Abstract
Introduction
In April 2017, the Tennessee Department of Health (TDH) was notified of an increase in the number of persons newly diagnosed with HIV in eastern Tennessee in the same month. Two were identified as persons with a history of injection drug use (IDU) and named each other as syringe-sharing partners, prompting an investigation into a possible HIV cluster among persons with a history of IDU.
Materials and Methods
TDH and public health staff members in eastern Tennessee collaborated to implement procedures outlined in TDH’s HIV/hepatitis C virus (HCV) Outbreak Response Plan, including conducting enhanced interviewing and using a preestablished database for data collection and management. To complement contact tracing and enhanced interviewing, TDH partnered with the Centers for Disease Control and Prevention to conduct molecular HIV analyses.
Results
By June 27, 2017, the investigation had identified 31 persons newly diagnosed with HIV infection; 8 (26%) self-reported IDU, 4 of whom were also men who have sex with men (MSM). Of the remaining 23 persons newly diagnosed with HIV infection, 10 were MSM who did not report IDU, 9 reported high-risk heterosexual contact, and 4 had other or unknown risk factors. Molecular analysis of the 14 HIV-1 polymerase genes (including 7 of the 8 persons self-reporting IDU) revealed 3 distinct molecular clusters, one of which included 3 persons self-reporting IDU.
Practice Implications
This investigation highlights the importance of implementing an established Outbreak Response Plan and using HIV molecular analyses in the event of a transmission cluster or outbreak investigations. Future HIV outbreak surveillance will include using Global Hepatitis Outbreak Surveillance Technology to identify HCV gene sequences as a potential harbinger for HIV transmission networks.
Keywords: HIV, investigation, risk factors, surveillance
In 2016, the Tennessee Department of Health (TDH) Viral Hepatitis Program, with support from other TDH programs, developed an HIV/hepatitis C virus (HCV) Outbreak Response Plan.1 This plan established a framework for TDH to prepare for, and respond to, an outbreak of HIV or HCV infection, and it outlined the roles and responsibilities of TDH staff members in the event of a transmission cluster or outbreak investigation. In April 2017, a regional disease intervention specialist (DIS) in eastern Tennessee notified TDH of an increase in the number of persons newly diagnosed with HIV in that region in the same month. From 2012 to 2016, an average of 3 persons were newly diagnosed with HIV in April. In April 2017, 6 persons were newly diagnosed with HIV. Two persons who inject drugs (PWID) who named each other as syringe-sharing partners were identified by an astute DIS, prompting an investigation into a possible HIV transmission cluster among PWID. TDH and local public health staff members collaborated to implement procedures outlined in the HIV/HCV Outbreak Response Plan, including enhanced interviewing (conducted only when investigating a possible transmission cluster) and data entry into a designated database.1 To complement contact tracing (conducted on every new HIV diagnosis), TDH partnered with the Centers for Disease Control and Prevention (CDC) to sequence HIV genes using serum specimens from persons with newly diagnosed HIV infection.
Materials and Methods
During this investigation, we defined a confirmed case as a person with (1) laboratory-confirmed HIV infection diagnosed on or after January 1, 2017 (by date of specimen collection) and (2) residence in Knox County or East Tennessee Public Health Region or an epidemiologic link (1 person reporting another person as a contact and/or vice versa) to another case as a syringe-sharing or sexual partner regardless of residence. Serum specimens from all persons who met the case definition underwent Bio-Rad HIV 1/2 antigen/antibody fourth-generation testing at the TDH State Public Health Laboratory in Nashville, Tennessee, and those specimens with positive test results and adequate specimen quantity were sent to the CDC HIV Laboratory Branch for gene sequencing. Partial HIV-1 polymerase (pol) genes were amplified by polymerase chain reaction from serum samples of persons who met the case definition (n = 14). CDC determined Tamura-Nei pairwise distances between HIV-1 sequences and subtyping by using the online tool COMET (https://comet.lih.lu) as previously described.2 We defined molecular clusters as a genetic distance of <1.5% between a pair of sequences; we could not infer directionality of transmission from this analysis.
Given the potential for rapid dissemination of HIV among PWID, as evidenced in the Scott County, Indiana, HIV outbreak in 2014,3 we prioritized interviewing of persons with newly diagnosed HIV infection who reported a history of injection drug use (IDU) and HIV testing of their named contacts. For the interviews, staff members used a questionnaire developed to interview persons in the event of an HIV transmission cluster. This questionnaire focuses on history of drug use, sexual behavior, partner elicitation (ie, determining partners for the person being interviewed,type and duration of exposure), and HCV testing. Outreach efforts (ie, efforts needed to find persons to test for HIV infection) included 16 adjoining counties and spanned 2 public health regions (Knox County and 15 counties in the East Tennessee Public Health Region).
TDH built a Research Electronic Data Capture (REDCap) database to house data on demographic characteristics, laboratory results, and risk factors for all persons newly diagnosed with HIV infection in the cluster investigation.4 Throughout the investigation, we developed social network analysis to visualize epidemiologic connections among cases and named contacts. For the social network analysis, although we asked for names of second-degree contacts (ie, contacts of named contacts linked to cases), we focused on monitoring the transmission network among cases and named contacts. TDH performed network analyses by using R version 3.2.4 and the SNA and igraph packages.5-7
Results
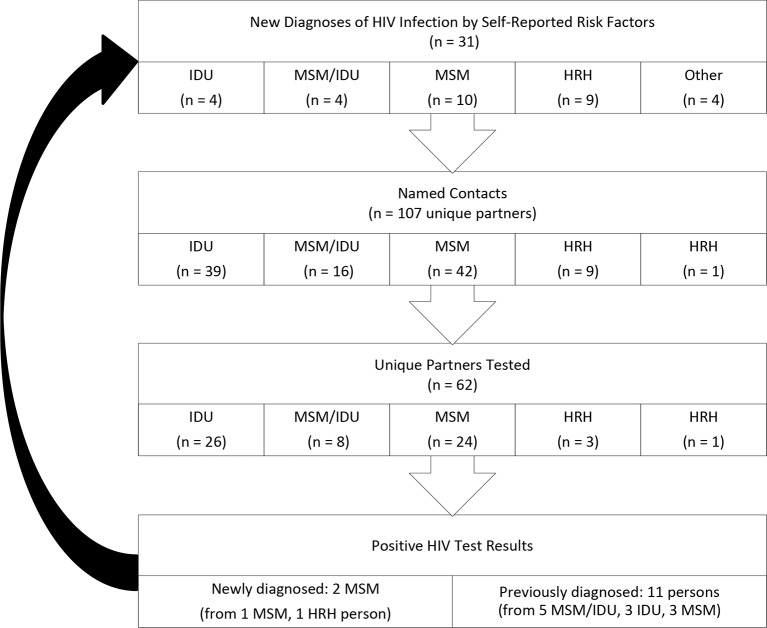
By June 27, 2017, the investigation had identified 31 persons with newly diagnosed HIV infection who met case criteria; 8 (26%) self-reported IDU, 4 (13%) of whom were also men who have sex with men (MSM) (Figure 1). Of the other 23 persons with newly diagnosed HIV infection, 10 were MSM with no self-reported IDU, 9 reported high-risk heterosexual contact, and 4 had other or unknown risks factors.
Figure 1.
Persons newly diagnosed with HIV infection in a cluster investigation in Tennessee, January 1–June 27, 2017. HRH is heterosexual contact with a person known to have, or to be at risk of, HIV infection. In total, 107 contacts were identified, and the 55 contacts elicited from persons who inject drug (PWID) and MSM/IDU cases were prioritized for interviewing and testing. Sixty-two contacts (34 from PWID or MSM/IDU cases) were located and underwent HIV testing. Two (3%) new HIV cases were identified from partners of MSM cases; however, no new HIV diagnoses were made among the partners of PWID or MSM/IDU cases. Abbreviations: HRH, high-risk heterosexual contact; IDU, injection drug use; MSM, men who have sex with men
Of the 31 persons newly diagnosed with HIV infection, 13 (42%) reported a history of incarceration and 14 (45%) were tested for HCV infection. Three persons newly diagnosed with HIV infection self-reporting IDU tested positive for HCV antibody, 1 of whom was confirmed to be positive for HCV RNA through reflex testing. The initial interviews with persons newly diagnosed with HIV infection identified 2 pairs of HIV-infected persons who were epidemiologically linked: the original pair of persons self-reporting IDU (Pair A) who prompted the investigation and a pair of MSM, 1 of whom self-reported IDU (Pair B). Of the 8 HIV-positive persons self-reporting IDU, 7 reported the type of drugs injected, which included (not mutually exclusive) methamphetamine (n = 6), heroin (n = 5), cocaine (n = 4), and non-heroin opioids (n = 3).
TDH identified 107 contacts during the investigation. Of these, 55 were contacts of the 8 persons self-reporting IDU or MSM self-reporting IDU. These 55 contacts were prioritized for an interview using the developed questionnaire and HIV testing. From identification of contacts, 62 (58%) contacts (34 from persons newly diagnosed with HIV infection self-reporting IDU or MSM self-reporting IDU) could be located and were offered HIV testing; the remaining 45 contacts could not be located despite numerous attempts. Of the 62 contacts located and tested, 46 (74%) tested negative for HIV, 11 (18%) had previously diagnosed HIV infection, and 3 (5%) had an insufficient quantity of sample for testing. TDH identified 2 (3%) new persons newly diagnosed with HIV infection from partners of MSM who met case criteria, but no new HIV diagnoses were made among the partners of persons newly diagnosed with HIV infection who self-reported IDU or who were MSM self-reporting IDU (Figure 1). Of the 34 contacts tested for HIV who self-reported IDU or were MSM self-reporting IDU, 25 (74%) reported a history of incarceration.
Analysis of the 14 HIV-1 pol sequences (including those of 7 of the 8 persons who reported IDU) revealed 3 distinct molecular clusters (Figure 2). One molecular cluster included 3 persons self-reporting IDU. CDC determined all 14 pol sequences to be sequences indicating HIV-1 subtype B infections.
Figure 2.
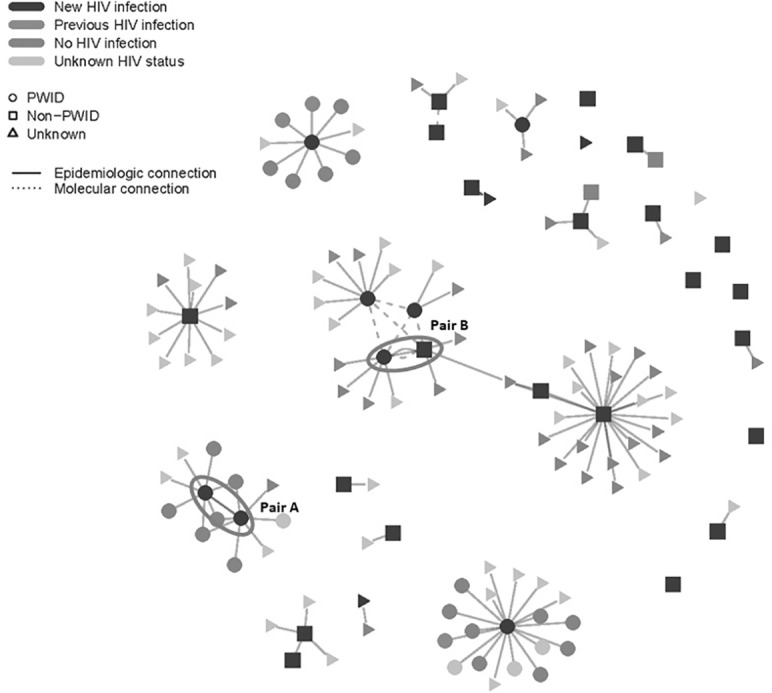
Social and molecular network of persons newly diagnosed with HIV infection and their named partners during an HIV cluster investigation in eastern Tennessee, January 1–June 27, 2017. The initial interviews with persons newly diagnosed with HIV infection identified 2 pairs of persons newly diagnosed with HIV who were linked: the original pair of persons who self-reported IDU (Pair A) and prompted the investigation and a pair of MSM, 1 of whom self-reported IDU (Pair B). The investigation found 33 new HIV infections: 31 persons with newly diagnosed HIV infection and 2 named contacts who received testing and were newly diagnosed with HIV infection. Of 107 named contacts, 62 were tested for HIV infection; of these 62 named contacts, 11 had previously diagnosed HIV infection, 46 had no HIV infection, the HIV status of 3 was not determined because of insufficient quantity of sample, and 2 were new positives. The remaining 45 named contacts were not tested. The transmission risk factors for the 62 tested were self-reported IDU (n = 34) and other non–IDU-related risk factor (eg, MSM, heterosexual contact) (n = 28). Molecular analysis of the 14 HIV-1 pol sequences (including 7 of 8 persons who reported IDU) revealed 3 molecular clusters: 1 cluster consisted of 4 persons (3 persons self-reporting IDU and 1 MSM with no history of IDU; center of figure); a second cluster consisted of a pair of persons who reported MSM as their risk factor and no reported history of IDU; and a third cluster consisted of 1 person who reported MSM as a risk factor but no reported history of IDU and 1 person whose risk factor information was unknown. Abbreviations: IDU, injection drug use; MSM, men who have sex with men.
Discussion
After the HIV outbreak in Scott County, Indiana, CDC conducted an analysis in 2016 to identify US counties where PWID are vulnerable to the rapid spread of HIV and HCV infections. Forty-one of the 220 (19%) US counties identified as being most vulnerable to such an outbreak are in Tennessee, including 10 counties in our cluster investigation.8 Our HIV investigation was triggered by the rapid identification of HIV in 2 PWID who shared syringes. We subsequently detected 31 persons with newly diagnosed HIV in eastern Tennessee, providing an opportunity to use a well-established HIV/HCV Outbreak Response Plan and HIV molecular analyses to identify linkage and prevent further transmission.
Stigmatized populations such as MSM and PWID often do not access health department services; therefore, cultivating relationships with community partners that work closely with MSM and PWID is critically important. Our cluster investigation validated the importance of health departments developing partnerships with external organizations that are likely to serve PWID, including community-based organizations and county jails, before an HIV transmission cluster or outbreak investigation. Most community-based organizations are well-established and historically trusted allies for PWID and MSM populations. In addition, because a high proportion of persons newly diagnosed with HIV infection and their contacts in this cluster investigation reported a history of incarceration, health department and jail partnerships were critically important to ensure contacts could be located, interviewed, tested, and provided necessary resources. The HIV/HCV Outbreak Response Plan facilitated community outreach in the context of classroom exercises before this investigation, and it led to the development of an enhanced interview questionnaire for PWID that could be expeditiously implemented.
Practice Implications
To respond to and interrupt a potential outbreak of HIV infection, CDC recommends ensuring that PWID have access to prevention services and routine HIV testing and are rapidly linked to care and treatment services.9 To ensure all persons at risk of HIV infection have access to testing resources, HIV testing is provided at all 95 local health departments in Tennessee, regardless of the patient’s ability to pay. In addition, care and treatment services through the Ryan White HIV/AIDS program are available throughout Tennessee, including a Ryan White–funded HIV Center of Excellence in the regions involved in this cluster investigation. Harmreduction strategies, especially syringe services programs (SSPs), are an essential component of prevention and outbreak response efforts for PWID. In May 2017, Tennessee passed legislation to legalize SSPs, and 1 of the first SSPs was established in Knox County in March 2018.10
Our investigation highlights the importance of establishing a statewide HIV/HCV Outbreak Response Plan and conducting HIV molecular analyses cluster and outbreak investigations. Future HIV outbreak surveillance will include using Global Hepatitis Outbreak Surveillance Technology to analyze HCV gene sequences and identify HCV transmission networks. This approach should allow TDH to identify persons at risk for rapid dissemination of HIV infection via networks detected in HCV molecular analyses. This information may be particularly useful in situations in which epidemiological connections are difficult to identify.
Acknowledgments
The Tennessee Department of Health recognizes the following persons for their contributions to this investigation: Mary E. Evans, MD; Allison Sanders, MPH; Roberta Sturm, MPH; Brittany S. Isabell, MPH; Sara Hall, MPH; Stephanie Paul, BSPH; Tara Sturdivant, MD; Hongwei Jia, PhD; Wei Luo, MS; Silvina Masciotra, MSc; Rendi Murphree, PhD; Julie Shaffner, MPH; Corinne M. Davis, MPH; Richard Steece, PhD, D(ABMM); Paula Gibbs, MPH; Kimberly Truss, MPH; Jalila Guy, MPH; Kimberly Gill, MPH; Shanell McGoy, PhD; Gary Messer, MPH; Jane Crowe, MSSW; Michael Rickles, PhD; Lindsay Jolly, MPH; Bryan P. Mason, MS; and staff members of the Tennessee Department of Health State Public Health Laboratory Serology.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This investigation was supported by the Centers for Disease Control and Prevention grant PS17-1703.
References
- 1. Centers for Disease Control and Prevention Managing HIV and hepatitis C outbreaks among people who inject drugs: a guide for state and local health departments. March 2018. https://www.cdc.gov/hiv/pdf/programresources/guidance/cluster-outbreak/cdc-hiv-hcv-pwid-guide.pdf. Accessed April 10, 2019.
- 2. Campbell EM., Jia H., Shankar A. et al. Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J Infect Dis. 2017;216(9):1053-1062. 10.1093/infdis/jix307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters PJ., Pontones P., Hoover KW. et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229-239. 10.1056/NEJMoa1515195 [DOI] [PubMed] [Google Scholar]
- 4. Harris PA., Taylor R., Thielke R. et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butts CT. Social network analysis with SNA. J Stat Softw. 2008;24(6). 10.18637/jss.v024.i06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Csardi G., Nepusz T. The Igraph software package for complex network research. Int J Complex Systems. 2005;1695(5):1-9. [Google Scholar]
- 7. R [statistical computing] Version 3.2.4. Vienna: R Foundation for Statistical Computing; 2016.
- 8. Van Handel MM., Rose CE., Hallisey EJ. et al. County-level vulnerability assessment for rapid dissemination of HIV and HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr. 2016;73(3):323-331. 10.1097/QAI.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention Outbreak of recent HIV and HCV infections among persons who inject drugs. Health Alert Network, CDCHAN-00377. April 24, 2015. https://emergency.cdc.gov/han/han00377.asp. Accessed April 10, 2019.
- 10. SB No 806, Public Chapter No. 413, an Act to amend TN Code Annotated Title 68.