Abstract
Background:
Modern molecular tools make it possible to manipulate neural activity in a reversible and cell-type specific manner. For rhesus monkey research, molecular tools are generally introduced via viral vectors. New instruments designed specifically for use in monkey research are needed to enhance the efficiency and reliability of vector delivery.
New Method:
A suite of multi-channel injection devices was developed to permit efficient and uniform vector delivery to cortical regions of the monkey brain. Manganese was co-infused with virus to allow rapid post-surgical confirmation of targeting accuracy using MRI. A needle guide was designed to increase the accuracy of sub-cortical targeting using stereotaxic coordinates.
Results:
The multi-channel injection devices produced dense, uniform coverage of dorsal surface cortex, ventral surface cortex, and intra-sulcal cortex, respectively. Co-infusion of manganese with the viral vector allowed for immediate verification of injection accuracy. The needle guide improved accuracy of targeting sub-cortical structures by preventing needle deflection.
Comparison with Existing Method(s):
The current methods, hand-held injections or single slow mechanical injection, for surface cortex transduction do not, in our hands, produce the density and uniformity of coverage provided by the injector arrays and associated infusion protocol.
Conclusions:
The efficiency and reliability of vector delivery has been considerably improved by the development of new methods and instruments. This development should facilitate the translation of chemo- and optogenetic studies performed in smaller animals to larger animals such as rhesus monkeys.
Keywords: chemogenetic, optogenetic, lentivirus, DREADD, rhesus monkey, non-human primates
1. INTRODUCTION
Modern molecular tools - chemogenetics, optogenetics, and calcium sensors - have introduced a new level of precision for examining neuronal circuits, both through manipulating and monitoring them (Boyden et al., 2005; Deisseroth et al., 2006; Armbruster et al., 2007; Pei et al., 2008; Alexander et al., 2009; Deisseroth, 2010; Stachniak et al., 2014; Oguchi et al., 2015; López et al., 2016; Stauffer et al., 2016; Pina & Cunningham, 2017; Ju et al., 2018; Choi et al., 2019). Application of these tools has been dramatically successful in rodents, especially mice, with a wide spectrum of applications examining the contributions of brain areas, individual pathways and specific cell types for behaviors such as memory, drug seeking and sleep have been exquisitely characterized (Pologruto et al., 2004; Nawaratne et al., 2008; Ferguson & Neumaier, 2011; Sasaki et al., 2011; Bull et al., 2014; López et al., 2016).
Although there is ongoing success, application of optogenetics, chemogenetics, and calcium sensors in rhesus monkey research has proceeded slowly (Han et al., 2009; Heider et al., 2010; Diester et al., 2011; Eldridge et al., 2016; Nagai et al., 2016; Ju et al., 2018; see Galvan et al., 2018 for review of more recent progress in optogenetics). Contributing reasons for the slower adoption of molecular tools in larger mammals include, but are not limited to:
The larger size of the rhesus monkey brain presents a challenge for delivering enough viral particles to large, discrete regions of cortex or subcortical structures for effective transduction.
Inter-species differences in viral tropism and transgene expression complicate vector/construct selection (Gray et al., 2011; Gerits et al., 2015; Scheyltjens et al., 2015; Watakabe et al., 2015; Galvan et al., 2019).
Off-target effects of chemogenetic ligands and/or their metabolites (Gomez et al., 2017; Raper et al., 2017; Manvich et al., 2018; Allen et al., 2019).
The difficulty in getting enough light to the site of expression of optogenetic receptors (Galvan et al., 2017; Stauffer et al., 2016).
Here, we address the first issue: the rhesus monkey brain is significantly larger than that of a mouse/rat (Chareyron et al., 2011). Thus, to achieve the desired vector penetrance and uniformity of coverage across a whole brain area requires the delivery of large quantities of virus in a precisely targeted fashion. Solving this issue requires the development of suitable surgical approaches to (i) achieve dense, uniform receptor expression across large brain structures, (ii) verify accuracy of virus delivery, and (iii) reduce the impact of mechanical failure at the site of injection (i.e., needle deflection or blockage). We focus on optimizing the delivery of one commonly used chemogenetic tool - the hM4Di receptor (a Designer Receptor Exclusively Activated by a Designer Drug, DREADD) - into deep subcortical structures and large cortical areas of the monkey brain. To obtain immediate verification of injection location on the day of surgery, the contrast reagent manganese MnCl2•4H2O (Mn2+) was co-infused with virus. To achieve widespread and uniform viral expression in cortical areas, a set of multi-channel injection devices was constructed that allows slow infusion of large volumes of viral vectors across the targeted tissue(s). To reduce needle deflections or bending during stereotactically guided injections to subcortical structures, a needle guide that attaches to the infusion apparatus was produced to improve mechanical stability. We show that combining these three innovations produces a more uniform level of expression of hM4Di across larger target areas within monkey brain that is comparable to expression levels previously achieved in rodent studies. All of these developments should be applicable to any viral construct.
2. MATERIALS & METHODS
2.1. Surgical Procedure
All experimental procedures conformed to the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and were performed under an Animal Study Proposal approved by the Animal Care and Use Committee of the National Institute of Mental Health. For subcortical injections, targets were derived from a pre-operative T1-weighted scan using a 3-Telsa MR scanner (Philips, Achieva DStream), (Saunders et al., 1990). All scans were acquired using the coronal orientation with a field of view of 100 × 100 × 60 mm3 and a matrix of 256 × 256 pixels. The slice thickness for each scan was 1 mm, the bandwidth was 260 Hz/pixel, the repetition time was 2720 ms, and the echo time was 4.3 ms using a modified driven equilibrium Fourier transform, and a flip angle of 11.6 degrees. For scanning, each Rhesus monkey was placed in a MRI-safe stereotaxic frame (Jerry Rig USA, NJ) for the pre-operative scan. A human knee coil was placed on top of the head. For the MR scan a tooth-marker (Walbridge et al., 2006) mounted on a stereotaxic arm was used to measure the position of the tip of the left canine in 3D space (i.e. mediolateral, dorsoventral, and anterior-posterior co-ordinates were recorded). For surgery, the monkey was placed in the stereotaxic frame so that the tooth-marker measurements of the position of the tip of the left canine were the same as the measurements recorded at the time of the MRI. All cortical injections were visualized under an operating microscope (Zeiss, OPMI Vario S88 Surgical Microscope). All surgical procedures were conducted using aseptic techniques in a dedicated operating suite. At least 12 hours before surgery, the subjects were treated with an antibiotic (cefazolin; 330 mg) and steroidal anti-inflammatory (dexamethasone; 0.5 – 4.0 mg i.m., SID to TID) to reduce the risk of post-operative infection, edema, and inflammation. Antibiotic and steroid treatment continued for 3 – 7 days post-surgery. On the day of surgery, a bone flap was removed and an incision made in the dura to provide access to the target region. Needles were inserted into the target region (cortical or subcortical) and the virus plus contrast reagent (see below) was infused at the rate of 0.5 μL/min, to a total volume of 10 μL of virus per injection (unless specifically stated otherwise). At the end of injections a 10-min wait time allowed pressure to dissipate, and virus to diffuse into the target tissue. All injections in this study used a lentivirus expressing an hM4Di-CFP fusion protein under a human synapsin promoter. The virus was made in-house at a titer of 1×109 i.u./mL (Lerchner et al., 2014). After the needles were removed, dura was sutured closed, the bone flap was sewn into position, and the soft tissue was closed in anatomical layers. Immediately following surgery, injections were visualized with a T1-weighted MRI scan. All constructs were allowed to express for a minimum of 6 weeks prior to tissue harvest, to allow protein expression to reach maximum stable levels (Nagai et al., 2016).
2.2. Injection visualization
To allow visualization of the injected area up to 24 hours after the injection procedure, the contrast reagent manganese MnCl2•4H2O (Mn2+) was co-infused with virus. Mn2+ is dissolved in double-distilled water to produce a 10 mM stock solution. The stock solution is sterile filtered through a 0.22 micron filter (Corning, NY). Once the virus is thawed to room temperature, the Mn2+ stock solution is added to the virus aliquot in the volume required to produce the desired final concentration. Concentrations of 0.1 and 5 mM Mn2+ were tested. The effect of Mn2+ on the viability of the virus was assessed using an in vitro assay; one Hamilton syringe was filled with virus and a second was filled with virus plus 10 mM Mn2+. The solutions from each syringe were injected into separate wells of cultured HEK cells at a series of time points (0.5, 1, 2, 4, 6, & 8 hrs) after initial loading. Time point 0 was pipetted directly into the well without loading in the Hamilton syringe to serve as a baseline.
2.3. Virus injection apparatus
At the core of all three injector arrays is a 3D-printed manifold (3D Systems Projet 3510, using Visijet M3 Crystal material). A Harvard Apparatus Pump 11 Elite Nanomite is used to infuse virus through the channels in the array at a controlled rate. Each array is attached to the infusion apparatus (Supplemental Figure 1) via Tygon ND100–80 microbore silicone tubing that has an inner diameter of 0.01mm, and an outer diameter of 0.03 mm (Component Supply Company, Tennessee). In surgery, the syringes are back loaded with the virus and Mn2+ solution, loaded into custom housing (Supplemental Figure 1), and attached to the pump. The pump is set to infuse at 1 μL/min until flow of virus is visible from all channels under the operating microscope; at high magnification (10x) it is possible to see the small droplets that form on the needle tips as the viral solution is effluxed. Once flow is confirmed, the infusion rate is lowered to the target rate (0.5 μL/min) and the apparatus is inserted into the target tissue under visual guidance.
2.3.1. Ventral Array Assembly:
To target the ventral surface of the cortex a manifold of 4 × 4.5 × 1.3 mm (see Supplemental Figure 2 for additional detail) was used. Four surface holes are printed with a diameter of 0.3 mm to allow the insertion of four 31-guage needles of 2.5 mm in length, countersunk by 0.5 mm to produce a 2 mm length needle for injections. The holes are arranged such that a single array placement will result in four separate injections at 2 mm spacing. The rear of the array contains four channels, with a diameter of 0.3 mm, into which 30-gauge hypodermic tubing of 30 mm length is inserted (Figure 1). All components are stabilized within the printed manifold with ‘Krazy Glue Maximum Bond’ (ethyl cyanoacrylate >95%, and 2,2’-Methylenebis(4-methyl-6-tert-butylphenol), 0.1%) with a viscosity of 85 cps at room temperature (Elmer’s Products, Inc., OH).
Figure 1:
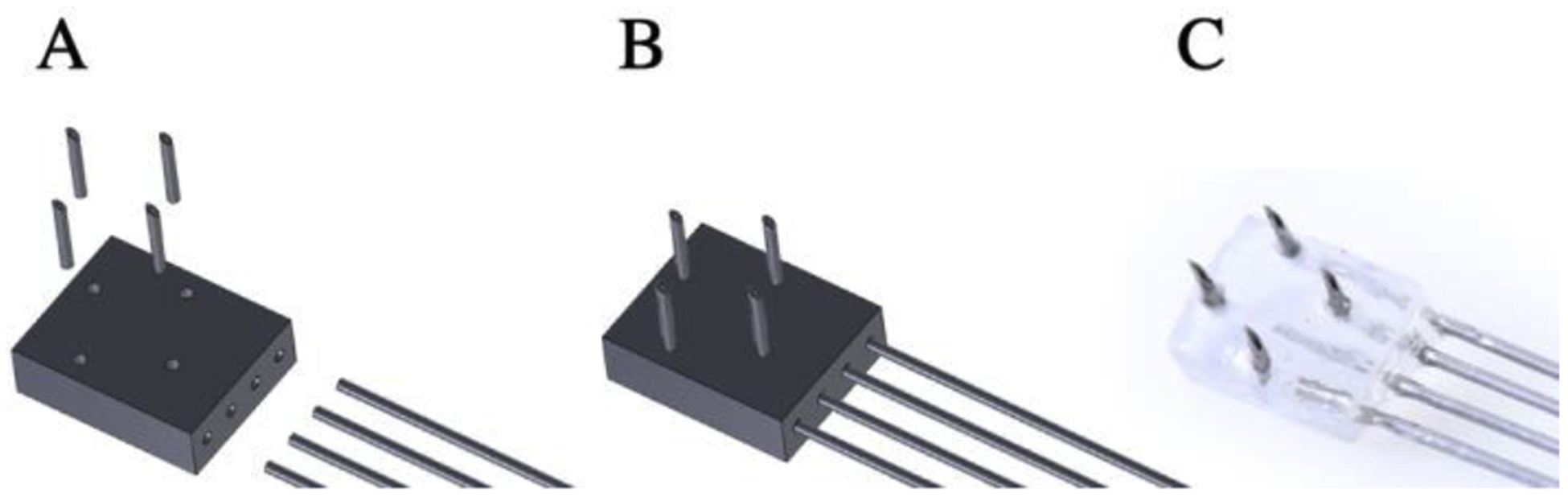
Multi-channel array for surface injections into ventral cortex. (A) Components: four needles inserted into the dorsal surface holes, 3D printed base, hypodermic tubing is inserted into the lateral surface (see Supplemental Figure 2 for additional detail). (B) Computer Aid Design (CAD) model of the assembled ventral array. (C) Photo of assembled ventral array. The array is connected to the infusion apparatus (see Supplemental Figure 1) by 10 cm silicone tubing for each channel, which is secured to the array using Krazy glue.
2.3.2. Lateral Array Assembly:
To target the lateral surface of the cortex a square manifold of 4 × 4 × 3 mm (see Supplemental Figure 2 for additional detail) is used. Four surface holes with a diameter of 0.46 mm accommodate four 26-gauge hypodermic tubes of 8 mm length. Four 31-gauge needles of 5.5 mm length are inserted into the hypodermic tubing and glued in place. A central hole of diameter 0.65 mm accommodates a 23-gauge wire that attaches to the injection apparatus (Supplemental Figure 1). The needles are arranged such that a single array placement will result in four separate injections at 2 mm square spacing (Figure 2). All components are secured to the array using Krazy glue.
Figure 2:
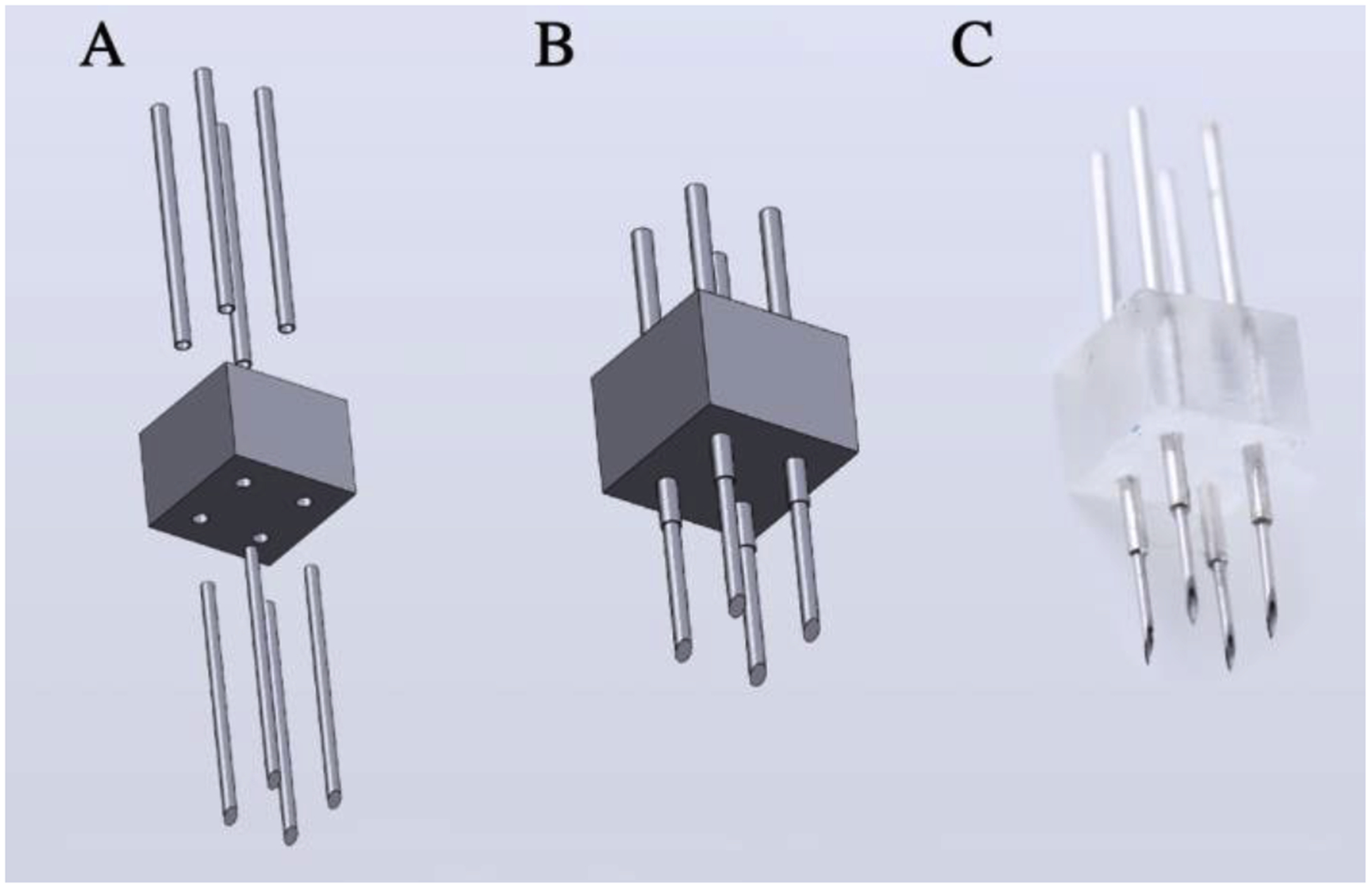
Multi-channel array for surface injections of dorsal cortex. (A) Components: 4 hypodermic tubing inserted from above, 3D printed base, 4 needles inserted from below (see Supplemental Figure 2 for additional detail). (B) CAD model of assembled array. (C) Photo of assembled array. The array is connected to the infusion apparatus (Supplemental Figure 1) by 5 cm silicone tubing. The center hole (not shown) in the 3D printed base allows for the insertion of 23-gauge wire to stabilize the connection to the infusion apparatus.
2.3.3. Linear-Lateral Array Assembly:
To target cortex in sulci on the lateral surface of the brain, a rectangular manifold of 6 × 2 × 3 mm is used (see Supplemental Figure 2 for additional detail) is used. Four holes with a diameter of 0.46 mm accommodate four 26-gauge hypodermic tubes of 8 mm length. Four 31-gauge needles of 5.5 mm length are inserted into the hypodermic tubing. A center surface hole with a diameter of 0.65 mm accommodates a 23-gauge wire that secures into the injection apparatus (Supplemental Figure 1). The holes are arranged such that a single array placement will result in four separate injections at 2 mm linear spacing (Figure 3). All components are secured to the array using Krazy glue.
Figure 3:

Needle array for injections into sulci. (A) Components: hypodermic tubing inserted from above, 3D printed base, needles inserted from below (see Supplemental Figure 2 for additional detail). (B) CAD model of assembled array. (C) Photo of assembled array. The array is connected to the infusion apparatus (Supplemental Figure 1) by 5 cm silicone tubing. The center hole (not shown) in the 3D printed base allows for the insertion of 23-gauge wire to stabilize the connection to the infusion apparatus.
2.4. Needle guide for subcortical targeting
A detachable needle guide made of anodized aluminum was built to mount onto the Nanomite pump. It is compatible with any system using 5/16” diameter stainless steel dowels. The arm can be sterilized with EtO prior to use. Once mounted, the aperture in the base of the needle guide is aligned to the trajectory of the needle. As the needle is lowered to target, the needle guide was adjusted to sit just touching the skull and the needle passes through the aperture as it is lowered (Figure 4C). The syringes were Hamilton (Cole-Parmer, Illinois) 81008 Gastight Syringes, 100μL, cemented needle, 30-gauge, 45-degree bevel. Needles were sheathed with TSP polyimide coating (product TSP320450; Molex, IL) to create a reflux resistant step (see Supplemental Figure 4 for details).
Figure 4:
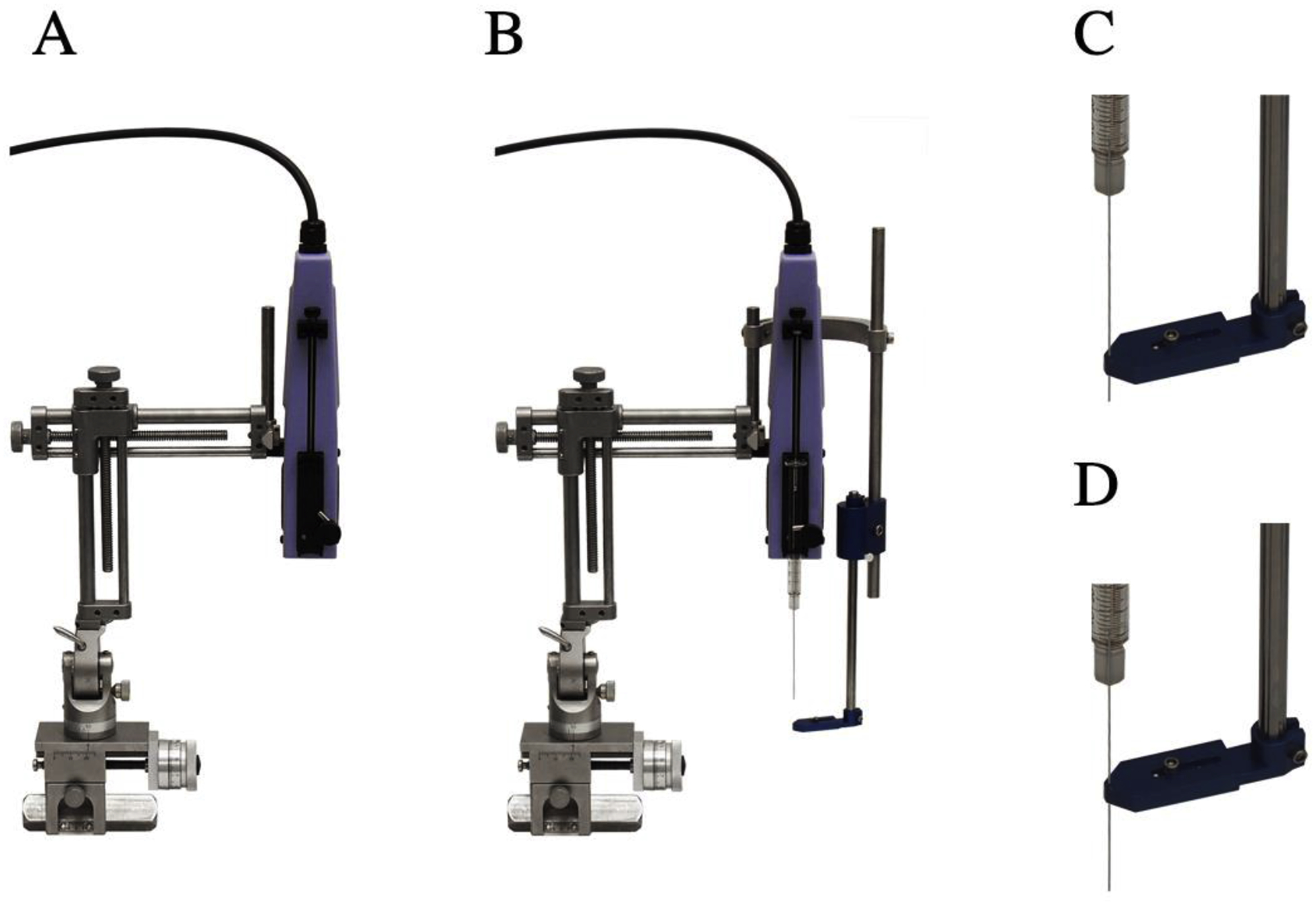
Needle guide for precise sub-cortical targeting. (A) The pump is mounted on a stereotaxic arm. (B) The needle guide is attached to the arm with a ‘C’-bracket. (C & D) The needle guide aperture allows the needle to pass through. Component dimensions of the foot can be found in Supplemental Figure 3.
3. RESULTS
3.1. Injection visualization
The contrast reagent, Mn2+, was co-infused with the virus, lenti-hSyn-hM4Di-CFP (titer of 1×109 i.u./mL), to provide post-operative confirmation that the virus was infused, and that it was targeted to the correct location. At each injection site, where 10 μL was injected at a rate of 0.5 μL/min, a MR signal was visible at the lowest concentration tested, 0.1 mM Mn2+ (Figure 5B). Higher concentrations produced a higher contrast (as expected), but also made it more difficult to localize the focus of the injection (Figure 5C). The addition of up to 10 mM Mn2+ did not interfere with the viability of the virus (Supplemental Figure 6); the toxicity of 10 mM Mn2+ was not assessed in vivo.
Figure 5:
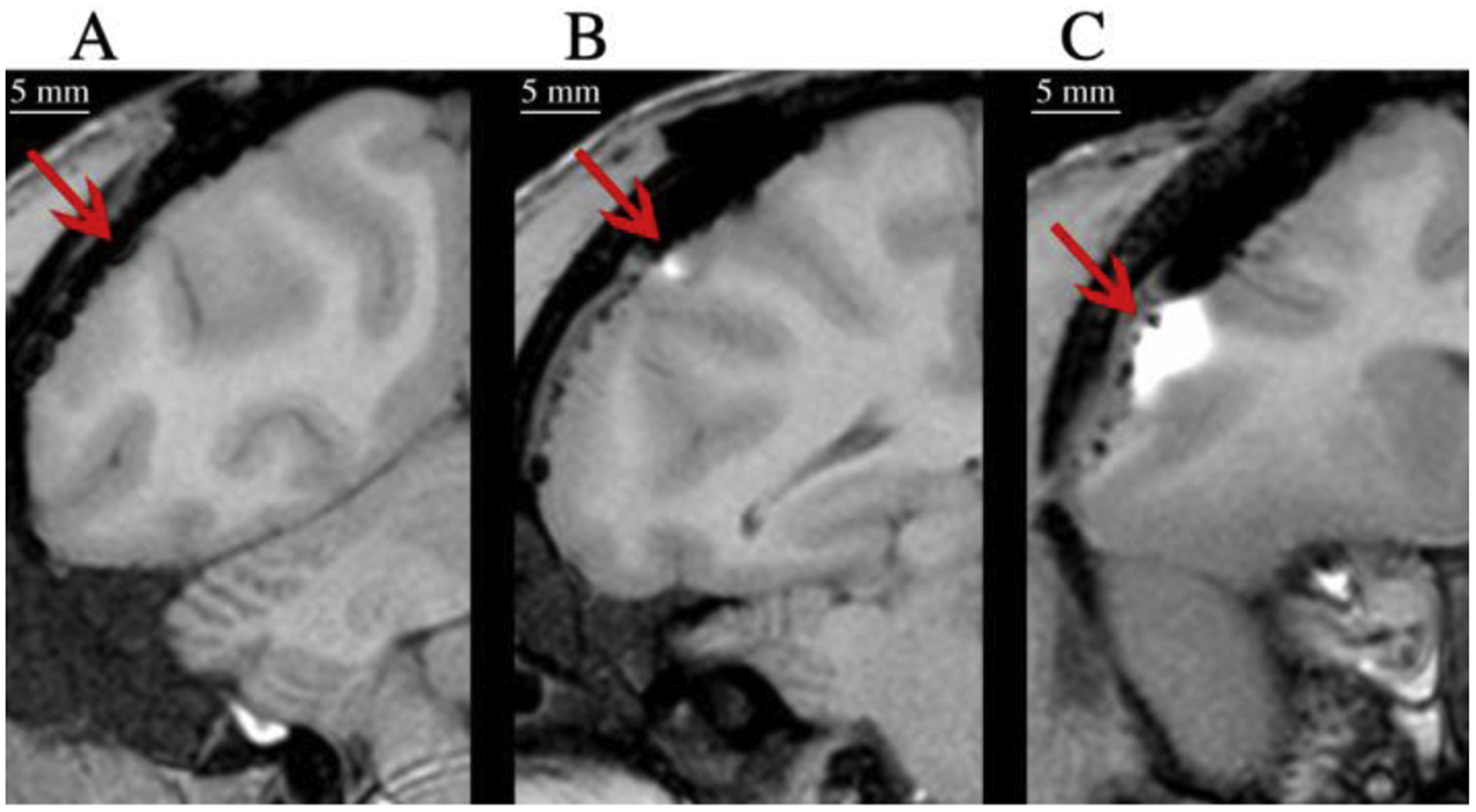
Visualization of cortical injections using MR contrast reagent. MR images acquired immediately after surgery of single injections in which virus was co-infused with 0.5 μL of (A) no Mn2+, (B) 0.1 mM Mn2+, or (C) 5 mM Mn2+.
3.2. Virus injection apparatus
Cortical injections were performed using a 2 × 2 multi-channel injector for targeting ventral cortex (Figure 6 AI), a 2 × 2 injector for targeting dorsal cortex (Figure 6 BI), and a 4 × 1 injector for targeting intra-sulcal cortex (Figure 6 CI). 5 μL per channel was injected into the ventral surface, and 10 μL per channel was injected into the dorsal surface. Accuracy of injections and approximate coverage was assessed with post-operative MRI (Figure 6, column II). Coverage was assessed at higher resolution (5X Magnification) post-mortem with a Diaminobenzidine (DAB) stain; bright-field images reveal the somatic and dendritic expression profiles of each injection type (Figure 6 III). Somatic expression at high resolution (5X Magnification) was examined with fluorescence immunohistochemistry under a confocal microscope (Figure 6 IV). A GFP antibody was used to detect CFP expression (Abcam, MA).
Figure 6:
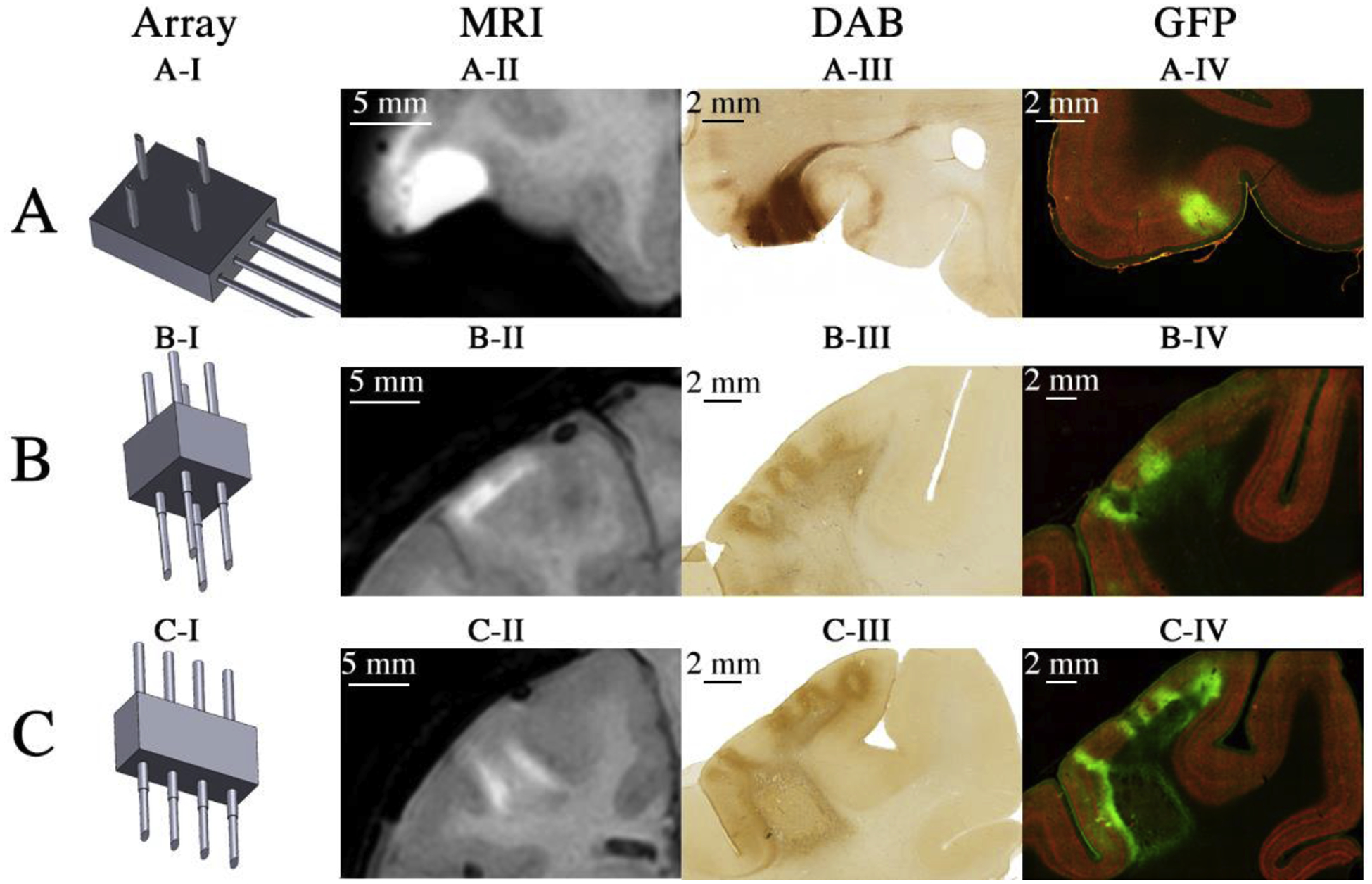
In vivo and ex vivo visualization of cortical viral injection/expression using injector arrays. (AI) Schematic representation of injector type used. (AII) Skull-stripped MR image of Monkey A acquired after virus co-infused with 5 mM Mn2+ into area 12 of ventrolateral prefrontal cortex (vlPFC). (AIII) Bright field visualization of CFP expression. (AIV) Fluorescent imaging of CFP expression. (BI – BIV) Same conventions as ‘A’, but illustrating the results of injections into primary somatosensory cortex (S1) using the needle array for surface injections of dorsal cortex of Monkey B using 0.1 mM Mn2+. (CI – CIV) Same conventions as ‘A’, but illustrating the results of two injections, one into central sulcus and one into intraparietal sulcus of Monkey B using the needle array for sulcal injections with 0.1 mM Mn2+.
3.3. Needle guide
Sub-cortical injections were performed using a single sheathed syringe. The custom-built needle guide (Figure 4) constrained the path of the needle to maximize accuracy of targeting. MR imaging was used to plan the location of the injection targets (Figure 7A, 7B). Virus (lenti-hSyn-hM4Di-CFP) was co-infused with 0.1 mM Mn2+ to allow immediate post-op visualization of needle patency and accuracy of placement (Figure 7C). Post-mortem visualization of hM4Di-CFP expression using bright field (Figure 7D) and confocal (Figure 7E) microscopy confirmed that injections accurately targeted the tail of the caudate nucleus. Contrasting this result with that of a surgery performed in the absence of the needle guide (Supplemental Figure 5) illustrates the utility of the needle guide.
Figure 7:

In vivo and ex vivo visualization of subcortical viral injection/expression using a needle guide for enhanced accuracy. Targeting Tail of Caudate: the pre-op scan of Monkey C (A) used to calculate the coordinates (B) for targeting of injections. A post-op scan (C) was acquired 8 hours after the injection of 10 μL of virus co-infused with 0.5 mM Mn2+. All MR images are skull stripped (D) Bright field visualization of CFP expression in Monkey D. (E) Confocal imaging of CFP expression in Monkey D.
4. DISCUSSION & CONCLUSIONS
Achieving efficient and reliable viral vector delivery is critical for increasing the adoption of modern molecular tools in research with rhesus monkeys; we have described three new methods to this end. Co-infusion of the contrast reagent, Mn2+, permits a verification process that is advantageous in primate research. Being off target by even small distances can result in vastly different behavioral and physiological effects. Being able to verify injection accuracy immediately post-operatively provides the means to proceed with assurance that targeting of the injection was as intended. Thus, the likelihood of missing targets due to needle deflection, or slight differences in animal positioning can be minimized.
The use of Mn2+ as a reporter for virus injection and localization allows rapid verification of injection accuracy. Intraoperative MRI is superior, in the sense that it provides feedback during the virus injection, thus permitting adjustments of targeting in real-time (Szerlip et al., 2007). However, the use of a contrast reagent for post-operative imaging overcomes the difficulty of verifying injection accuracy when surgical procedures can only be completed with instruments that are not MR compatible (such as those involving the injector arrays described herein), when real-time MR visualization of the injection is not feasible. The longevity of the contrast signal produced by Mn2+ allows injection accuracy to be verified several hours after the end of the procedure; an option that is not reliable when using contrast reagents with a shorter half-life, such as gadolinium. It is known that Mn2+ is toxic at high concentrations (120 mM) (Simmons et al., 2008). However, the concentrations of Mn2+ reported here (<= 5 mM) produced no detectable cell loss, in vivo. We have used 0.1 mM Mn2+ to visualize injections into other brain regions not reported in this study, including ventral tegmental area, substantia nigra pars compacta, superior colliculus, amygdala, and head of caudate nucleus; we have never observed any reduction in transfection efficiency, cell loss, or other markers of tissue inflammation when using the Mn+2. Furthermore, concentrations up to 10 mM caused no loss of virus viability in vitro (Supplemental Figure 6).
Intracerebral virus injections in monkeys are often performed via visually guided hand-held injections for surface cortex (Eldridge et al., 2016; Upright et al., 2018) or stereotaxic injections of deeper cortical structures (Saunders et al., 1990). Hand-held injections of viral vectors can, in our experience, produce patchy expression in the target region (Eldridge et al., 2016). The use of the multi-channel injector array allowed the needles to remain stable within cortex, while virus was infused slowly. The needles remained situated for several minutes after the end of the injection, to allow residual pressure to dissipate. This approach appears to result in more uniform coverage of the target regions (Figure 6), particularly for the ventral injector (Figure 6A). The higher concentration of Mn2+ tested, 5 mM, produced an over-estimation of the area transfected (Figure 6A-II cf. 6A-IV), although this higher concentration more closely approximated the region of dendritic + somatic expression (Figure 6A-II cf. 6A-III). The lower concentration of Mn2+, 0.1 mM, more closely approximated the somatic expression profile of the virus (Figure 6B-II and 6C-II cf. 6B-IV and 6c-IV). The difference in the density of staining between the DAB (Figure 6 III) and fluorescent images (Figure 6 IV) results from different staining protocols; we routinely visualize the fluorescent sections under high magnification to resolve individual somata, hence a lower density of staining is preferable for this application. As a result, dendritic expression tends to be less clearly visible in the fluorescent sections under lower magnifications (such as that used in Fig. 6).
Using stereotaxic co-ordinates to target subcortical structures is a method that has proven useful in past studies (Saunders et al., 1990). However, mistargeting can occur as a result of deflections, for example if the needle deviates following contact with a blood-vessel, or if the needle slides along the pial surface when inserted at a tangent. Needle deflections can be mitigated by using a guide tube. This approach is only convenient when the subject has a previously implanted chamber to provide access through a cranial window. The needle guide described herein seems to prevent the needle from deviating from the intended track during an acute surgery, providing the precision required to deliver virus to small subcortical structures.
In summary, co-infusion of manganese with the viral vector allowed for immediate post-operative verification of injection accuracy. The multi-channel injection devices increased the efficiency and uniformity of viral delivery to cortical regions of the monkey brain. The needle guide improved targeting accuracy for sub-cortical structures by preventing needle deflection. The combination of these three innovations allowed for the transduction of hM4Di in a reliable and reproducible manner across different regions of the monkey brain. These developments should facilitate the translation of molecular methods used in small animal research to studies with rhesus monkeys
Supplementary Material
Supplemental 1: Multi-channel injector apparatus. (A) The computer driven pump is fitted with custom components (B): a multi-syringe mount, multi-plunger depressor, a dowel, and syringes to produce (D) a system capable of simultaneously delivering virus through four channels. (C1) C-arm connects (C2) the metal dowel, to the pump. (C3) Glass syringes are loaded into the mount and can be simultaneously depressed by fitting the pump with (C4, C5) a multi-plunger adaptor.
Supplemental 2: Dimensions of (A) ventral injector array, with a transparent image of the array to demonstrate how the channels are printed, (B) the lateral array, and (C) the linear array. Stl. files are available to download from the NIH 3D Print Exchange at: https://3dprint.nih.gov/discover/3dpx-013591.
Supplemental 3: Dimensions of Needle guide components including (A) needle clamp, and (B) the base of the needle guide.
Supplemental 4: (A) Gastight syringe with cemented needle without modification (model number: 81008, Hamilton, ME). (B) Same Hamilton syringe depicted in (A), sheathed with TSP polyimide coating (product TSP320450, Molex, IL). The sheathing begins 2mm distal to the needle tip. (C) The dimensions of the sheathing and needle.
Supplemental 5: In vivo visualization of subcortical viral injection without the use of a needle guide. Targeting Tail of Caudate in Monkey X. A post-op scan was acquired 8 hours after the injection of 10 μL of virus co-infused with 0.5 mM Mn2+. The MR images have been manually skull stripped. The green lines show the targeted injections. (A) In the right hemisphere the Mn2+ contrast reagent is visible, however in the left hemisphere nothing appears in the tail of the caudate nucleus. (B) The red dashed line indicates the apparent needle deflection, resulting in an injection into the ventricle.
Supplemental 6: Stability of lentivirus in the presence of 10 mM Mn2+ measured in cell culture. All values normalized to time-point 0 (see methods for details). The addition of 10 mM Mn2+ did not alter transfection efficacy (ANOVA, p > 0.05 for main effects of Group, Time, and for the Group*Time interaction).
Supplemental Table 1: Materials
HIGHLIGHTS.
Manganese contrast reagent for safe, rapid post-operative injection visualization
Multi-channel injector arrays
Precise sub-cortical injections
Acknowledgements
We thank Ms. Damara Miller for help with histology, and Dr. Krystal Allen-Worthington and Mr. Justin Golomb for veterinary assistance and anesthesia monitoring. This work was supported by the Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services under project number: 1ZIAMH002619-28, and by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (MAGE). The opinions expressed in this article are the authors’ own and do not necessarily reflect the view of the US National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No competing interests declared
REFERENCES
- 1.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, … Roth BL (2009). Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron, 63(1), 27–39. 10.1016/j.neuron.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DC, Carlson TL, Xiong Y, Jin J, Grant KA, & Cuzon Carlson VC (2019). A Comparative Study of the Pharmacokinetics of Clozapine N-Oxide and Clozapine N-Oxide Hydrochloride Salt in Rhesus Macaques. Journal of Pharmacology and Experimental Therapeutics, 368(2), 199 10.1124/jpet.118.252031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armbruster BN, Li X, Pausch MH, Herlitze S, & Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences, 104(12), 5163 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden ES, Zhang F, Bamberg E, Nagel G, & Deisseroth K (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience, 8(9), 1263–1268. 10.1038/nn1525 [DOI] [PubMed] [Google Scholar]
- 5.Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, … Bowers MS (2014). Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence. Neuropsychopharmacology, 39, 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chareyron LJ, Banta Lavenex P, Amaral DG, & Lavenex P (2011). Stereological analysis of the rat and monkey amygdala. The Journal of Comparative Neurology, 519(16), 3218–3239. 10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi EA, Jean-Richard-dit-Bressel P, Clifford CWG, & McNally GP (2019). Paraventricular Thalamus Controls Behavior during Motivational Conflict. The Journal of Neuroscience, 39(25), 4945 10.1523/JNEUROSCI.2480-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deisseroth K (2010). Optogenetics. Nature Methods, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, & Schnitzer MJ (2006). Next-Generation Optical Technologies for Illuminating Genetically Targeted Brain Circuits. The Journal of Neuroscience, 26(41), 10380 10.1523/JNEUROSCI.3863-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, … Shenoy KV (2011). An optogenetic toolbox designed for primates. Nature Neuroscience, 14(3), 387–397. 10.1038/nn.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldridge MAG, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, … Richmond BJ (2015). Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nature Neuroscience, 19, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson SM, & Neumaier JF (2011). Grateful DREADDs: Engineered Receptors Reveal How Neural Circuits Regulate Behavior. Neuropsychopharmacology, 37, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvan A, Raper J, Hu X, Paré J-F, Bonaventura J, Richie CT, … Smith Y (2019). Ultrastructural localization of DREADDs in monkeys. European Journal of Neuroscience, 0(0). 10.1111/ejn.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvan A, Caiola MJ, & Albaugh DL (2018). Advances in optogenetic and chemogenetic methods to study brain circuits in non-human primates. Journal of Neural Transmission, 125(3), 547–563. 10.1007/s00702-017-1697-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerits A, Vancraeyenest P, Vreysen S, Laramée M-E, Michiels A, Gijsbers R, Van den Haute C, Moons L, Debyser Z, Baekelandt V, Arckens L, & Vanduffel W (2015). Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics, 2(3), 031209–031209. 10.1117/1.NPh.2.3.031209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, … Michaelides M (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science, 357(6350), 503 10.1126/science.aan2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, & Samulski RJ (2011). Preclinical Differences of Intravascular AAV9 Delivery to Neurons and Glia: A Comparative Study of Adult Mice and Nonhuman Primates. Molecular Therapy, 19(6), 1058–1069. 10.1038/mt.2011.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Qian X, Bernstein JG, Zhou H, Franzesi GT, Stern P, … Boyden ES (2009). Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron, 62(2), 191–198. 10.1016/j.neuron.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heider B, Nathanson JL, Isacoff EY, Callaway EM, & Siegel RM (2010). Two-Photon Imaging of Calcium in Virally Transfected Striate Cortical Neurons of Behaving Monkey. PLOS ONE, 5(11), e13829 10.1371/journal.pone.0013829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju N, Jiang R, Macknik SL, Martinez-Conde S, & Tang S (2018). Long-term all-optical interrogation of cortical neurons in awake-behaving nonhuman primates. PLOS Biology, 16(8), e2005839 10.1371/journal.pbio.2005839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerchner W, Corgiat B, Minassian V, Saunders R, & Richmond B (2014). Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain. Gene Therapy, 21 10.1038/gt.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López AJ, Kramár E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, … Wood MA (2016). Promoter-Specific Effects of DREADD Modulation on Hippocampal Synaptic Plasticity and Memory Formation. The Journal of Neuroscience, 36(12), 3588 10.1523/JNEUROSCI.3682-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, & Weinshenker D (2018). The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Scientific Reports, 8(1), 3840 10.1038/s41598-018-22116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai Y, Kikuchi E, Lerchner W, Inoue K-I, Ji B, Eldridge MAG, … Minamimoto T (2016). PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nature Communications, 7, 13605–13605. 10.1038/ncomms13605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawaratne V, Leach K, Suratman N, Loiacono RE, Felder CC, Armbruster BN, … Christopoulos A (2008). New Insights into the Function of M4 Muscarinic Acetylcholine Receptors Gained Using a Novel Allosteric Modulator and a DREADD (Designer Receptor Exclusively Activated by a Designer Drug). Molecular Pharmacology, 74(4), 1119 10.1124/mol.108.049353 [DOI] [PubMed] [Google Scholar]
- 26.Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N, … Sakagami M (2015). Double Virus Vector Infection to the Prefrontal Network of the Macaque Brain. PLOS ONE, 10(7), e0132825 10.1371/journal.pone.0132825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei Y, Rogan SC, Yan F, & Roth BL (2008). Engineered GPCRs as Tools to Modulate Signal Transduction. Physiology, 23(6), 313–321. 10.1152/physiol.00025.2008 [DOI] [PubMed] [Google Scholar]
- 28.Pina MM, & Cunningham CL (2017). Ethanol-seeking behavior is expressed directly through an extended amygdala to midbrain neural circuit. Neurobiology of Learning and Memory, 137, 83–91. 10.1016/j.nlm.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pologruto TA, Yasuda R, & Svoboda K (2004). Monitoring Neural Activity and [Ca2+] with Genetically Encoded Ca2+ Indicators. The Journal of Neuroscience, 24(43), 9572 10.1523/JNEUROSCI.2854-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, & Galvan A (2017). Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chemical Neuroscience, 8(7), 1570–1576. 10.1021/acschemneuro.7b00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, & Sakurai T (2011). Pharmacogenetic Modulation of Orexin Neurons Alters Sleep/Wakefulness States in Mice. PLOS ONE, 6(5), e20360 10.1371/journal.pone.0020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders RC, Aigner TG, & Frank JA (1990). Magnetic resonance imaging of the rhesus monkey brain: Use for stereotactic neurosurgery. Experimental Brain Research, 81(2), 443–446. 10.1007/BF00228139 [DOI] [PubMed] [Google Scholar]
- 33.Scheyltjens I, Laramée M, Van den Haute C, Gijsbers R, Debyser Z, Baekelandt V, Vreysen S, & Arckens L (2015). Evaluation of the expression pattern of rAAV2/1, 2/5, 2/7, 2/8, and 2/9 serotypes with different promoters in the mouse visual cortex. The Journal of Comparative Neurology, 523(14), 2019–2042. [DOI] [PubMed] [Google Scholar]
- 34.Simmons JM, Saad ZS, Lizak MJ, Ortiz M, Koretsky AP, & Richmond BJ (2008). Mapping Prefrontal Circuits In Vivo with Manganese-Enhanced Magnetic Resonance Imaging in Monkeys. The Journal of Neuroscience, 28(30), 7637 10.1523/JNEUROSCI.1488-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stachniak TJ, Ghosh A, & Sternson SM (2014). Chemogenetic Synaptic Silencing of Neural Circuits Localizes a Hypothalamus→Midbrain Pathway for Feeding Behavior. Neuron, 82(4), 797–808. 10.1016/j.neuron.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, & Schultz W (2016). Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell, 166(6), 1564–1571.e6. 10.1016/j.cell.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szerlip NJ, Walbridge S, Yang L, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. Journal of Neurosurgery. 2007. September;107(3):560–567. DOI: 10.3171/jns-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 38.Upright NA, Brookshire SW, Schnebelen W, Damatac CG, Hof PR, Browning PGF, … Baxter MG (2018). Behavioral Effect of Chemogenetic Inhibition Is Directly Related to Receptor Transduction Levels in Rhesus Monkeys. The Journal of Neuroscience, 38(37), 7969 10.1523/JNEUROSCI.1422-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walbridge S, Murad GJA, Heiss JD, Oldfield EH, & Lonser RR (2006). Technique for enhanced accuracy and reliability in non-human primate stereotaxy. Journal of Neuroscience Methods, 156(1), 310–313. 10.1016/j.jneumeth.2006.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, Ozawa K, Isa T, & Yamamori T (2015). Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Marmoset Neuroscience, 93, 144–157. 10.1016/j.neures.2014.09.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental 1: Multi-channel injector apparatus. (A) The computer driven pump is fitted with custom components (B): a multi-syringe mount, multi-plunger depressor, a dowel, and syringes to produce (D) a system capable of simultaneously delivering virus through four channels. (C1) C-arm connects (C2) the metal dowel, to the pump. (C3) Glass syringes are loaded into the mount and can be simultaneously depressed by fitting the pump with (C4, C5) a multi-plunger adaptor.
Supplemental 2: Dimensions of (A) ventral injector array, with a transparent image of the array to demonstrate how the channels are printed, (B) the lateral array, and (C) the linear array. Stl. files are available to download from the NIH 3D Print Exchange at: https://3dprint.nih.gov/discover/3dpx-013591.
Supplemental 3: Dimensions of Needle guide components including (A) needle clamp, and (B) the base of the needle guide.
Supplemental 4: (A) Gastight syringe with cemented needle without modification (model number: 81008, Hamilton, ME). (B) Same Hamilton syringe depicted in (A), sheathed with TSP polyimide coating (product TSP320450, Molex, IL). The sheathing begins 2mm distal to the needle tip. (C) The dimensions of the sheathing and needle.
Supplemental 5: In vivo visualization of subcortical viral injection without the use of a needle guide. Targeting Tail of Caudate in Monkey X. A post-op scan was acquired 8 hours after the injection of 10 μL of virus co-infused with 0.5 mM Mn2+. The MR images have been manually skull stripped. The green lines show the targeted injections. (A) In the right hemisphere the Mn2+ contrast reagent is visible, however in the left hemisphere nothing appears in the tail of the caudate nucleus. (B) The red dashed line indicates the apparent needle deflection, resulting in an injection into the ventricle.
Supplemental 6: Stability of lentivirus in the presence of 10 mM Mn2+ measured in cell culture. All values normalized to time-point 0 (see methods for details). The addition of 10 mM Mn2+ did not alter transfection efficacy (ANOVA, p > 0.05 for main effects of Group, Time, and for the Group*Time interaction).
Supplemental Table 1: Materials


