Abstract
Conventional oxygen therapy (COT) and noninvasive ventilation (NIV) have been considered for decades as frontline treatment for acute or chronic respiratory failure. However, COT can be insufficient in severe hypoxaemia whereas NIV, although highly effective, is poorly tolerated by patients and its use requires a specific expertise. High-flow nasal cannula (HFNC) is an emerging technique, designed to provide oxygen at high flows with an optimal degree of heat and humidification, which is well tolerated and easy to use in all clinical settings. Physiologically, HFNC reduces the anatomical dead space and improves carbon dioxide wash-out, reduces the work of breathing, and generates a positive end-expiratory pressure and a constant fraction of inspired oxygen. Clinically, HFNC effectively reduces dyspnoea and improves oxygenation in respiratory failure from a variety of aetiologies, thus avoiding escalation to more invasive supports. In recent years it has been adopted to treat de novo hypoxaemic respiratory failure, exacerbation of chronic obstructive pulmonary disease (COPD), postintubation hypoxaemia and used for palliative respiratory care. While the use of HFNC in acute respiratory failure is now routine as an alternative to COT and sometimes NIV, new potential applications in patients with chronic respiratory diseases (e.g. domiciliary treatment of patients with stable COPD), are currently under evaluation and will become a topic of great interest in the coming years.
Keywords: high-flow nasal cannula, oxygen therapy, respiratory failure
Introduction
In patients with respiratory failure due to a variety of chronic and acute conditions, different kinds of respiratory supports are used to improve oxygenation and ventilation. Among these, high-flow nasal cannula (HFNC) oxygen therapy is an emerging form of noninvasive respiratory support gaining increasing attention among clinicians.1,2 Conventional oxygen therapy (COT), i.e. the administration of oxygen via a nasal cannula or face mask, has been considered the frontline treatment for acute and chronic hypoxaemia for a long time. However, only low flows of oxygen (up to 15 l/min) can be provided via a traditional cannula or mask due to insufficient heating and humidification of the inhaled gas that can give discomfort to the patient as the flow increases.3 Noninvasive mechanical ventilation (NIV), usually coupled with COT, is a more advanced option to improve ventilation, reduce the work of breathing and improve gas exchange across a variety of aetiologies.4 In particular, there is compelling evidence that NIV improves outcomes in acute life-threatening hypercapnic respiratory failure in patients with chronic obstructive lung disease (COPD), and the use of NIV in these patients is now strongly recommended.4 Long-term home treatment with NIV is also recommended by guidelines for hypercapnic patients with COPD.5 Despite its effectiveness, NIV may be poorly tolerated, and even frightening, for a number of patients, due to the high pressures delivered in the airways, difficulty in synchronising breathing, claustrophobia, stomach distension and mask-related side effects such as nose sores and skin lesions over the bridge of the nose.6
HFNC is an emerging technique designed to provide oxygen at high flows (above 30 l/min) with an optimal degree of heat and humidification via an interface consisting of a silicone cannula that fits, without occluding, the nose. This offers better comfort, compared with NIV, and a more efficient oxygenation than COT.3 HFNC was first introduced into clinical practice in the early 2000s as a noninvasive system to manage apnoea in premature neonates and since then its use in paediatrics, particularly in respiratory failure caused by bronchiolitis, is well established.7 Successively, HFNC has been investigated in adults with acute respiratory failure, gaining increasing popularity among intensivists.8,9 More recently, the use of this new device has spread out of intensive care units (ICUs), particularly the respiratory units where it is widely and increasingly used, often replacing NIV in the management of respiratory failure from a variety of aetiologies. Finally, in the last few years the development of simpler and user-friendly HFNC devices, suitable for low-level healthcare settings or home use, has provided a new opportunity, still to be explored, for managing patients with chronic respiratory conditions or for domiciliary palliative care.
Articles and reviews/meta-analysis on HFNC have been constantly increasing. However, most of the available literature focuses on the current use of HFNC, which is limited to hospitalised patients with respiratory failure. We now feel that in the next few years this technique will be rapidly implemented for the treatment of patients with chronic respiratory failure from different aetiologies, some of them never explored so far (e.g. bronchiectasis). Therefore, in this review we report the rationale and current clinical use of HFNC in adults and we discuss potential new fields of application of HFNC in patients with chronic respiratory diseases.
Brief description of the device
The HFNC device is an open-circuit system consisting of a flow generator (air/oxygen blender, turbine or Venturi mask), an active heated humidifier and a single heated noncondensing circuit (Figure 1). The circuit is connected to a silicone nasal cannula of different sizes to fit the patient’s nostrils. The original device, intended to be used in environments of high-level healthcare, needed to be connected to a source of oxygen and a source of high-pressure air in order to generate flows. Recently, new devices have been produced which can be used at home, as high flows can be obtained by a turbine (Figure 2). Parameters that can be set include the flow/min, fraction of inspired oxygen (FiO2) and temperature. The flow of gas can be regulated up to 60 l/min (depending on the device), whereas the FiO2 can be regulated up to 60% or 100%, again depending on the device. The recommended temperature is 37°C to warrant a content of 44 mg H2O/l, which is 100% relative humidity. Currently the most marketed devices are the Optiflow and Airvo 2 (Fisher & Paikel Healthcare Ltd, Maidenhead, UK) and Precision Flow Plus and Flowrest (Vapotherm, Exeter, NH, USA). A recent review by Nishimura accurately describes technical details for all available devices.10
Figure 1.
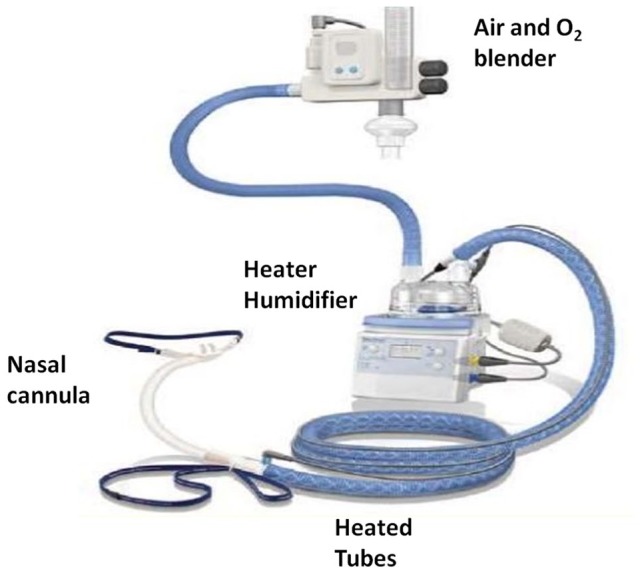
Optiflow nasal high-flow system (Fisher & Paikel Healthcare). The air–oxygen blender allows a fraction of inspired oxygen from 21% to 100% and generates a flow of up to 60 l/min. The gas is heated and humidified through an active heated humidifier and delivered through a heated tube.
Figure 2.
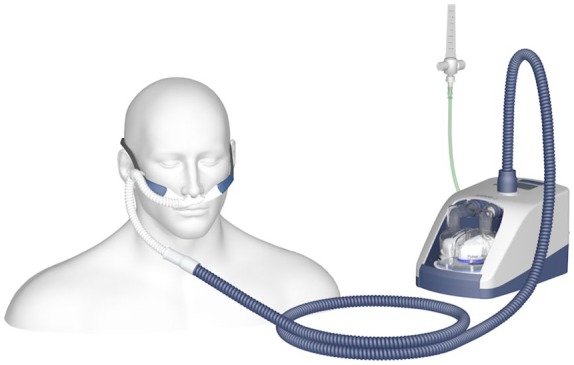
Airvo 2 high-flow system (Fisher & Paikel Healthcare). The high flow (up to 60 l/min) is generated by a turbine and the system can be used at home.
Physiological effects
The HFNC treatment exerts its benefits on the respiratory system by a number of different physiological mechanisms. Some of them have been extensively explored, but others still remain unclear (Table 1). It is intriguing that, differently from NIV, which has been physiologically studied for many years before implementation in clinical practice, HFNC has been rapidly introduced with enthusiasm by clinicians in their daily practice, while physiological studies are still ongoing.
Table 1.
Beneficial effects of the high-flow nasal cannula.
| Physiological | Clinical |
|---|---|
| Reduction of anatomical dead space | Reduction of dyspnoea |
| Generation of a positive end-expiratory pressure | Improved oxygenation |
| Maintenance of a constant fraction of inspired oxygen | Improvement in patient’s comfort due to: |
| Reduction in work of breathing | • optimal humidification |
| Improvement in mucociliary clearance | • possibility of eating or talking while under treatment |
| • lack of deleterious effects from the nasal/facial mask |
Effect on anatomical dead space and carbon dioxide wash-out
In the respiratory system ‘dead space’ refers to the space in which oxygen and carbon dioxide (CO2) are not exchanged across the alveolar membrane. Specifically, ‘anatomical dead space’ refers to the volume of air located in the segments of the respiratory tract responsible for conducting air to the alveoli and respiratory bronchioles but which do not take part in the process of gas exchange itself. Reduction of dead space is one of the mechanisms by which the high flows produced by HFNC improve the removal of CO2.
In patients with severe respiratory distress, HFNC reduces the respiratory rate.11,12 In addition, compared with low-flow oxygen, HFNC improves thoracoabdominal synchrony in adults with mild to moderate respiratory impairment.13 Although HFNC decreases minute ventilation, the partial pressure of CO2 (PaCO2) remains steady or even decreases. This indicates that HFNC produces a more efficient ventilation, facilitating the clearance of CO2 from the anatomical dead space.2,14,15 This is an extremely relevant effect, as during physiological human ventilation, around 30% of the tidal volume is wasted in anatomical space that does not participate in gas exchanges. The contribution of dead space is particularly important in patients with acute hypercapnia in which the dead space volume is increased by the high respiratory rate. This is a peculiar effect of HFNC in contrast with traditional NIV. The face mask used for NIV has the disavantage to increase anatomical dead space.2
Effect on work of breathing
Work of breathing (WOB) is the energy expended by the respiratory muscles to perform their physiological activity and the main effect of NIV is to assist respiratory muscles by reducing WOB. Theoretically, the reduction of respiratory rate and the improved thoracicabdominal coordination should, as a consequence, reduce WOB. A reduction in WOB by HFNC has been shown directly in infants with respiratory distress.16 In adults with COPD it has been reported that HFNC treatment decreases WOB compared with COT.17–19 In a group of patients with exacerbation of COPD, the electrical activity of the diaphragm significantly decreased during postextubation HFNC treatment compared with COT.18 In another study diaphragm activation was assessed by ultrasound in a group of patients recovering from acute hypercapnic respiratory failure and previously treated with NIV. Again, after NIV interruption, diaphragm activation increased during COT, but not HFNC.19 This effect on WOB might be particularly useful in hypercapnic patients in which HFNC can represent an alternative to COT in order to avoid escalation to NIV.
Generation of a positive end-expiratory pressure
The HFNC device is an open system (while NIV is a closed system), however the high flows produced are sufficient to prevail against the expiratory flow and generate a small positive pressure in the airways, known as positive end-expiratory pressure (PEEP).20,21
If the patient is breathing with closed mouth, so that there is little escape of gas, the pressure measured at the nasopharynx, increases with increasing flows. With a maximum flow of 60 l/min, the pharyngeal pressure will be around 3 cm H2O, however, if the mouth is open the pressure will remain below 3 cm.2,22 In a lung simulator model, factors affecting the level of PEEP were the set flow, peak inspiratory flow and tidal volume.23 This positive pressure, although small, is sufficient to increase the end-expiratory lung volume in a linear fashion with increasing gas flow.21,24 Therefore, the PEEP effect of HFNC, which is lacking with COT, has undoubtedly some advantages in different clinical conditions. In acute hypoxaemic failure, PEEP causes alveolar recruiting (increase in the number of ‘open’ alveoli participating in ventilation) and the shift of lung water from the alveoli to the perivascular interstitial space (e.g. in pulmonary oedema). In patients with expiratory flow obstruction such as COPD, intrinsic PEEP, generated by lung hyperinflation, can be effectively counteracted by the application of a small extrinsic PEEP.25 This will contribute further to reduce WOB.
Regulation of FiO2
One problem with COT is that relatively low flows of oxygen can be delivered and FiO2 is inconstant when using a traditional cannula or mask.26 This occurs because physiologically the inspiratory flow varies with each breath and so does the FiO2, although it is often erroneously assumed that at a set FiO2 all the oxygen given is inhaled by the patient. On the contrary, if the patient is in respiratory distress, his inspiratory flow will exceed the oxygen flow delivered by traditional devices.26,27 Moreover, in severe hypoxaemia masks are generally preferred to cannulas. Therefore, the high patient’s respiratory rate and flows will produce an entrainment of room air into the mask, so that the inhaled oxygen will be further diluted, resulting in an insufficient FiO2.27,28 When oxygen is administered via HFNC the flow is high and constant and this overcomes the issue of gas dilution, allowing the patient to inhale a constant FiO2.29 Studies show that the set FiO2 is more constant at higher flows23 and tends to be higher during open-mouth breathing.30
Airway humidification and heating
The HFNC device has an incorporated active heated humidifier which allows the delivery of warm and humidified gas into the airways (Figure 1). At the optimal temperature of 37°C, with flows in the range of 20–60 l/min, a 100% humidification can be achieved and maintained constant, although minor variations may occur at different flow levels, with changes in the patient’s breathing pattern and with different devices.31 With higher flows (>80 l/min), which are currently under evaluation, conventional humidifiers may not be reliable.32 The conditioned gas is delivered through a heated circuit which avoids condensation when the atmospheric temperature is lower.33
Indeed, the application of such conditioned gas may affect positively the airway function. It is well known that inhalation of dry and cold air increases airway resistance and WOB.34 A proper humidification is also necessary to optimise mucosal function, and to maintain gas exchange and host defense.35,36 When the respiratory mucosa dries, mucus becomes thicker and the ciliary function is impaired so that it will be more difficult to clear secretions from the airways.37 With such premises, the use of COT or NIV without sufficient humidification may cause discomfort to the patient and produce an adverse physiological effect.38 On the contrary, in different clinical conditions, delivery of water vapour to the airways may improve airway clearance, increase the amount of secretions and preserve mucus rheological properties.36,38 This will prevent the occurrence of atelectasis improving the ventilation–perfusion ratio.39 This effect of HFNC is particularly promising in those chronic hypersecretive states requiring an optimisation of airway clearance. In addition, patients with acute distress may have excessive secretions thus making a big effort in expelling them. At the moment, data on the effect of HFNC on mucus secretion are lacking, but indeed this is worthy of further investigation.
Current clinical applications of HFNC oxygen therapy
Hypoxic respiratory failure
The respiratory system is functionally composed of two parts, the lungs, which provide a gas-exchange surface, and the pump, which ventilates the lungs. In general, respiratory failure from lung dysfunction, caused by a variety of diseases (e.g. pneumonia, interstitial disease or pulmonary oedema), leads to hypoxaemia with normocapnia or hypocapnia. Acute hypoxic respiratory failure (ARF) was the first condition treated with HFNC in adults. Its use has been widely explored, particularly in ICUs and emergency departments (EDs) (Table 2).2,27
Table 2.
Current clinical applications of the high-flow nasal cannula system.
| Clinical effects | |
|---|---|
| Acute hypoxaemic respiratory failure | • Reduces dyspnoea • Improves oxygenation • Decreases escalation to invasive support |
| Hypoxaemic failure in immunocompromised patients | • Reduces dyspnoea • Improves oxygenation • Reduces intubation rate • Reduces mortality (?) |
| Cardiogenic pulmonary oedema | • Improves oxygenation • Reduces cardiac afterload |
| Exacerbation of chronic obstructive pulmonary diseases | • Improves gas exchange • Decreases partial pressure of CO2 |
| Postextubation | • Improves gas exchange • Decreases reintubation rates |
| Respiratory procedures | • Improves oxygenation during endoscopy |
The first studies in patients with different degrees of acute hypoxaemia focused on physiological parameters. These studies showed that HFNC oxygen therapy, compared with COT, improved oxygenation without affecting PaCO2, and reduced the respiratory rate and clinical signs of respiratory distress.2,9,11,12 In addition HFNC offered better comfort and was better tolerated than conventional therapy.2,9 The rationale of using HFNC in ARF is to avoid escalation to more advanced respiratory supports such as NIV or endotracheal intubation. In one early study in patients with ARF treated with HFNC compared with those treated with COT, escalation to NIV was less likely.40 In another group of patients, developing ARF after lung transplantation, treatment with HFNC at admission to the ICU was the only variable associated with a reduced risk of NIV.41 HFNC has been compared also with NIV itself in the management of ARF.42–44 In a large trial performed in 2015, Frat et al. randomised 310 patients with ARF, mainly affected by pneumonia (those with acute heart failure were excluded), to standard oxygen therapy, NIV or HFNC treatment.43 They found no difference in the intubation rate among groups, but there was a significant difference in favour of high-flow oxygen in 90-day mortality. Interestingly, a post hoc analysis of this study showed that HFNC treatment was significantly superior to NIV or COT in a subgroup of immunocompromised patients.44 After an early implementation in ICUs, the use of HFNC has spread in EDs for the treatment of ARF, most commonly in patients with pneumonia or pulmonary oedema. Some studies confirm that in the ED HFNC, compared with conventional treatment, improves oxygenation, dyspnoea and comfort,45,46 and reduces escalation to mechanical ventilation.47 Conversely, in one report HFNC did not reduce the need for mechanical ventilation compared with COT in the ED.48 In addition, a more recent study showed a higher mortality rate among patients treated with HFNC in non-ICU wards.49 The authors warned that care should be exercised when a patient with ARF, treated with HFNC, is not continuously monitored. At the moment the effect of HFNC on intubation or mortality rate in the ED remains unclear.
Although results from single studies published so far on the effect of HFNC on ARF seem encouraging, the attempt to merge the available data in meta-analysis has given conflicting results. Two meta-analyses in 2016 failed to find superiority of HFNC compared with NIV or COT in terms of mortality or intubation rate.50,51 In contrast, in 2017 two further meta-analyses comprising 3881 and 1891 patients reported that HFNC was superior to COT and similar to NIV in reducing the intubation rate in ARF.52,53 In the same year a Cochrane review was unable to establish whether HFNC is more effective or safe than COT in ICU patients.54 Again, in 2019 Zayed et al. failed to show an effect of HFNC on mortality rate in such patients.55 Inconclusive data from these meta-analyses are likely due to the heterogeneous patients included in the studies (e.g. different aetiologies of ARF), nonhomogeneous data, so that pooling is difficult, and some bias among studies (e.g. lack of blinding).54,56 While HFNC is generally indicated for patients with mild to moderate hypoxia, a clear association between the efficacy of HFNC and the aetiology of ARF has not been established. Escalation to more invasive treatment is more likely to occur in patients whose hypoxaemia is strongly dependent in alveolar collapse and in patients with acute respiratory distress syndrome.
Immunocompromised patients
Among the variety of conditions that may be effectively treated with HFNC it is worth mentioning ARF in patients with immunodepression. These include patients with active cancer, organ transplant, use of immunosuppressive agents and chemotherapy or affected by HIV. When ARF occurs in such patients, NIV has been widely used as the treatment of choice.4,57 HFNC treatment has been tested in immunocompromised patients, but often these patients were included in groups with ARF from different aetiologies. In a subset of immunocompromised patients with ARF, from the FLORALI study, the use of HFNC was compared with NIV and COT. Treatment with HFNC compared with NIV and COT significantly reduced the intubation rate, mortality at 90 days and the number of ventilator-free days at day 28.44 Successively, a large single-centre observational study comprising 115 immunocompromised patients confirmed the superiority of HFNC, compared with NIV, in reducing intubation rate.58 It is noteworthy that both these studies revealed poor outcomes associated with the use of NIV. It has been speculated that, in some conditions, NIV could be harmful due to potential ventilator-induced lung injury. This injury is generated by a high-pressure support that increases tidal volume leading, in turn, to high transpulmonary pressure. Successively, other studies have outlined the usefulness of HFNC in managing ARF in immuodepressed patients.59,60 One recent meta-analysis by Cheng et al. showed the superiority of HFNC versus COT in reducing the intubation rate, without benefit on survival.60 Another meta-analysis confirmed the superiority of HFNC versus NIV in reducing the intubation rate, again without effect on mortality in the ICU.59 Other studies failed to show advantages of HFNC over standard treatment.61,62 Lemiale et al. showed that in immunocompromised patients with hypoxaemic ARF, a 2-h trial with HFNC improved neither mechanical ventilatory assistance nor patient comfort compared with oxygen delivered via a Venturi mask. However, the study was underpowered because of the low event rate and the one-sided hypothesis.61 In a more recent randomised trial comprising 776 immunocompromised patients with ARF, HFNC did not significantly decrease day-28 mortality compared with standard oxygen therapy.62 A large multicentre trial (FLORALI IM protocol) to assess the effect of HFNC compared with NIV on mortality rate in immunocompromised patients is ongoing.63
Other applications in the acute setting
Of great interest is the potential use of HFNC in acute heart failure. So far, continuous positive airway pressure has been the first-line treatment for pulmonary oedema as it improves oxygenation and reduces cardiac afterload. It has been suggested that similar effects may be obtained by HFNC with less discomfort for the patient.64,65 Unfortunately, few specific studies are available on the topic, as many patients with pulmonary oedema have been included in the large group of ‘hypoxaemic respiratory failure’. Other applications of HFNC include the prevention of postextubation hypoxaemia, pre-oxygenation before sedation/anaesthesia and oxygenation during bronchoscopy.2,27
Hypercapnic respiratory failure
Exacerbation in patients with COPD
Up to 64% of patients with acute hypercapnic failure do not improve with optimal medical treatment, and guidelines strongly recommend the use of NIV to treat exacerbations of COPD if acidosis is present.4,17 As HFNC is better tolerated by patients and improves gas exchange, a number of studies has addressed the question of whether HFNC provides a valuable alternative to NIV or COT, particularly when NIV is not feasible, in exacerbated COPD. Since a report in 2014, which described a patient with acute hypercapnia noncompliant to NIV who was successfully treated with HFNC,66 the number of studies has grown exponentially. Some of these studies, all including patients with mild to moderate hypercapnia, showed a reduction of CO2 levels whereas others showed a steady CO2.2 A recent systematic review analysed five randomised trials and 198 patients with exacerbated COPD treated with HFNC as an alternative to NIV or COT.17 Four studies showed that treatment with HFNC, compared with NIV, reduced PaCO2 levels and WOB by a similar extent.17,18,67,68 In one study the transcutaneous CO2 was slightly decreased by HFNC compared with COT.65 The comfort of HFNC treatment reported by patients was generally superior to NIV, also considering that with this latter treatment, some patients developed skin breakdown due to the mask pressure.19,68 The comfort of HFNC compared with COT was similar or superior.19,68,69 In one recent study the level of CO2 reduction produced by HFNC significantly correlated with initial CO2 levels.67 In a group of exacerbated hypercapnic patients, stabilised with NIV, oesophageal pressure, which indicates the WOB, was measured during HFNC at different flows. At a flow of 30 l/min, WOB was reduced to a similar extent compared with NIV at an inspiratory/expiratory pressure of 11/5 cm H2O, but higher flows increased the breathing effort.70 This is in contrast with data obtained in patients with acute hypoxaemic failure, in which higher flows were associated with a decrease in respiratory effort.71 Clearly, further physiological studies are needed to address this discrepancy. Unfortunately, we have few data on the effect of HFNC on clinical outcomes in hypercapnic patients with COPD. Interestingly, Lee et al. in groups with severe exacerbation of COPD and moderate hypercapnia did not find differences in the intubation rate and 30-day mortality between NIV and HFNC treatment.72 Jing et al. more recently confirmed that there was no difference in the intubation rate and 28-day mortality between the two treatments in hypercapnic COPD.68 At present, another randomised trial by Cortegiani et al. is ongoing in order to establish the noninferiority of HFNC compared with NIV in terms of functional parameters and clinical outcomes in patients hospitalised for acute hypercapnic COPD.73 Sound evidence that HFNC is as effective as NIV in such patients would be of great interest, as the clinician dealing with COPD exacerbation will be provided with a further respiratory support which is simpler to use and better tolerated. The idea is that HFNC and NIV may be used in a rotational strategy to improve tolerance and comfort. This approach has never been assessed in the studies available in the literature, however in our respiratory ward we currently use the rotational strategy, particularly in those patients who need >18-h ventilation and suffer from mask-associated skin lesions. Finally, it is noteworthy that all the studies mentioned have been performed in patients with moderate hypercapnia (generally around 50–55 mmHg) and no studies so far have been performed in severe hypercapnia.
Stable patients with COPD
The use of HFNC in patients with stable COPD and a chronic mild-moderate degree of hypercapnia is at a very early stage of investigation. A first study by Fraser et al. reported that in patients with stable COPD and mild hypercapnia, who had indications for long-term oxygen therapy, short-term use of HFNC at 30 l/min improved oxygenation and slightly reduced PaCO2 compared with COT (46 mmHg versus 43 mmHg PaCO2).74 In more severe hypercapnic (PaCO2 56 mmHg) stable patients with COPD, 2 h of treatment with HFNC at 30 l/min or NIV decreased CO2 by a similar extent.75 In contrast, in normocapnic hypoxaemic stable patients with COPD, treatment with HFNC reduced the respiratory effort, without affecting blood gases levels.76 Recently, Bonnevie et al. performed a meta-analysis including six randomised trials on the use of HFNC in patients with stable COPD.77 The studies on short-term treatment with HFNC consistently found a decrease of around 3 mmHg in PaCO2 levels without any change in PaO2.74,77–79 Similar changes were observed after long-term treatment.80,81 Of course, the clinical implication of this finding still remains unclear and more long-term studies to assess clinical outcomes are needed.
Contraindications and disadvantages
Without sufficient evidence clear contraindications for HFNC are lacking, however some authors suggest avoiding HFNC in patients for whom NIV is contraindicated.2 Owing to the side effects of the applied low pressure such as abdominal distension and barotrauma should be more rare in HFNC than in NIV. One important issue when using a noninvasive respiratory support, such as HFNC, is that recourse to more invasive management may be delayed and this may be deleterious in patients with respiratory instability. The precise time to give up HFNC and escalate respiratory support is not standardised yet and the decision is left to the judgement of the physician. In ARF, prolonged attempts with HFNC may delay intubation with adverse outcomes.27,82 Conversely, intubation following early failure (before 48 h) of HFNC is associated with lower mortality in the ICU.82 In patients who respond to the treatment generally an improvement is observed within the first 1–2 h.2 Low SaO2, high respiratory rate and thoracoabdominal asynchrony should be considered predictors of HFNC failure.2,41
The future: potential use of HFNC oxygen therapy and fields to be explored
In the last 3 years treatment with HFNC has gained great attention among clinicians. It is peculiar that, although large clinical trials are still lacking, the treatment is already prescribed for home use, so that prescriptions of NIV have decreased over the last year in favour of HFNC (confidential, unpublished data). Indeed, while we are scientifically at a very preliminary stage, the success of this therapy when tested in the field warrants further studies to explore new clinical applications (Table 3).
Table 3.
Future potential applications of the high-flow nasal cannula.
| Clinical effects | |
|---|---|
| Domiciliary treatment of chronic COPD | • Decreases PaCO2
• Reduces exacerbations • Improves quality of life |
| Support during exercise in COPD | • Improves oxygenation • Reduces dyspnoea • Increases endurance time |
| Bronchiectasis and cystic fibrosis | • Improves muco-ciliary clearance • Improves ventilation |
| Palliative care | • Reduces dyspnoea • Improves oxygenation |
COPD, chronic obstructive pulmonary disease; PaCO2, partial pressure of CO2.
Domiciliary treatment of patients with COPD and respiratory failure
Chronic management of patients with COPD includes, in addition to medical treatment, long-term oxygen therapy and domiciliary NIV, if hypercapnia is present, and both are effective in reducing mortality.83,84 Long-term treatment with NIV, although highly effective, may have problems with patients’ compliance and adaptation to interfaces, that are negligible in the acute setting. Therefore, domiciliary treatment with HFNC would avoid the discomfort associated with the long-term use of a mask for NIV. In addition, the administration of oxygen via HFNC, providing optimal heat and humidification of the inhaled gas, may increase chest drainage, acting as a sort of respiratory physiotherapy, while the patient is simply breathing. At the moment only two studies have examined long-term treatment with HFNC in COPD.80,81 Nagata et al. followed 32 patients with hypoxaemic hypercapnic COPD treated with HFNC for a period of 6 weeks. HFNC significantly reduced the levels of PaCO2 and improved health-related quality of life compared with treatment with COT.82 Storgaard et al. followed 200 hypoxaemic patients with COPD treated with HFNC for 12 months. These authors found that HFNC treatment, compared with COT, reduced the rate of exacerbations by about 40%, and reduced hospital admission and symptoms, without any effect on all-causes mortality.81 It has been speculated that the reduction of exacerbations is likely due to the effect of humidification on airway clearance,36 although a direct study measuring the effect of HFNC on sputum production is lacking. Whatever is the underlying mechanism, the reduction of exacerbation rate in COPD is a major clinical outcome, as exacerbations are an important determinant of disease progression and quality of life.83 More long-term studies are therefore needed to clarify these aspects. In addition some technical aspects need consideration when HFNC is prescribed for home use. Among these, the risk of contamination of the tubes since, differently from NIV, warm and humidified gas is delivered and condensation cannot be avoided.
Support treatment during exercise in COPD
Exercise limitation, due to dyspnoea, is a major symptom in patients with COPD.85 In such patients, during exercise, the development of hypoxaemia and the increase in dead space can produce an abnormal rise in minute ventilation, so that the ventilatory reserve is rapidly reached.85 It has been speculated that HFNC, by improving the efficiency of ventilation, may increase exercise capacity. In addition, the high flow provided may improve gas exchange in a condition in which the patients unavoidably breathe at very high flows. In an early study, Chatila et al. administered heated and humidified high-flow oxygen at 20 l/min to 10 patients with COPD with advanced airflow obstruction and severe oxygen dependency during exercise. Compared with low-flow oxygen, high-flow oxygen decreased the respiratory rate, the TI/TTOT ratio and the rapid shallow breathing index, improving SaO2 and endurance time.86 More recently, Cirio et al. evaluated the effect of HFNC on exercise performance (incremental exercise test on cycle ergometer) in 12 patients with severe COPD with exercise limitation.87 They found that HFNC at 55–60 l/min, compared with room air, increased endurance time, improved oxygenation and reduced dyspnoea. Exercise training represents an important part in the rehabilitation programme of patients with COPD and benefits are proportional to the applied intensity.88 Although high-intensity exercise is advised, this may be difficult to achieve for patients with severe obstruction. The use of HFNC may allow a given high-intensity load to be sustained for a longer time with fewer symptoms, improving the effect of exercise training in pulmonary rehabilitation programmes. Although these are interesting preliminary findings, no other studies are available so far. At present, the first randomised controlled trial on the effect of HFNC during exercise training in patients with COPD with respiratory failure is ongoing.89
Management of chronic mucus hypersecretion: bronchiectasis and cystic fibrosis
Chronic bronchitis, bronchiectasis and cystic fibrosis are all associated with a chronic increase in mucus secretion. Although there are differences in aetiologies, for all these conditions improving the muco-ciliary clearance is a pivotal approach in order to preserve airway patency, long-term function and to prevent recurrent infections. The expulsion of secretions can be facilitated by a number of manual and instrumental airway clearance techniques; however, maintenance of a sufficient airway surface liquid is crucial for effective ciliary function. In addition, as water is a main constituent of mucus, optimal hydration of the mucosa may reduce mucus viscosity, facilitating excretion.90 One early study showed that 30 min of airway humidification facilitates postural drainage in patients with bronchiectasis.91 It is now well established that the optimal level of temperature and humidity to maintain mucosal function is core temperature and 100% relative humidity. Out of these values a progressive mucosal dysfunction begins and the greater the humidity deviation, the faster the mucosal dysfunction progresses.85 It has been reported that in a group of patients with bronchiectasis, inhalation of high-flow saturated air at 37°C for 3 h/day for 6 days, significantly improved lung clearance.36 Unfortunately, this is the only study available so far, but it is clear that the benefits of HFNC in bronchiectasis deserve further investigation. The use of HFNC is particularly promising in the management of patients with cystic fibrosis. There is one study showing that HFNC in stable patients with cystic fibrosis reduces the work of breathing to a similar extent compared with NIV, however the effect on mucus secretion has not been assessed in this or any other study.92 It is conceivable that in cystic fibrosis HFNC may have combined advantages improving both ventilation and airway clearance.
Palliative care: from hospital to home
In the last years, although with a lack of clear recommendations, NIV has been used to improve dyspnoea in patients at the end of life with respiratory failure and a do-not-intubate status.93 As HFNC is more comfortable than NIV, particularly in patients with a poor general condition, studies have evaluated whether this device can be used as a palliative treatment in hypoxaemic patients at the end of life. In one study, comprising 183 patients with advanced cancer, HFNC improved clinical outcomes (SaO2, comfort and the need for a step-up to other respiratory support) in 41% of patients, while 44% remained stable and 15% deteriorated during the therapy.94 In another group of patients who refused intubation, affected by a variety of conditions, such as pulmonary fibrosis, pneumonia and malignancies, treatment with HFNC significantly improved oxygenation and dyspnoea so that 82% were maintained by HFNC and only 18% escalated to NIV.95 The use of HFNC was also compared with NIV in patients with end-stage interstitial lung disease, a condition associated with particularly severe hypoxaemia and devastating dyspnoea. While no difference was reported in the survival rate, HFNC was better tolerated as the patients could eat and converse until just before death.96 In another group with idiopathic pulmonary fibrosis admitted to a respiratory ICU for ARF, short-term mortality fell to below 50% when a treatment algorithm incorporating HFNC was implemented.97 One putative advantage of a palliative treatment with HFNC is that severe patients in the last phases of their disease who require a high FiO2, can be more easily treated at home, reducing their stay in the hospital. A recent study retrospectively examined a cohort of severe patients with end-stage respiratory failure, including interstitial lung diseases, cancer and COPD, who were discharged home on long-term HFNC. The survival of these patients was poor, but HFNC allowed the patients to return home and to be treated at reasonable cost.98 The role of this device, therefore, among other options for palliative care, needs further consideration.
Conclusion
HFNC oxygen therapy is a promising technique that is changing the management of patients with acute and chronic respiratory failure. The delivery of heated and humidified oxygen at high-flow rates has a number of positive effects on the airways and respiratory function. In addition, the device, using a comfortable nasal cannula, is exceptionally well tolerated by most patients. In acute respiratory failure, HFNC therapy can be placed above conventional oxygen therapy in order to improve outcomes and avoid escalation to more invasive support. Future potential applications of HFNC oxygen therapy include chronic care of patients with COPD, management of mucus hypersecretion in bronchiectasis and cystic fibrosis, and palliative care for end-stage lung disease. Whether the high-flow techniques are the dawn of a new era for respiratory support is difficult to say. The advantages and disadvantages of this therapy remain largely to be determined.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lucia Spicuzza  https://orcid.org/0000-0002-8246-8357
https://orcid.org/0000-0002-8246-8357
Contributor Information
Lucia Spicuzza, Dipartimento di Medicina Clinica e Sperimentale, University of Catania, UO Pneumologia, Azienda Policlinico-OVE, Via S. Sofia, Catania 95123, Italy.
Matteo Schisano, Dipartimento di Medicina Clinica e Sperimentale, University of Catania, Catania, Italy.
References
- 1. Hernández G, Roca O, Colinas L. High-flow nasal cannula support therapy: new insights and improving performance. Crit Care 2017; 21: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care 2015; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chanques G, Constantin JM, Sauter M, et al. Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med 2009; 35: 996–1003. [DOI] [PubMed] [Google Scholar]
- 4. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 31: 50: 1602426. [DOI] [PubMed] [Google Scholar]
- 5. Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J 2019; 28: 54: 1901003. [DOI] [PubMed] [Google Scholar]
- 6. Smith TA, Davidson PM, Jenkins CR, et al. Life behind the mask: the patient experience of NIV. Lancet Respir Med 2015; 3: 8–10. [DOI] [PubMed] [Google Scholar]
- 7. Sreenan C, Lemke RP, Hudson-Mason A, et al. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001; 107: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 8. Williams AB, Ritchie JE, Gerard C. Evaluation of a high-flow nasal oxygen delivery system: gas analysis and pharyngeal pressures. Intensive Care Med 2006; 32: S219 [Google Scholar]
- 9. Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010; 55: 408–413. [PubMed] [Google Scholar]
- 10. Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care 2019; 64: 735–742. [DOI] [PubMed] [Google Scholar]
- 11. Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 2011; 37: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 12. Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 2012; 27: 324.e9–e13. [DOI] [PubMed] [Google Scholar]
- 13. Itagaki T, Okuda N, Tsunano Y, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care 2014; 59: 70–74. [DOI] [PubMed] [Google Scholar]
- 14. Biselli P, Fricke K, Grote L, et al. Reductions in dead space ventilation with nasal high flow depend on physiological dead space volume: metabolic hood measurements during sleep in patients with COPD and controls. Eur Respir J 2018; 30: 51: 1702251. [DOI] [PubMed] [Google Scholar]
- 15. Onodera Y, Akimoto R, Suzuki H, et al. A high-flow nasal cannula system with relatively low flow effectively washes out CO2 from the anatomical dead space in a sophisticated respiratory model made by a 3D printer. Intensive Care Med Exp 2018; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavizzari A, Veneroni C, Colnaghi M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch Dis Child Fetal Neonatal 2014; 99: F315–F320. [DOI] [PubMed] [Google Scholar]
- 17. Pisani L, Astuto M, Prediletto I, et al. High flow through nasal cannula in exacerbated COPD patients: a systematic review. Pulmonology 2019; 25: 348–354. [DOI] [PubMed] [Google Scholar]
- 18. Di Mussi R, Spadaro S, Stripoli T, et al. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit Care 2018; 22: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Longhini F, Pisani L, Lungu R, et al. High-flow oxygen therapy after noninvasive ventilation interruption in patients recovering from hypercapnic acute respiratory failure: a physiological crossover trial. Crit Care Med 2019; 47: e506–e511. [DOI] [PubMed] [Google Scholar]
- 20. Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care 2013; 58: 1621–1624. [DOI] [PubMed] [Google Scholar]
- 21. Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011; 107: 998–1004. [DOI] [PubMed] [Google Scholar]
- 22. Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009; 103: 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun YH, Dai B, Peng Y, et al. Factors affecting FiO2 and PEEP during high-flow nasal cannula oxygen therapy: a bench study. Clin Respir J 2019; 13: 758–764. [DOI] [PubMed] [Google Scholar]
- 24. Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care 2015; 60: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 25. Van den Berg B, Stam H, Bogaard JM. Effects of PEEP on respiratory mechanics in patients with COPD on mechanical ventilation. Eur Respir J 1991; 4: 561–567. [PubMed] [Google Scholar]
- 26. Wagstaff TA, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia 2007; 62: 492–503. [DOI] [PubMed] [Google Scholar]
- 27. Renda T, Corrado A, Iskandar G, et al. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth 2018; 120: 18–27. [DOI] [PubMed] [Google Scholar]
- 28. Sim MA, Dean P, Kinsella J, et al. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia 2008; 63: 938–940. [DOI] [PubMed] [Google Scholar]
- 29. Ritchie JE, Williams AB, Gerard C, et al. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care 2011; 39: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 30. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care 2005; 50: 604–609. [PubMed] [Google Scholar]
- 31. Chikata Y, Izawa M, Okuda N, et al. Humidification performance of two high-flow nasal cannula devices: a bench study. Respir Care 2014; 59: 1186–1190. [DOI] [PubMed] [Google Scholar]
- 32. Chikata Y, Morinishi K, Nishimura M. Humidification in very-high-flow nasal-cannula therapy in an adult lung model. Respir Care 2019; 64: 809–817. [DOI] [PubMed] [Google Scholar]
- 33. Chikata Y, Unai K, Izawa M, et al. Inspiratory tube condensation during high-flow nasal cannula therapy: a bench study. Respir Care 2016; 61: 300–305. [DOI] [PubMed] [Google Scholar]
- 34. Fontanari P, Zattara-Hartmann MC, Burnet H, et al. Nasal eupnoeic inhalation of cold, dry air increases airway resistance in asthmatic patients. Eur Respir J 1997; 10: 2250–2254. [DOI] [PubMed] [Google Scholar]
- 35. Esquinas Rodriguez AM, Scala R, Soroksky A, et al. Clinical review: humidifiers during non-invasive ventilation–key topics and practical implications. Crit Care 2012; 16: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis 2008; 5: 81–86. [DOI] [PubMed] [Google Scholar]
- 37. Salah B, Dinh Xuan AT, Fouilladieu JL, et al. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J 1988; 1: 852–855. [PubMed] [Google Scholar]
- 38. Kilgour E, Rankin N, Ryan S, et al. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med 2004; 30: 1491–1494. [DOI] [PubMed] [Google Scholar]
- 39. Solomita M, Palmer LB, Daroowalla F, et al. Humidification and secretion volume in mechanically ventilated patients. Respir Care 2009; 54: 1329–1335. [PubMed] [Google Scholar]
- 40. Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011; 56: 265–270. [DOI] [PubMed] [Google Scholar]
- 41. Roca O, de Acilu MG, Caralt B, et al. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation 2015; 99: 1092–1098. [DOI] [PubMed] [Google Scholar]
- 42. Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol 2014; 14: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 44. Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016; 4: 646–652. [DOI] [PubMed] [Google Scholar]
- 45. Rittayamai N, Tscheikuna J, Praphruetkit N, et al. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care 2015; 60: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 46. Huang CC, Lan HM, Li CJ, et al. Use high-flow nasal cannula for acute respiratory failure patients in the emergency department: a meta-analysis study. Emerg Med Int 2019; 2019: 2130935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bell N, Hutchinson CL, Green TC, et al. Randomised control trial of humidified high flow nasal cannulae versus standard oxygen in the emergency department. Emerg Med Australas 2015; 27: 537–541. [DOI] [PubMed] [Google Scholar]
- 48. Jones PG, Kamona S, Doran O, et al. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care 2016; 61: 291–299. [DOI] [PubMed] [Google Scholar]
- 49. Zemach S, Helviz Y, Shitrit M, et al. The use of high-flow nasal cannula oxygen outside the ICU. Respir Care 2019; 64: 1333–1342. [DOI] [PubMed] [Google Scholar]
- 50. Maitra S, Som A, Bhattacharjee S, et al. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care 2016; 35: 138–144. [DOI] [PubMed] [Google Scholar]
- 51. Monro-Somerville T, Sim M, Ruddy J, et al. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med 2017; 45: e449–e456. [DOI] [PubMed] [Google Scholar]
- 52. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation? A systematic review and meta-analysis. Chest 2017; 151: 764–775. [DOI] [PubMed] [Google Scholar]
- 53. Ou X, Hua Y, Liu J, et al. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ 2017; 189: E260–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Corley A, Rickard CM, Aitken LM, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev 2017; 5: CD010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zayed Y, Barbarawi M, Kheiri B, et al. Initial noninvasive oxygenation strategies in subjects with de novo acute hypoxemic respiratory failure. Respir Care 2019; 64: 1433–1444 [DOI] [PubMed] [Google Scholar]
- 56. Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev 2017; 6: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Keenan SP, Sinuff T, Burns KE, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011; 183: E195–E214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coudroy R, Jamet A, Petua P, et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care 2016; 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kang H, Zhao Z, Tong Z. Effect of high-flow nasal cannula oxygen therapy in immunocompromised subjects with acute respiratory failure. Respir Care. Epub ahead of print November 2019. DOI: 10.4187/respcare.07205. [DOI] [PubMed] [Google Scholar]
- 60. Cheng LC, Chang SP, Wang JJ, et al. The impact of high-flow nasal cannula on the outcome of immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. Medicina (Kaunas) 2019; 55: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care 2015; 2: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA 2018; 27: 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coudroy R, Frat JP, Ehrmann S, et al. High-flow nasal oxygen therapy alone or with non-invasive ventilation in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure: the randomised multicentre controlled FLORALI-IM protocol. BMJ Open 2019; 9: e029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care 2013; 28: 741–746. [DOI] [PubMed] [Google Scholar]
- 65. Makdee O, Monsomboon A, Surabenjawong U, et al. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: a randomized controlled trial. Ann Emerg Med 2017; 70: 465–472. [DOI] [PubMed] [Google Scholar]
- 66. Millar J, Lutton S, O’Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis 2014; 8: 63–64. [DOI] [PubMed] [Google Scholar]
- 67. Lee HW, Choi SM, Lee J, et al. Reduction of PaCO(2) by high-flow nasal cannula in acute hypercapnic respiratory failure patients receiving conventional oxygen therapy. Acute Crit Care 2019; 34: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jing G, Li J, Hao D, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health 2019; 42: 217–225. [DOI] [PubMed] [Google Scholar]
- 69. Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology 2017; 22: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 70. Rittayamai N, Phuangchoei P, Tscheikuna J, et al. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care 2019; 9: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 72. Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J 2018; 12: 2046–2056. [DOI] [PubMed] [Google Scholar]
- 73. Cortegiani A, Longhini F, Carlucci, et al. High-flow nasal therapy versus noninvasive ventilation in COPD patients with mild-to-moderate hypercapnic acute respiratory failure: study protocol for a noninferiority randomized clinical trial. Trials 2019; 20: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fraser JF, Spooner AJ, Dunster KR. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax 2016; 71: 759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Atwood CW, Jr, Camhi S, Little KC, et al. Impact of heated humidified high flow air via nasal cannula on respiratory effort in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis 2017; 4: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bonnevie T, Elkins M, Paumier C, et al. Nasal high flow for stable patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD 2019; 16: 368–377. [DOI] [PubMed] [Google Scholar]
- 78. Nilius G, Domanski U, Franke KJ, et al. Nasal high flow oxygen therapy attenuates nocturnal hypoventilation in COPD patients with hypercapnic respiratory failure . Am J Respir Crit Care Med 2013; 187: A3102. [Google Scholar]
- 79. McKinstry S, Pilcher J, Bardsley G, et al. Nasal high flow therapy and PtCO(2) in stable COPD: a randomized controlled cross-over trial. Respirology 2018; 23: 378–384. [DOI] [PubMed] [Google Scholar]
- 80. Nagata K, Kikuchi T, Horie T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. a multicenter randomized crossover trial. Ann Am Thorac Soc 2018; 15: 432–439. [DOI] [PubMed] [Google Scholar]
- 81. Storgaard LH, Hockey HU, Laursen BS, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018; 13: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015; 41: 623–632. [DOI] [PubMed] [Google Scholar]
- 83. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019; 53: pii: 1900164. [DOI] [PubMed] [Google Scholar]
- 84. Chen H, Liang BM, Xu ZB, et al. Long-term non-invasive positive pressure ventilation in severe stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J (Engl) 2011; 124: 4063–4070. [PubMed] [Google Scholar]
- 85. Killian KJ, Leblanc P, Martin DH, et al. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis 1992; 146: 935–940. [DOI] [PubMed] [Google Scholar]
- 86. Chatila W, Nugent T, Vance G, et al. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest 2004; 126: 1108–1115. [DOI] [PubMed] [Google Scholar]
- 87. Cirio S, Piran M, Vitacca M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med 2016; 118: 128–132. [DOI] [PubMed] [Google Scholar]
- 88. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013. 15; 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 89. Vitacca M, Pietta I, Lazzeri M, et al. Effect of high-flow nasal therapy during exercise training in COPD patients with chronic respiratory failure: study protocol for a randomised controlled trial. Trials 2019; 20: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 1996; 24: 1920–1929. [DOI] [PubMed] [Google Scholar]
- 91. Conway JH, Fleming JS, Perring S, et al. Humidification as an adjunct to chest physiotherapy in aiding tracheo-bronchial clearance in patients with bronchiectasis. Respir Med 1992; 86: 109–114. [DOI] [PubMed] [Google Scholar]
- 92. Sklar MC, Dres M, Rittayamai N, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care 2018; 8: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Davies JD. Noninvasive respiratory support at the end of life. Respir Care 2019; 64: 701–711. [DOI] [PubMed] [Google Scholar]
- 94. Epstein AS, Hartridge-Lambert SK, Ramaker JS, et al. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med 2011; 14: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care 2013; 58: 597–600. [DOI] [PubMed] [Google Scholar]
- 96. Koyauchi T, Hasegawa H, Kanata K, et al. Efficacy and tolerability of high-flow nasal cannula oxygen therapy for hypoxemic respiratory failure in patients with interstitial lung disease with do-not-intubate orders: a retrospective single-center study. Respiration 2018; 96: 323–329. [DOI] [PubMed] [Google Scholar]
- 97. Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat acute respiratory failure in patients with acute exacerbation of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 2019; 13: 1753466619847130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dolidon S, Dupuis J, Molano Valencia LC, et al. Characteristics and outcome of patients set up on high-flow oxygen therapy at home. Ther Adv Respir Dis 2019; 13: 1753466619879794. [DOI] [PMC free article] [PubMed] [Google Scholar]


