95 | 0.56‐1.60 | 0.950 | ||||||
| Bone metastasis (yes vs. no) | 0.79 | 0.57‐1.09 | 0.146 | 0.95 | 0.60‐1.49 | 0.806 | ||||||
| Lymph nodes metastasis (yes vs. no) | 1.12 | 0.81‐1.54 | 0.497 | 1.73 | 1.10‐2.73 | 0.019 | 2.07 | 1.28‐3.34 | 0.003 | |||
| Liver metastasis (yes vs. no) | 1.51 | 1.07‐2.13 | 0.020 | 1.43 | 1.00‐2.04 | 0.046 | 1.65 | 1.01‐2.70 | 0.045 | 1.94 | 1.16‐3.24 | 0.012 |
| Lung metastasis (yes vs. no) | 1.00 | 0.72‐1.41 | 0.983 | 1.00 | 0.62‐1.62 | 0.994 | ||||||
| Brain metastasis (yes vs. no) | 0.70 | 0.42‐1.17 | 0.176 | 1.78 | 0.98‐3.24 | 0.060 | 2.83 | 1.48‐5.40 | 0.002 | |||
| Disease status prior to CM administration | 0.235 | 0.254 | 0.054 | |||||||||
| PD | 1.00 | 1.00 | 1.00 | |||||||||
| SD | 0.62 | 0.34‐1.14 | 0.123 | 0.89 | 0.53‐1.49 | 0.656 | ||||||
| CR/partial response | 0.99 | 0.58‐1.70 | 0.966 | 0.49 | 0.21‐1.15 | 0.099 | 0.34 | 0.14‐0.83 | 0.017 | |||
| CM therapy for metastatic diseases | 0.161 | 0.513 | 0.029 | 0.005 | ||||||||
| 1st‐line | 0.51 | 0.23‐1.11 | 0.088 | 0.60 | 0.27‐1.34 | 0.211 | 0.13 | 0.02‐0.92 | 0.041 | 0.22 | 0.03‐1.70 | 0.147 |
| 2nd‐line | 1.02 | 0.64‐1.64 | 0.924 | 1.14 | 0.58‐2.28 | 0.702 | ||||||
| 3rd‐line | 1.23 | 0.83‐1.83 | 0.302 | 1.73 | 1.03‐2.88 | 0.037 | 2.39 | 1.38‐4.16 | 0.002 | |||
| ≥4th‐line | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| CM therapy (maintenance vs. salvage) | 0.61 | 0.44‐0.85 | 0.003 | 0.60 | 0.42‐0.84 | 0.003 | 0.75 | 0.47‐1.20 | 0.232 | |||
| Combination | 0.226 | 0.863 | ||||||||||
| CM alone | 1.00 | 1.00 | 1.00 | 0.792 | ||||||||
| CM + endocrine therapy | 1.26 | 0.80‐1.98 | 0.324 | 0.76 | 0.44‐1.33 | 0.338 | ||||||
| CM + targeted therapy | 1.41 | 0.99‐2.00 | 0.055 | 1.19 | 0.71‐2.00 | 0.499 | 0.87 | 0.55‐1.66 | 0.871 | |||
| CM + others | 0.70 | 0.25‐1.93 | 0.486 | 0.94 | 0.29‐3.11 | 0.920 | ||||||
Abbreviations: PFS, progression‐free survival; OS, overall survival; HR‐hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor receptor 2; CM, cyclophosphamide and methotrexate; PD, progressive disease; SD, stable disease; CR, complete response.
Fifty‐eight (34.5%) of the 186 patients achieved PFS longer than 6 months, and 19 (10.2%) still had response to metronomic CM therapy at 12 months. Patients without liver metastasis before metronomic CM therapy were more likely to have a PFS ≥ 6 months than those with liver metastasis (P = 0.022, Table S1). No other factors were significantly associated with the likelihood of PFS over 6 months. In 19 patients who had responses at 12 months, 12 had received more than three lines of prior therapy for metastatic disease, and 11 had visceral metastasis.
Cox regression analysis was also performed to evaluate the prognostic factors for OS. Lymph node, liver, and brain metastases, disease progression prior to metronomic CM therapy, and receiving the CM regimen as the third‐line therapy for advanced diseases were unfavorable prognostic factors for OS (Table 4).
3.5. Safety
Forty‐four patients reported at least one AE after metronomic CM therapy (Table 5). The most frequently reported AE was nausea (7.1%). Two patients reported grade 2 elevated aspartate aminotransferase (AST)/alanine aminotransferase (ALT), which were considered to be related to liver metastasis and recovered to grade 1 after symptomatic treatments. All other AEs were classified as grade 1.
TABLE 5.
Adverse events among all patients with advanced breast cancer after different treatment protocols
| Adverse event | Any grade [cases (%)] | Grade 1 [cases (%)] | Grade 2 [cases (%)] |
|---|---|---|---|
| Any | 44 (23.6) | 42 (22.4) | 2 (1.2) |
| Nausea | 12 (7.1) | 12 (7.1) | 0 |
| Pain | 11 (6.5) | 11 (6.5) | 0 |
| Loss of appetite | 8 (4.8) | 8 (4.8) | 0 |
| Fatigue | 7 (4.2) | 7 (4.2) | 0 |
| Elevated AST/ALT | 5 (3.0) | 3 (1.8) | 2 (1.2) |
| Skin rash | 5 (3.0) | 5 (3.0) | 0 |
| Cough | 4 (2.4) | 4 (2.4) | 0 |
| Arthralgia | 4 (2.4) | 4 (2.4) | 0 |
| Peripheral neuropathy | 3 (1.8) | 3 (1.8) | 0 |
| Weight loss | 1 (0.6) | 1 (0.6) | 0 |
| Insomnia | 1 (0.6) | 1 (0.6) | 0 |
| Decreased WBC/neutrophil count | 1 (0.6) | 1 (0.6) | 0 |
| Decreased platelet count | 1 (0.6) | 1 (0.6) | 0 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase;
WBC, white blood cell.
Fifteen (8.1%) of the 186 patients had dose reduction (discontinuation of either MTX or CTX), and 1 had temporary break of both CTX and MTX due to AEs. No patient reported death due to AEs. The reasons for drug discontinuation or temporary break were elevated AST/ALT (n = 4), nausea (n = 5), loss of appetite (n = 4), skin rashes (n = 1), peripheral neuropathy (n = 1), and diarrhea (n = 1).
4. DISCUSSION
The present study demonstrated the real‐world effectiveness of metronomic CM therapy in advanced breast cancer patients who were previously heavily treated. The DCR at 12 weeks was 41.4% and remained 31.2% at 24 weeks, which was relatively lower than the reported DCR (31%‐62.5%) in both clinical trials [18, 22, 24] and retrospective studies [21, 25]. It is acceptable when considering that more than 60% of the patients had visceral metastasis and were treated with multiple lines of prior therapy. Consistent with the results of previous studies [18, 21, 22, 24], the median PFS of all patients was 4.0 months; however, the median DCB was 9.5 months in the present study. Colleoni et al. [18, 22] reported that the median TTP of the CM regimen were 2.8 and 3.8 months in two phase II studies. A meta‐analysis [26] including all metronomic regimens for advanced breast cancer and prostate cancer showed that the mean ORR of mCHT was 26.0% and the median PFS was 4.6 months. Studies of other metronomic regimens with or without targeted therapy have reported the ORR of 18%‐62% and PFS ranging from 4.3 to 10.8 months [16, 17, 27, 28, 29]. No clinical trials have compared the efficacy among these regimens.
The present study revealed that 10.2% of patients have a durable response to the CM regimen. This phenomenon was also observed in other studies. In the VICTOR‐2 study [17], 24.3% of patients in first‐line and 22.2% of patients in second‐line therapy setting had a durable response lasting at least 12 months. Another study has also shown that 15.7% of patients achieved a prolonged clinical benefit from metronomic CM therapy, in which prolonged clinical benefit was defined as disease control of longer than 12 months [19]. They also have discovered that hormonal receptor status and achievement of objective response were factors predicting prolonged clinical benefit. However, in the present study, the absence of liver metastasis was the only factor that was associated with a PFS ≥ 6 months.
The combination of mCHT with targeted therapy, such as trastuzumab [30], pertuzumab [15], and bevacizumab [27, 28], has been studied in several phase II trials. The addition of targeted therapy yielded good efficacy without extra toxicity in patients with limited lines (< 3 lines) of therapy for advanced disease. In the present study, the combined use of anti‐Her2 therapy yielded a CBR of 26.4% and a median PFS of 3.7 months with good tolerability in patients who received at least 3 lines of therapies.
Subgroup analysis showed that the CM regimen as maintenance therapy and no liver metastasis were the factors associated with response to metronomic CM therapy. This might be because patients that did not benefit from prior therapy or with liver metastasis usually have poor response to chemotherapy and poor prognosis. Therefore, patients with relatively good prognosis would be more likely to benefit from the subsequent treatments. Additionally, due to the unique mechanisms of metronomic therapy, the time from initiation use of mCHT to best tumor response may be longer than that in conventional chemotherapy, and the anti‐tumor effects may be stronger in patients with low tumor burden than in those with high tumor burden.
Researchers have tried to find predictive factors of response to mCHT in order to select the patients who will be most likely to benefit. Colleoni et al. [18, 22] have demonstrated the association of serum vascular endothelial growth factor (VEGF) level and possibility of response, although a significant drop in VEGF level was observed after 2 months of treatment in patients with response. Circulating endothelial cells and their progenitor counterparts have been studied as predictors for response to mCHT combined with the antiangiogenetic agent bevacizumab [28, 29]. Standardization of these biomarkers should be confirmed before wide application in clinical practice. In the VEX study, increased tumor‐infiltrating lymphocytes were associated with shorter TTP [31]. Clinical subtypes have also been reported to be associated with response to mCHT (no specific regimen referred), in which study no triple‐negative breast cancer patients responded to mCHT, whereas hormone receptor‐positive patients had higher ORR [32]. However, the present study showed no difference in the effectiveness among patients with different molecular subtypes. Previous studies have shown that factors associated with good prognosis (sensitive to prior therapy or ER/PR expression) were also associated with better response to mCHT [19, 32]. Biomarkers related to angiogenesis or the tumor‐immune system may be predictors of response to specific mCHT regimens. The present study also discovered that metronomic CM regimen as maintenance therapy and the absence of liver metastasis were associated with responsiveness and prolonged PFS. Other clinical factors were not related to PFS in the present study.
In a meta‐analysis in 2011, maintenance therapy for advanced breast cancer was shown to be associated with prolonged PFS and OS [33]. Experts and guidelines recommend advanced breast cancer patients who have unacceptable AEs after standard chemotherapy should consider change to maintenance therapy, using low‐toxicity endocrine therapy or convenient oral cytotoxic agents. mCHT is an option for maintenance therapy because of its low toxicity, durable effectiveness, and tolerability [34]. The present study further demonstrated that the use of metronomic CM therapy as maintenance therapy achieved a median DCB of 12.2 months.
In terms of safety, metronomic CM therapy was well‐tolerated and with low toxicity. In patients receiving the CM regimen in combination with endocrine therapy, targeted therapy, or local therapy such as radiotherapy, no new AEs were reported.
There were limitations in the present study. First, all data were retrospectively collected, which could lead to selection bias. Prospective observational study of this topic would provide more detailed data. Second, because only a few patients were treated with the CM regimen in combination with endocrine therapy or targeted therapy, we could not precisely evaluate the efficacy of combination treatments according to specific molecular subtypes. However, the present study supplemented more real‐world data of mCHT to help physicians in clinical practice. As most of the advanced breast cancer patients have longer survival than patients with other types of advanced cancer, a durable, effective, low‐toxicity therapeutic option is important. mCHT can be a good option for selected patients.
5. CONCLUSIONS
Metronomic CM therapy showed durable effect in selected patients. We recommend its use as maintenance therapy and in patients without liver metastasis in clinical practice. Further follow‐up investigation should be performed to identify patients who be most likely to benefit from this therapeutic regimen.
DECLARATION
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of Sun Yat‐sen Cancer Center (No. B2019‐033‐01), and exception to the requirement of informed consent was approved.
CONSENT OF PUBLICATION
Not applicable.
FUNDING
This work was supported by the Joint Fund of National Natural Science Foundation of China (No. U1601224).
AUTHORS’ CONTRIBUTIONS
Study concept and design: QYL, SSW, FX, ZYY, and YXS; data collection:QYL, QLZ, KPL, KKJ, RXH, and YL; data analysis: QYL, QFZ, WX, and KPL; manuscript writing: all authors.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGEMENT
We sincerely thank the reviewers and the editor for their suggestions and insights to improve the manuscript. We would like to thank Editage (http://www.editage.cn) for English language editing.
Lu Q, Lee K, Xu F, et al. Metronomic chemotherapy of cyclophosphamide plus methotrexate for advanced breast cancer: Real‐world data analyses and experience of one center. Cancer Communications. 2020;40:222–233. 10.1002/cac2.12029
AVAILABILITY OF DATA AND MATERIALS
The data generated and/or analyzed in the current study are available in the Research Data Deposit (http://rdd.sysucc.org.cn/, RDDA2020001375).
REFERENCES
- 1. Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105(8):1405‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerbel RS, Kamen BA. The anti‐angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423‐36. [DOI] [PubMed] [Google Scholar]
- 3. Cazzaniga ME, Camerini A, Addeo R, Nole F, Munzone E, Collova E, et al. Metronomic oral vinorelbine in advanced breast cancer and non‐small‐cell lung cancer: current status and future development. Future Oncol. 2016;12(3):373‐87. [DOI] [PubMed] [Google Scholar]
- 4. Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti‐tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358(2):100‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerbel RS, Shaked Y. The potential clinical promise of ‘multimodality’ metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett. 2017;400:293‐304. [DOI] [PubMed] [Google Scholar]
- 6. Cazzaniga ME, Biganzoli L, Cortesi L, De Placido S, Donadio M, Fabi A, et al. Treating advanced breast cancer with metronomic chemotherapy: what is known, what is new and what is the future? Onco Targets Ther. 2019;12:2989‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandi G, Venturi M, De Lorenzo S, Garuti F, Frega G, Palloni A, et al. Sustained complete response of advanced hepatocellular carcinoma with metronomic capecitabine: a report of three cases. Cancer Commun (London). 2018;38(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO‐ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28(1):16‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cazzaniga ME, Munzone E, Bocci G, Afonso N, Gomez P, Langkjer S, et al. Pan‐European Expert Meeting on the Use of Metronomic Chemotherapy in Advanced Breast Cancer Patients: The PENELOPE Project. Adv Ther. 2018;36(2):381‐406. [DOI] [PubMed] [Google Scholar]
- 10. Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12(11):631‐44. [DOI] [PubMed] [Google Scholar]
- 11. Dellapasqua S, Mazza M, Rosa D, Ghisini R, Scarano E, Torrisi R, et al. Pegylated liposomal doxorubicin in combination with low‐dose metronomic cyclophosphamide as preoperative treatment for patients with locally advanced breast cancer. Breast. 2011;20(4):319‐23. [DOI] [PubMed] [Google Scholar]
- 12. Masuda N, Higaki K, Takano T, Matsunami N, Morimoto T, Ohtani S, et al. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5‐fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple‐negative or low hormone receptor expressing/HER2‐negative primary breast cancer. Cancer Chemother Pharmacol. 2014;74(2):229‐38. [DOI] [PubMed] [Google Scholar]
- 13. Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low‐dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24(22):3623‐8. [DOI] [PubMed] [Google Scholar]
- 14. Colleoni M, Gray KP, Gelber S, Lang I, Thurlimann B, Gianni L, et al. Low‐Dose Oral Cyclophosphamide and Methotrexate Maintenance for Hormone Receptor‐Negative Early Breast Cancer: International Breast Cancer Study Group Trial 22‐00. J Clin Oncol. 2016;34(28):3400‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wildiers H, Tryfonidis K, Dal Lago L, Vuylsteke P, Curigliano G, Waters S, et al. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2‐positive metastatic breast cancer (EORTC 75111‐10114): an open‐label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 2018;19(3):323‐36. [DOI] [PubMed] [Google Scholar]
- 16. Cazzaniga ME, Torri V, Riva F, Porcu L, Cicchiello F, Capici S, et al. Efficacy and safety of vinorelbine‐capecitabine oral metronomic combination in elderly metastatic breast cancer patients: VICTOR‐1 study. Tumori. 2017;103(1):e4‐e8. [DOI] [PubMed] [Google Scholar]
- 17. Cazzaniga ME, Cortesi L, Ferzi A, Scaltriti L, Cicchiello F, Ciccarese M, et al. Metronomic chemotherapy with oral vinorelbine (mVNR) and capecitabine (mCAPE) in advanced HER2‐negative breast cancer patients: is it a way to optimize disease control? Final results of the VICTOR‐2 study. Breast Cancer Res Treat. 2016;160(3):501‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, et al. Low‐dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 19. Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006;17(8):961‐7. [DOI] [PubMed] [Google Scholar]
- 20. Cazzaniga ME, Pinotti G, Montagna E, Amoroso D, Berardi R, Butera A, et al. Metronomic chemotherapy for advanced breast cancer patients in the real world practice: Final results of the VICTOR‐6 study. Breast. 2019;48:7‐16. [DOI] [PubMed] [Google Scholar]
- 21. Krajnak S, Battista M, Brenner W, Almstedt K, Elger T, Heimes AS, et al. Explorative Analysis of Low‐Dose Metronomic Chemotherapy with Cyclophosphamide and Methotrexate in a Cohort of Metastatic Breast Cancer Patients. Breast Care (Basel). 2018;13(4):272‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, et al. Metronomic low‐dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17(2):232‐8. [DOI] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228‐47. [DOI] [PubMed] [Google Scholar]
- 24. Hussein MM, Gaafar RM, Abdel‐Warith AM, Ahmed WA, Allahloubi NMA, Salem SE, et al. Efficacy and Toxicity of Metronomic Chemotherapy in Metastatic Breast Cancer: Egyptian Experience. Clin Breast Cancer. 2017;17(8):618‐28. [DOI] [PubMed] [Google Scholar]
- 25. Munzone E, Di Pietro A, Goldhirsch A, Minchella I, Verri E, Cossu Rocca M, et al. Metronomic administration of pegylated liposomal‐doxorubicin in extensively pre‐treated metastatic breast cancer patients: a mono‐institutional case‐series report. Breast. 2010;19(1):33‐7. [DOI] [PubMed] [Google Scholar]
- 26. Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low‐dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49(16):3387‐95. [DOI] [PubMed] [Google Scholar]
- 27. Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26(30):4899‐905. [DOI] [PubMed] [Google Scholar]
- 28. Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2‐negative breast cancer: clinical and biological activity. Clin Breast Cancer. 2012;12(3):207‐14. [DOI] [PubMed] [Google Scholar]
- 29. Torrisi R, Bagnardi V, Cardillo A, Bertolini F, Scarano E, Orlando L, et al. Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER‐ and/or PgR‐positive breast cancer: clinical and biological activity. Br J Cancer. 2008;99(10):1564‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER‐2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montagna E, Vingiani A, Maisonneuve P, Cancello G, Contaldo F, Pruneri G, et al. Unfavorable prognostic role of tumor‐infiltrating lymphocytes in hormone‐receptor positive, HER2 negative metastatic breast cancer treated with metronomic chemotherapy. Breast. 2017;34:83‐8. [DOI] [PubMed] [Google Scholar]
- 32. Kontani K, Hashimoto S‐I, Murazawa C, Norimura S, Tanaka H, Ohtani M, et al. Indication of metronomic chemotherapy for metastatic breast cancer: Clinical outcomes and responsive subtypes. Mol Clin Oncol. 2016;4(6):947‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gennari A, Stockler M, Puntoni M, Sormani M, Nanni O, Amadori D, et al. Duration of Chemotherapy for Metastatic Breast Cancer: A Systematic Review and Meta‐Analysis of Randomized Clinical Trials. J Clin Oncol. 2011;29(16):2144‐9. [DOI] [PubMed] [Google Scholar]
- 34. Malik PS, Raina V, André N. Metronomics as Maintenance Treatment in Oncology: Time for Chemo‐Switch. Front Oncol. 2014;4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data generated and/or analyzed in the current study are available in the Research Data Deposit (http://rdd.sysucc.org.cn/, RDDA2020001375).


