Abstract
Background:
A significant component of ethanol dependence is the disruption to decision-making processes. Prior work has shown ethanol dependence biases habitual seeking of ethanol, and disrupts neural mechanisms supporting decision-making. This has contributed to the hypothesis that habitual ethanol seeking in ethanol dependence may promote excessive habitual or compulsive ethanol consumption. However, decision-making and behavioral processes underlying seeking and consummatory behaviors differ. Here we examine the microstructure of ethanol consummatory behavior in the context of habitual ethanol seeking.
Methods:
Following home-cage pre-exposure to ethanol, C57Bl/6J mice underwent 4 rounds of chronic intermittent ethanol (CIE) or air exposure. Following acute withdrawal, mice began training for operant self-administration of 15% ethanol. Training consisted of 16-hour sessions in which mice were trained in a random ratio (RR) schedule of reinforcement for 30 second access to the ethanol sipper. To test for CIE-induced changes in action control, we used sensory-specific satiation and assessed the effect of outcome devaluation on ethanol seeking. Importantly, the use of a lickometer during operant training allowed us to measure the microstructure of lick behavior.
Results:
Prior induction of ethanol dependence led to increased ethanol seeking, consumption, and an insensitivity to outcome devaluation, the latter indicative of habitual ethanol seeking. We also found altered consummatory lick patterns in CIE exposed mice compared to Air controls. While CIE mice had significantly more licks in a burst and a longer burst duration, there were no differences in the total number of bursts compared to Air controls. Furthermore, these ethanol consummatory behaviors correlated with blood ethanol concentrations (BECs), while ethanol seeking responses did not.
Conclusions:
Our results confirm that ethanol dependence can produce habitual ethanol seeking and suggests the increased ethanol consummatory behaviors following ethanol dependence are separable from decision-making processes controlling ethanol seeking.
Keywords: Alcohol, CIE, Operant, Devaluation, Habits
Introduction
Flexible decision-making processes are essential for everyday behavioral functions. Ethanol dependence has been suggested to involve a shift in decision-making processes controlling our actions, from goal-directed to habitual control. One hypothesis is that habitual processes support excessive ethanol seeking and consumption, even in the face of negative consequences. Indeed, much work has shown that ethanol seeking can be biased toward habitual control (Corbit et al., 2014, 2012; Dickinson et al., 2002; Lopez et al., 2014; Mangieri et al., 2014, 2012) and that ethanol dependence itself can bias reward-based decision-making towards reliance on habitual processes (Renteria et al., 2018). These previous works show that ethanol dependence can bias habitual decision-making control over seeking processes. However, it is not clear whether these dependence-induced changes in decision-making contribute to any observed changes in ethanol consumption (Becker, 2013; Lopez and Becker, 2014). Indeed, previous findings suggest processes controlling seeking and consumption differ (Gremel and Lovinger, 2017; Travers et al., 1997). It could be that any effect of ethanol dependence on decision-making is independent from processes driving increased consumption.
Analysis of consummatory behaviors and the underlying processes that drive such behaviors could help in our understanding of excessive ethanol consumption. In primates, specific patterns of ethanol intake have been shown to be predictive of excessive ethanol drinking (Grant et al., 2008). Furthermore, repeated abstinence in chronically drinking non-human primates produced increased drinking bout volumes and durations, resulting in higher blood ethanol concentrations (Cuzon Carlson et al., 2011). In rodents, ethanol dependence induced by chronic intermittent ethanol (CIE), followed by an operant choice paradigm, was found to alter the licking microstructure of ethanol self-administration in which CIE exposed rats showed increases in the number of bursts, burst size and lick rate (Robinson and McCool, 2015). Importantly, the schedule of reinforcement or the pattern of responding required to get access to ethanol can also influence licking microstructure of ethanol consumption (Ford et al., 2007). When transitioned from a fixed ratio (FR) schedule to a single response requirement for extended sipper access, mice showed increases in burst frequency, burst duration, licks in a burst, and burst lick rate. This raises the hypothesis that the effects of ethanol dependence on ethanol consummatory behaviors may be related to the type of ethanol seeking behavior reinforced. Thus, it could be that inducing habitual ethanol-seeking would correspond to a related change in ethanol consummatory behaviors. On the other hand, the effects of ethanol dependence on ethanol seeking and consummatory processes may be separable. However, previous studies have used operant paradigms in which response strategies were not assessed or studies where response strategies were assessed but with limited analyses of consummatory behaviors (Lopez et al., 2014), leaving it unclear as to whether the effects of ethanol dependence on habitual seeking behavior and consumption patterns and licking microstructure are related.
Decision-making and consummatory behaviors are thought to be controlled by distinct neural processes (Gremel and Lovinger, 2017; Travers et al., 1997). Thus, identifying effects of ethanol dependence on both is key to isolating disrupted neural mechanisms supporting each of the behaviors. In the current study, mice were exposed to CIE in order to investigate the effects of ethanol dependence on habitual ethanol seeking behaviors concurrent with licking microstructures during operant self-administration of ethanol.
Materials and Methods
Mice
Adult (> 8 weeks) male and female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed in groups of 2–4, with mouse chow (Purina 5015) and water ad libitum and were kept on a 14-hour light/10-hour dark cycle. All experiments were approved by the Institutional Animal Care and Use Committees of the University of California San Diego and experiments were conducted according to NIH guidelines.
Chronic intermittent ethanol
In three replicates, mice were exposed to 4 rounds of ethanol vapor or air and repeated withdrawal (Becker, 1994; Becker and Lopez, 2004; Griffin 3rd et al., 2009; Lopez and Becker, 2005). Each round consisted of 16 hours of vapor exposure followed by an 8-hour withdrawal period, repeated for 4 consecutive days. Ethanol was volatilized by bubbling air through a flask containing 95% ethanol at a rate of 2–3 L/min. The resulting ethanol vapor was combined with a separate air stream to give a total flow rate of approximately 10 L/min, which was delivered to the mice housed in Plexiglas chambers (Plas Labs Inc, Lansing, MI, USA). Blood ethanol concentrations were collected at the end of each round from sentinel mice (mean BEC = 36.13 ± 2.62 mM). No pyrazole or loading ethanol injections were given prior to placement in vapor chambers (Renteria et al., 2018).
Operant self-administration
Mice were placed in sound attenuating operant boxes and were trained to press a single lever (left or right) for an ethanol (15% v/v) reinforcer. Each chamber was equipped with retractable sippers (ENV-252M, Med Associates, St. Albans, VT, USA). Training consisted of 16-hour sessions beginning 3 hours prior to the start of the dark cycle. The first day of training, the lever was retracted, and the sipper was extended throughout the session. This was followed by 3 days of FR1 training in which one lever press resulted in chamber insertion of the ethanol sipper for 60 seconds which was shortened to 30 seconds on the third day. After FR1, mice were trained on a random ratio (RR) schedule of reinforcement, previously found to bias the use of goal-directed strategies (Dickinson et al., 1983; Gremel and Costa, 2013) which consisted of two days of RR2 (on average the 2nd lever press produces the outcome) followed by 4 days of RR4 (on average the 4th lever press produces the outcome). The sipper tubes were connected to analog lickometers (ENV-250B, Med Associates, St. Albans VT, USA) interfaced with an Arduino Uno to a computer using open-source Bonsai software for data acquisition (Lopes et al., 2015). Analog signals for lick and lever press behaviors were acquired simultaneously, thresholded, and timestamped for later analyses. Drinking tubes were weighed before and after each session. Leakage and evaporation were averaged across three sessions in which the sipper was not extended and was subtracted from the weight measured at the end of each session.
Outcome devaluation
Devaluation testing through sensory-specific satiation was conducted across two days: a valued day and a devalued day. Mice were allowed 1-hour access to 15% ethanol (Devalued day) or 1% sucrose as a control outcome (Corbit et al., 2012) (Valued day). Mice that did not consume ethanol or sucrose during the free access period were excluded from subsequent analysis. On both the Valued and Devalued days, immediately following the free access period, mice were placed into the operant chamber for a 30 min extinction test in which lever presses were recorded but no sipper was extended, and hence no outcome was delivered. In a subset of mice, satiety was assessed by allowing an additional 1-hour free access period to ethanol or sucrose, immediately after the extinction test.
Behavioral and statistical analysis
Behavioral data (lever presses and licks) were imported to Matlab (Mathworks Inc., Natick, MA, USA) and were analyzed using custom scripts. Statistical significance was defined as an alpha of p < 0.05. Two-way repeated measures analysis of variance (ANOVA), linear regressions, or Student’s t test were used to analyze data using GraphPad Prism 6 (GraphPad Prism, San Diego, CA, USA). Repeated measures correlation (RMcorr) is a statistical package implemented in R (R Core Team, 2014) and was used to assess the within-subject association of two measures across multiple training days (Bakdash and Marusich, 2017). This provides a statistical measure of the overall strength and existence of any associations common across a group, independent of variability between subjects. The effect size (Rm) represents the degree to which each subject’s data is reflected by the common slope of the best fit line. When there is a good fit (bounded between −1 and 1), the effect size will be large and have tight confidence intervals. P-values for post-hoc comparisons were Bonferroni corrected for multiple comparisons. Post-hoc power analysis were completed using G*Power 3.1 (Faul et al., 2007) to ensure sufficient power in group comparisons.
Results
CIE induced enhancement of operant ethanol self-administration
For two weeks prior to the start of CIE, mice were given home cage access to 15% ethanol (Figure 1A, three vapor cohorts, Air n = 14, CIE n = 16). As chronic stress itself can bias the use of habitual processes (Dias-Ferreira et al., 2009), we group housed mice to reduce stress and were unable to measure individual home cage drinking. Ethanol dependence was induced using CIE procedures, in which mice were exposed to ethanol vapor while control animals received air. To limit the effects of acute withdrawal, ethanol self-administration training began seventy-two hours after the last vapor exposure. Mice began operant training in which during the first day the sipper was extended for the entire 16 hr session with the levers retracted (Figure 1D), thus all mice had free access to ethanol. For the next three days of training, mice were required to press the lever under an FR1 schedule. The following 6 days of training proceeded under a random ratio (RR) schedule of reinforcement to bias towards goal-directed control (Dickinson et al., 1983; Gremel and Costa, 2013). In comparison to Air controls, CIE mice pressed the lever significantly more during acquisition of lever press behavior (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(8, 224) = 2.07, p < 0.05; main effect of Training Day = F(8, 224) = 22.16, p < 0.0001; no main effect of CIE exposure F(1, 28) = 2.56, p = 0.12) (Figure. 1B). Although both Air and CIE mice earned a similar number of reinforcers (access to sipper) (repeated measures two-way ANOVA: no interaction (Training Day × CIE exposure) = F(8, 224) = 1.98, p = 0.06; main effect of Training Day = F(8, 224) = 7.49, p < 0.0001; no main effect of CIE exposure, F(1, 28) = 3.30, p = 0.07) (Figure 1C), CIE mice consumed significantly more ethanol compared to Air controls (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 252) = 4.68, p < 0.0001; main effect of Training Day = F(9, 252) = 21.21, p < 0.0001; main effect of CIE exposure = F(1, 28) = 14.89, p < 0.001; Bonferroni-corrected, ****p < 0.0001 vs. Air; ***p < 0.001 vs. Air **p < 0.01 vs. Air; *p < 0.05 vs. Air) (Figure 1D).
Figure 1. Escalation of operant ethanol self-administration after CIE.

A. Experimental timeline includes home cage ethanol drinking followed by CIE and subsequent operant self-administration using a random ratio schedule of reinforcement and outcome devaluation (DV) testing. B. Lever presses during operant training. C. Reinforcers earned across training days. D. The amount of ethanol consumed in both Air and CIE groups. Data points represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
CIE induced changes in organization of licking microstructure
To understand whether ethanol dependence altered patterns of consummatory behavior to contribute to the increase in ethanol consumption, we examined licking microstructure across training (Darevsky et al., 2019; Davis, 1989; Griffin et al., 2009; Lin et al., 2013; Robinson and McCool, 2015) (Figure 2A, B). Compared to Air controls, CIE mice had a greater number of licks as expected given the observed increase in consumption (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 252) = 4.51, p < 0.0001; main effect of Training Day = F(9, 252) = 39.41, p < 0.0001; main effect of CIE exposure = F(1, 28) = 15.93, p < 0.001; Bonferroni-corrected, ****p < 0.0001 vs. Air, ***p < 0.001 vs. Air, *p < 0.05 vs. Air) (Figure. 2C). Notably, Air and CIE mice had a similar number of bursts (Figure 2D) in which a burst of licks was defined as 3 or more licks with an interlick interval (ILI) of less than one second. However, CIE mice had significantly more licks in a burst than air controls (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 243) = 6.06, p < 0.0001; main effect of Training Day = F(9, 252) = 5.33, p < 0.0001; main effect of CIE exposure = F(1, 28) = 6.00, p < 0.05; Bonferroni-corrected, ****p < 0.0001 vs. Air, **p < 0.01 vs. Air *p < 0.05 vs. Air) (Figure 2E). Furthermore, burst durations were longer in CIE mice compared to Air controls (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 243) = 6.56, p < 0.0001; main effect of Training Day = F(9, 252) = 4.35, p < 0.0001; main effect of CIE exposure = F(1, 28) = 5.64, p < 0.05; Bonferroni-corrected, ****p < 0.0001 vs. Air, **p < 0.01 vs. Air *p < 0.05 vs. Air) (Figure 2F). Importantly, the CIE induced increases in the number of licks in a burst and burst duration were not due to changes in licking speed as there was no difference in ILI (Figure 2G) or lick duration (Figure 2H) compared to Air controls.
Figure 2. Licking microstructure in Air and CIE exposed mice.
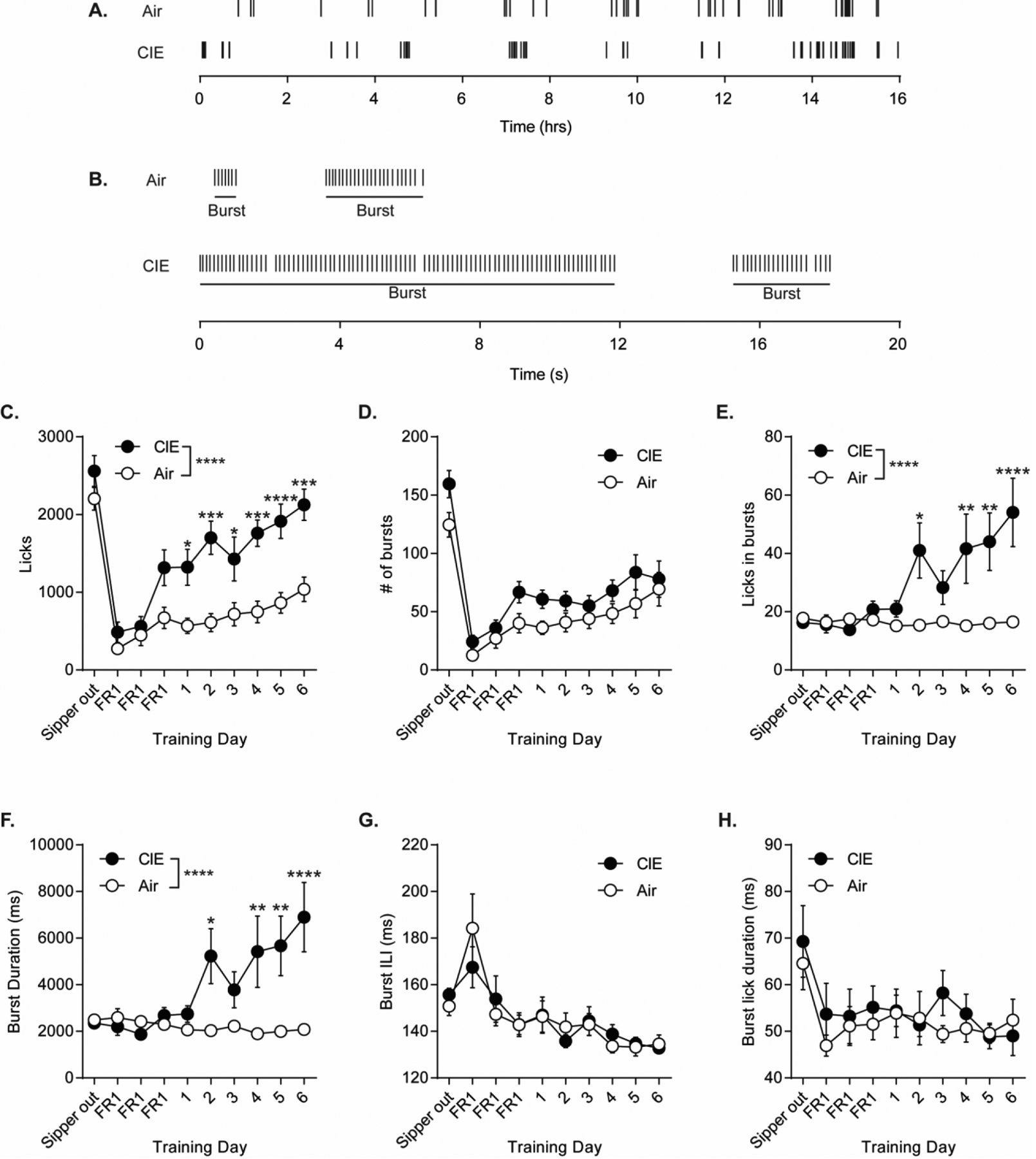
A. Example licks throughout 16-hour session in Air (top) and CIE (bottom) mice. B. Example bursts (≥ 3 licks, ˂ 1 sec) in Air (top) and CIE (bottom) mice. C. Total number of licks across training days. D. Number of bursts across training days. E. Number of licks within a burst and F. burst duration across training days. G. The interlick interval (ILI) within a burst. H. The duration of a lick within a burst. Data points represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Increased variability in licking microstructure in CIE exposed mice
As consumption behaviors progress from goal-directed to habitual or compulsive, it has been suggested they may become more stereotyped and less variable (Darevsky et al., 2019). To test this, we examined the variance in the relationship between licks and ethanol consumed. We examined the relationship between licks and consumption across training days in which we observed a difference in consumption between Air and CIE (Figure 1C). First, we accounted for the potential within-subject dependencies across days independent of the between subjects distribution. To do this, we used a repeated measures correlation which takes into account all repeated observations across mice while controlling for non-independence of observations collected within each mouse (Bakdash and Marusich, 2017). Thus, it finds the most common linear regression slope across subjects independent of the variance between subjects. With this test, we also found a strong significant correlation between licks and ethanol consumed in both Air (Rm = 0.92, degrees of freedom (DF) = 69, 95% confidence interval (CI) = 0.89, 096, p < 0.0001; slope = 142.76) (Figure 3A) and CIE mice (Rm = 0.90, DF = 79, CI = 0.85, 0.93, p < 0.0001; slope = 164.80) (Figure 3B). We then examined the distance of the observed data from each mouse’s regression line. CIE mice showed significantly greater variability in this relationship compared to Air controls (Air = 0.58 ± 0.05, CIE = 1.12 ± 0.11, p < 0.0001) (Figure 3C). Next, to account for the distribution of individuals within a group, we performing a simple linear regression on individually averaged training data (given the common significant relationship observed across individuals) in both Air and CIE groups. We found that both Air (Figure 3D) and CIE (Figure 3E) showed a significant correlation and CIE mice showed a trend towards a greater distance from the regression (Figure 3F, p = 0.08).
Figure 3. Increased variability in consumption patterns of CIE exposed mice.

A. Repeated measures correlation of licks and ethanol consumed in Air and B. CIE exposed mice (includes data across all training days). C. The distance of each point from their regression line. D. Simple correlation of the average licks and ethanol consumed in Air and E. CIE mice across training days. F. Distance of each point from the regression line. G. Coefficient of variance (CV) across training days for the number of licks in a burst, H. burst duration, and I. burst interlick interval (ILI). *p < 0.05, ****p < 0.0001.
To further examine these findings, we used the coefficient of variance (CV) of the microstructural patterns of licking in each mouse by finding the standard deviation across the session and dividing by the mean. We first looked at licks in a burst and burst duration, two measures that we found were increased in CIE mice (Figure 2E, F). Compared to Air controls, there was an increase in variance in licks made during a burst (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 252) = 1.98, p < 0.05; no main effect of Training Day = F(9, 252) = 1.46, p = 0.17; main effect of CIE exposure = F(1, 28) = 4.60, p < 0.05) (Figure 3G) as well as in the burst duration (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 252) = 2.05, p < 0.05; no main effect of Training Day = F(9, 252) = 1.43, p = 0.18; main effect of CIE exposure = F(1, 28) = 15.47, p < 0.001) (Figure 3H) in CIE mice. When examining burst ILI, a measure that was unchanged in CIE mice compared to Air controls (Figure 2G), we similarly observed no difference in variance (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(9, 252) = 1.16, p = 0.32; main effect of Training Day = F(9, 252) = 4.36, p < 0.0001; no main effect of CIE exposure = F(1, 28) = 2.51, p = 0.13) (Figure 3I). Thus, in our task, the variance in ethanol consummatory behaviors increased following the induction of ethanol dependence.
Habitual ethanol seeking in CIE exposed mice
To assess whether CIE exposure alters decision-making control over ethanol seeking behavior in our task, we used a sensory-specific satiation outcome devaluation procedure. Mice were allowed one-hour free access to either sucrose (valued) or ethanol (devalued) followed by a 30-minute extinction test. Air and CIE mice consumed similar amounts of sucrose and ethanol during the free access periods (repeated measures two-way ANOVA: no interaction (Devaluation day × CIE exposure) = F(1, 26) = 0.11, p = 0.74; no main effect of Devaluation day = F(1, 26) = 0.85, p = 0.37; no main effect of CIE exposure = F(1, 26) = 0.31, p = 0.58) (Figure 4A). Devaluation of ethanol did significantly reduce responding in Air controls, while CIE exposed mice continued to lever press at a similar rate, indicative of habitual control (repeated measures two-way ANOVA: interaction (Training Day × CIE exposure) = F(1, 26) = 9.54, p < 0.01; main effect of Training Day = F(1, 26) = 5.73, p < 0.05; no main effect of CIE exposure = F(1, 26) = 0.92, p = 0.19; Bonferroni-corrected, **p < 0.01) (Figure 4B). Our data shows that ethanol dependence biased the use of habitual decision-making processes in our task to control ethanol seeking.
Figure 4. Habitual responding does not predict increased ethanol consumption.

A. Total sucrose and ethanol consumed during outcome devaluation. B. Normalized lever presses showing the distribution of lever presses between the valued and devalued day. ** p < 0.01.
Relationship between BECs and seeking and consummatory behaviors.
Importantly, we wanted to probe whether the level of seeking behaviors is related to the escalation in ethanol self-administration observed. We first examined whether there would be a common significant relationship between lever pressing and consumption within individuals in Air and CIE groups. Its presence would suggest that individual mice exhibit similar patterns of seeking and consummatory behaviors across training days, instead of more stochastic distribution of behaviors. Indeed, we found a significant moderate positive common relationship in both Air mice (Rm = 0.41, DF = 41, CI = 0.11, 0.64, p < 0.01) (Figure 5A), and CIE mice (Rm = 0.63, DF = 47, CI = 0.41, 0.77 p < 0.0001) (Figure 5B), although the common slope was more shallow in CIE mice (Air common slope = 17.63; CIE common slope = 11.91). Given this, we averaged training data in individual mice, and performed a simple linear regression between consumption and lever pressing for Air and CIE groups. Taking into account the distribution of individual subjects, we found a significant relationship in Air mice; Air mice that on average lever pressed more, also consumed on average more alcohol (R2 = 0.45, p < 0.01) (Figure 5C). In contrast, no such positive relationship was observed in CIE mice (R2 = 0.01, p > 0.05) (Figure 5D). We then examined whether this disruption would be observed in a single training day. We performed simple linear regression on the last day of training and once again found, albeit weaker, a significant positive relationship in Air mice (R2 = 0.29, p < 0.05) (Figure 5E) that was absent in CIE mice (R2 = 0.20, p > 0.05) (Figure 5F). Hence, the level of lever pressing that produced access to ethanol was directly related to the level of ethanol consumption of Air mice but not CIE mice.
Figure 5.

A. Repeated measures correlation of licks and ethanol consumed in Air and B. CIE exposed mice (includes data across all training days). C. Simple correlation of the average licks and ethanol consumed in Air and D. CIE mice across training days. E. Correlation of lever presses to ethanol consumed on the last day of training in Air and F. CIE mice. *p < 0.05, **p < 0.01, ****p < 0.0001.
These findings raise the hypothesis that while ethanol seeking is under habitual control in dependence, CIE induced increases in ethanol consumption (Figure 1D) may be mediated in part through a distinct mechanism. We examined this hypothesis in a subset of mice, which following devaluation procedures underwent an additional two days of training to return response and consummatory behaviors to pre-devaluation levels (data not shown). One hour into self-administration sessions, we took blood samples to measure BECs achieved. BECs differed significantly between groups (Student’s t test, t11 = 2.21, p < 0.05) and were on average 3.48±0.18 mM in Air control mice and 34.95±11.08 mM in CIE mice. We then ran correlations between seeking and consummatory behaviors and BECs achieved for Air and CIE mice combined. As expected, the number of licks (Figure 6A) and ethanol consumed (Figure 6B) tightly correlated with BECs achieved. However, we found that lever presses and reinforcers earned showed no correlation with BECs (Figure 6C, D). This suggests consummatory behaviors, not ethanol seeking responses, are the main drivers of increasing levels of intoxication.
Figure 6. Consummatory behaviors but not seeking correlates with BEC.
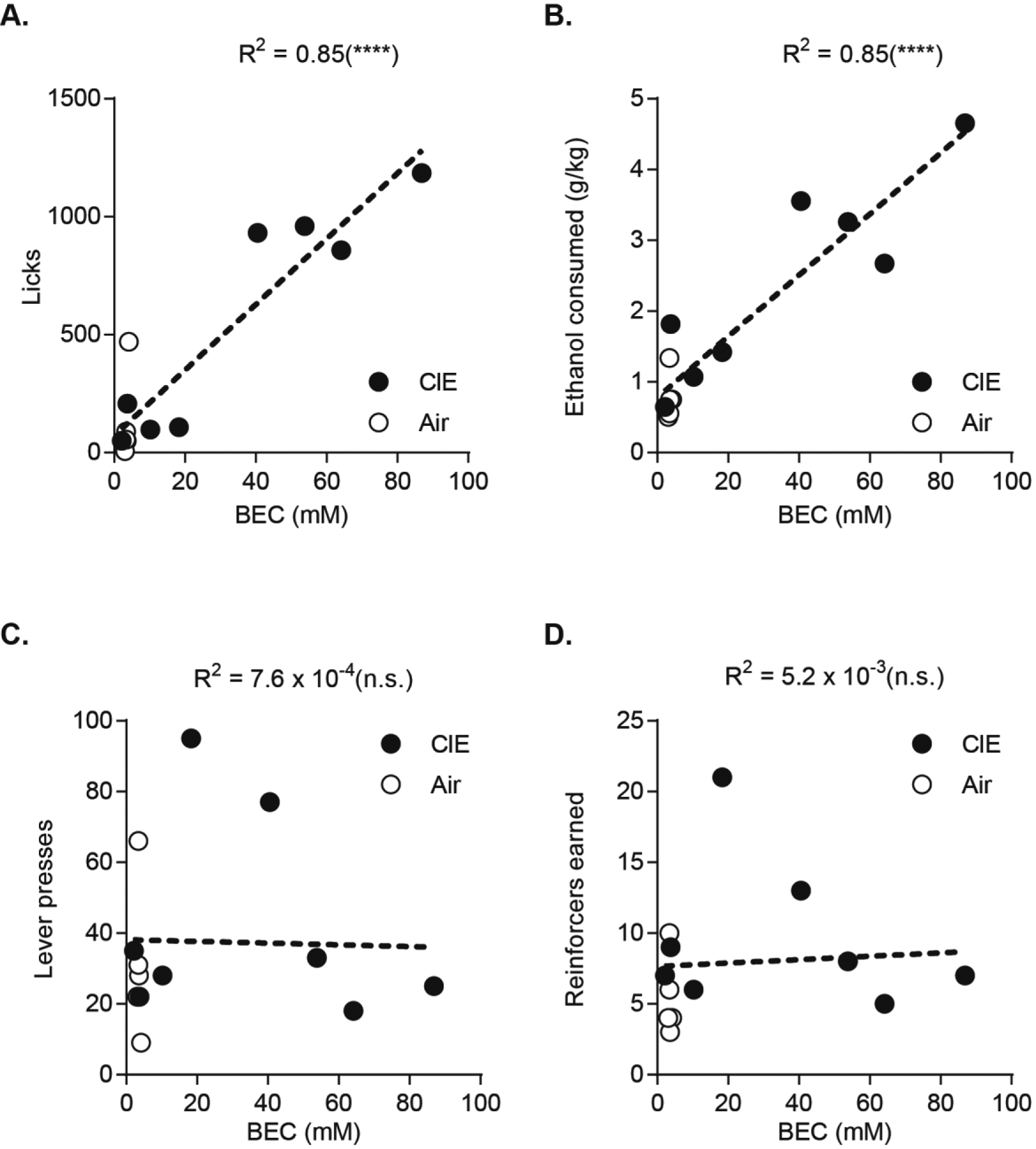
A. Correlation between licks and BECs and B. total ethanol consumed and BECs. C. Correlation between lever presses and BECs and D. reinforcers earned and BECs. Tail blood samples collected one hour into operant session. Correlations include data points from both Air and CIE exposed mice. ****p < 0.0001.
Discussion
In the present study, we found that mice exposed to CIE showed an escalation of ethanol consumption (Figure 1D), consistent with previous studies that observed a similar dependence-induced effect on operant ethanol self-administration (Becker, 2013; Lopez and Becker, 2014). In addition, we found that CIE mice show an increase in operant ethanol seeking (Figure 1B) that was found to be insensitive to outcome devaluation, a procedure often used to measure habitual control (Figure 4B). This replicates a previous report in CIE mice (Lopez and Becker, 2014) suggesting that alcohol dependence can bias the use of habitual control over decision-making ethanol seeking processes. However, we found no relationship between the degree of habitual ethanol seeking and the escalated consumption observed. Thus, our data does suggest a dissociation in behavioral, and likely neural, mechanisms between decision-making and consumption phenotypes associated with ethanol dependence.
The observed shift in action control from goal-directed to habitual is consistent with our previous report in which we found that CIE exposed mice no longer used value to guide action control for a food reinforcer (Renteria et al., 2018). Here we show that sensory specific devaluation of ethanol has no effect on ethanol seeking behavior, suggesting a similar loss of goal-directed control. In rats using an operant self-administration model, ethanol seeking has been shown to be habitual after prolonged operant training of up to 8 weeks (Corbit et al., 2014, 2012). Importantly, since the transition to habits can occur with extended training of an initially goal-directed decision-process, we wanted to limit the amount of instrumental training (Adams, 1982; Adams and Dickinson, 1981). Here we show that a 4-week cycle of CIE is sufficient to induce habitual ethanol seeking following limited training on a random ratio schedule of reinforcement that biases towards goal-directed control (Dickinson et al., 1983; Gremel and Costa, 2013).
The accelerated transition from what is often termed goal-directed to habitual decision-making is likely mediated through chronic exposure to ethanol itself. This loss of goal-directed control has been observed after both passive procedures like CIE (Lopez et al., 2014), as well as through prolonged operant ethanol self-administration (Corbit et al., 2014, 2012). This suggests that ethanol-induced changes in decision-making and action control circuits facilitate the transition to habitual responding. We previously have shown that ethanol dependence disrupts neural circuits supporting responding sensitive to outcome devaluation (Renteria et al., 2018). Importantly, artificially boosting neural activity within that goal-directed circuit, namely the orbital frontal cortex, was sufficient to restore goal-directed control independent of any manipulation to habit-related circuits. However, the dorsolateral striatum (DLS) has been shown to be necessary for habitual instrumental responding (Yin et al., 2006, 2004). Furthermore, habitual ethanol self-administration requires excitatory signaling in the DLS (Corbit et al., 2014). CIE has been shown to disrupt excitatory signaling and plasticity in the DLS (DePoy et al., 2015, 2013) and it may be these changes contribute to the bias towards habitual control. Thus, the extent of alterations to goal-directed and habitual neural circuits that support escalated ethanol consumption and habitual ethanol seeking is not fully clear.
Interestingly, prior work showed that the schedule of reinforcement can alter licking microstructure (Ford et al., 2007), suggesting that ethanol effects on action control could similarly affect ethanol consumption. Licking behaviors are mediated by central pattern generators in the brainstem and are under the control of regions of the motor cortex (Travers et al., 1997). Preparatory activity of voluntary licking behavior has been shown to be localized to the anterior lateral motor cortex (ALM) (Chen et al., 2017; Guo et al., 2014). Furthermore, ALM activity and performance of licking is modulated by midbrain dopaminergic inputs (Chen et al., 2019). Therefore, dopamine, which has a prominent role in instrumental learning (Coddington and Dudman, 2019), may be a potential point of modulation by chronic ethanol exposure that could influence both action control and licking behavior. However, we found that lever press behavior does not correlate with ethanol consumption (Figure 5D, F) in CIE exposed mice or BECs (Figure 6C) across all mice. Instead, only licking and ethanol consumption significantly correlated with BECs (Figure 6A, B). Others have reported a similar finding in tasks that did not assess decision-making controllers. Using a drinking in the dark (DID) paradigm followed by operant ethanol self-administration, neither lever press nor nose pokes were good predictors of ethanol consumption or BECs (Blegen et al., 2018). Together this suggests that although seeking behaviors in CIE mice may be under habitual control, the CIE induced changes in licking microstructure that result in excessive ethanol drinking, are likely mediated by a mechanism distinct from that controlling decision-making.
One explanation for the differences we observed in licking microstructure in CIE mice compared to Air controls could be attributable to a change in the rewarding value of ethanol and/or its associated hedonic value (i.e. palatability) (Dwyer, 2012). We found that CIE mice showed more licks in a burst and longer burst durations. Burst size has been shown to increase monotonically with increasing concentrations of a palatable solution such as sucrose (Davis and Smith, 1992; Spector et al., 1998). Conversely, increasing concentrations of an unpalatable solution such as quinine shows a negative monotonic relationship to burst size (Hsiao and Fan, 1993; Spector and St John, 1998). Hence, increases in the palatability of ethanol (achieved either through an increase in pleasantness of the ethanol or decreased aversion to the ethanol solution), could support the increases in licking behaviors observed. Another explanation could be that ethanol dependence alters the rewarding or conditioned rewarding aspects of ethanol. A more appropriate way to assess the latter hypothesis would be using tasks that more directly get at ethanol reward such as conditioned place preference paradigms (Cunningham et al., 2006).
Drug-seeking and taking have been hypothesized to progress from initial control by goal-directed processes, to habitual control and then finally to compulsive use (Everitt and Robbins, 2005). Compulsive ethanol drinking has been modeled as ethanol consumption that persists despite being paired with quinine, an aversive bitter tastant (Hopf and Lesscher, 2014). Indeed, ethanol dependence, induced by CIE, has been shown to result in the persistence of ethanol drinking despite being paired with quinine (den Hartog et al., 2016). In previous work examining ethanol drinking in rats, aversion-resistant ethanol drinking was characterized by differences in licking microstructure when compared to ethanol consumption alone (Darevsky et al., 2019). The authors found that aversion-resistant drinking showed less variability in the lick-consumption relationship, suggesting greater automaticity in the consumption pattern. In contrast, we found that CIE mice showed more variability in licking microstructure (Figure 3). This could be due to the high level of intoxication obtained as CIE mice consumed significantly more ethanol compared to Air controls (Figure 6). Intoxication will produce motor effects that could introduce variability into behavioral measures independent of any underlying behavioral or decision-making process (Gremel and Cunningham, 2007).
In using ethanol self-administration models in which mice are not food or water deprived, it is often difficult to establish adequate instrumental responding. The difficultly may arise from the delayed onset of the pharmacological and reinforcing properties of ethanol with oral self-administration (Meisch, 2001). To properly assess how action control contributes to excessive ethanol consumption we used a limited access model in order to increase instrumental responding to ensure that mice have sufficient lever press experience to learn the action-outcome contingency. Other models that have been used to evaluate the reinforcing properties of ethanol include a “sipper” model in which rats have a response requirement, that once met, allows uninterrupted sipper access for an extended period of time (Samson et al., 2000). Such a model works well to separate appetitive and consummatory behaviors, but its use makes it difficult to assess the effect of response strategies on consumption, as rats could accumulate ethanol drinking experience less contingent on instrumental responding during the long sipper access period. In another study by Robinson and McCool, lever pressing lead to sipper access that was maintained by continuous licking until a long pause in licking resulted in sipper retraction. The authors found that CIE exposed rats showed an increase in ethanol consumption as well as changes in licking microstructure including an increase in the number of bursts, burst size and lick rate. This nice demonstration showed how licking contingency controlled access to ethanol. Hence, it is important to reiterate that the manner in which animals attain access to ethanol can alter patterns of ethanol consumption (Ford et al., 2007). Our particular use of effort and shorter time contingencies of lever press and access to sipper, respectively, could be contributing to the microstructure of licking we observed. We want to emphasize that in Air controls, consumption was correlated with effort, and that effort (i.e. lever pressing) was sensitive to outcome devaluation procedures. Hence data from control mice suggests that the sipper duration used in the present studies did not disrupt the action-outcome contingency or expected-value relationship. However, licking and consumption was related to levels of intoxication achieved, while lever pressing was not (Figure 6C). Importantly, this was observed in a model that produced habitual seeking.
The observation that the prior induction of ethanol dependence disrupted the correlation between lever pressing and consummatory behaviors observed in control subjects (Figure 5C, E), raises the intriguing hypothesis that ethanol dependence induces changes in brain circuits thereby divorcing consumption from the oversight of decision-making processes. This suggests that restoring the ability of decision-making circuits to influence consummatory behaviors may be effective at reducing ethanol consumption. However, what type of decision-making process should be recruited to reduce consumption is not clear. Alcohol research with regards to the habit hypothesis has focused more on disrupting habitual ethanol seeking behaviors. Our data suggests that such a disruption may be less effective at reducing consumption. Future work could aim to illuminate and target restoration of decision-making influence on specific consummatory processes in the hopes of alleviating deleterious consequences associated with ethanol dependence.
References
- Adams CD (1982) Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol Sect B 34:77–98. [Google Scholar]
- Adams CD, Dickinson A (1981) Instrumental responding following reinforcer devaluation. Q J Exp Psychol Sect B 33:109–121. [Google Scholar]
- Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2013) Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci 13:355–77. [DOI] [PubMed] [Google Scholar]
- Becker HC (1994) Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology (Berl) 116:26–32. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838. [DOI] [PubMed] [Google Scholar]
- Blegen MB, da Silva E Silva D, Bock R, Morisot N, Ron D, Alvarez VA (2018) Alcohol operant self-administration: Investigating how alcohol-seeking behaviors predict drinking in mice using two operant approaches. Alcohol 67:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Vincis R, Fontanini A (2019) Disruption of Cortical Dopaminergic Modulation Impairs Preparatory Activity and Delays Licking Initiation. Cereb Cortex 29:1802–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Li N, Daie K, Svoboda K (2017) A Map of Anticipatory Activity in Mouse Motor Cortex. Neuron 94:866–879.e4. [DOI] [PubMed] [Google Scholar]
- Coddington LT, Dudman JT (2019) Learning from Action: Reconsidering Movement Signaling in Midbrain Dopamine Neuron Activity. Neuron 104:63–77. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2014) Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2012) Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry 72:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–70. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA (2011) Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36:2513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW (2019) Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict Biol 24:426–437. [DOI] [PubMed] [Google Scholar]
- Davis JD (1989) The Microstructure of Ingestive Behavior. Ann N Y Acad Sci 575:106–19. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP (1992) Analysis of the Microstructure of the Rhythmic Tongue Movements of Rats Ingesting Maltose and Sucrose Solutions. Behav Neurosci 106:217–28. [PubMed] [Google Scholar]
- den Hartog C, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Eaton B, Fedarovich H, Motts A, Woodward JJ (2016) Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology 107:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A 110:14783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, Lovinger D, Holmes A (2015) Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol 20:345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic Stress Causes Frontostriatal Reorganization and Affects Decision-Making. Science (80- ) 325:621–625. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, Adams CD (1983) The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q J Exp Psychol Sect B 35:35–51. [Google Scholar]
- Dickinson A, Wood N, Smith JW (2002) Alcohol seeking by rats: action or habit? Q J Exp Psychol B 55:331–48. [DOI] [PubMed] [Google Scholar]
- Dwyer DM (2012) Licking and liking: The assessment of hedonic responses in rodents. Q J Exp Psychol 65:371–94. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–9. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–91. [DOI] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA (2007) Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol 41:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32:1824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL (2007) Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 191:195–202. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Lovinger DM (2017) Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes, Brain Behav 16:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Becker HC (2009) Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res 33:1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC, Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC (2009) Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacol 201:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K (2014) Flow of cortical activity underlying a tactile decision in mice. Neuron 81:179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HMB (2014) Rodent models for compulsive alcohol intake. Alcohol 48:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S, Fan RJ (1993) Additivity of Taste-Specific Effects of Sucrose and Quinine: Microstructural Analysis of Ingestive Behavior in Rats. Behav Neurosci 107:317–26. [DOI] [PubMed] [Google Scholar]
- Lin X Bin, Pierce DR, Light KE, Hayar A (2013) The fine temporal structure of the rat licking pattern: What causes the variabiliy in the interlick intervals and how is it affected by the drinking solution? Chem Senses 38:685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes G, Bonacchi N, Frazão J, Neto JP, Atallah BV, Soares S, Moreira L, Matias S, Itskov PM, Correia PA, Medina RE, Calcaterra L, Dreosti E, Paton JJ, Kampff AR (2015) Bonsai: an event-based framework for processing and controlling data streams. Front Neuroinform 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2014) Operant ethanol self-administration in ethanol dependent mice. Alcohol 48:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacol 181:688–696. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, Chandler LJ (2014) Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol 48:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresí RU, Gonzales RA (2014) Ethanol exposure interacts with training conditions to influence behavioral adaptation to a negative instrumental contingency. Front Behav Neurosci 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresí RU, Gonzales RA (2012) Ethanol seeking by long evans rats is not always a goal-directed behavior. PLoS One 7:e42886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA (2001) Oral drug self-administration: An overview of laboratory animal studies. Alcohol 24:117–28. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R Core Team (2014). R: A language and environment for statistical computing. R Found Stat Comput; Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Renteria R, Baltz ET, Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, McCool BA (2015) Microstructural analysis of rat ethanol and water drinking patterns using a modified operant self-administration model. Physiol Behav 149:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ (2000) A new assessment of the ability of oral ethanol function as a reinforcing stimulus. Alcohol Clin Exp Res 24:766–73. [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM (1998) Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112:678–94. [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ (1998) Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol - Regul Integr Comp Physiol 274:R1687–703. [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H (1997) Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21:631–47. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW (2006) Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res 166:189–196. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW (2004) Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19:181–189. [DOI] [PubMed] [Google Scholar]


