Abstract
Objectives:
Robust data on the impact of comorbidities on health in people with osteoarthritis (OA) are lacking, despite its potential importance for patient management. Objectives were to determine coexisting conditions in people with OA in primary care and whether more comorbidities were linked with individual health status.
Methods:
A retrospective analysis of 23,892 patients with knee and hip OA was conducted to determine comorbidities present (number/clusters) and how these linked with pain intensity (0–100), widespread pain (site numbers), medication usage (paracetamol, nonsteroidal anti-inflammatory drugs, opioids), quality of life EuroQol five dimension scale (EQ-5D), and physical function (walking speed) using independent t-tests or χ 2 test.
Results:
Sixty-two percent of people with OA treated in primary care had at least one comorbidity; hypertension (37%), heart disease (8%), and diabetes (7%) being most common. Outcome measures worsened with more comorbidities (0–4+ comorbidities); pain intensity [mean (SD)] 46(22)–57(21); number of painful sites 3.7(3.0)–6.3(5.4); quality of life 0.73(0.10)–0.63(0.15); walking speed 1.57 m/s (0.33)–1.24 m/s (0.31), while the proportion of people using pain medication increased from 0 to 2 comorbidities (58–69%; p < 0.001), with an increase in opioid use from 4.6% to 19.5% with more comorbidities (0–4+ comorbidities).
Conclusion:
Most people with knee or hip OA in primary care have at least one other long-term condition. A greater number of comorbidities is linked with worsening health, highlighting the importance of screening for comorbidities when treating patients with OA. It is important for clinicians to consider how OA treatments will interact and affect other common comorbidities.
Keywords: Osteoarthritis, multimorbidity, comorbidity, health status
Introduction
Multimorbidity, defined as the co-occurrence of at least two long-term conditions,1,2 affects up to a fifth of the world population3,4 and accounts for 78% of all consultations in primary care.5 A recent report from the Academy of Medical Sciences6 highlighted the urgent need to address multimorbidity through a range of research priorities, including increased knowledge about common clusters of conditions and their impact on health care utilization and health status.
Although osteoarthritis (OA) is the disease with the highest occurrence of comorbidities,7 robust large-scale data on common clusters of long-term conditions in OA and their impact on individual health status in primary care is missing. Previous smaller cohort studies including patients through advertisements8 and from secondary care9 suggest that at least two of three people with OA have one or more other comorbidities8,9 with hypertension, heart disease, type 2 diabetes mellitus, and lung disease being some of the most common comorbidities.8,10
People with OA and comorbidities, as well as other people affected by multiple long-term conditions, are treated using multiple single-disease guidelines.4 However, the recent National Institute for Health and Care Excellence (NICE) guidelines on multimorbidity question this single-disease care approach and highlight the importance of accounting for all conditions when informing the patient about available treatment options and treating the individual patient.1 To best inform patients with OA, it is important to understand what other conditions coexist in primary care and how these conditions affect the patient’s health status.1
Therefore, the aim of this study was to determine which diseases typically cluster in people with knee or hip OA in a large-scale registry in primary care and to identify whether the number of comorbidities present is linked with symptom severity, usage of pain medication, widespread pain, quality of life, physical activity level, and walking speed.
We hypothesized that people with a higher number of long-term conditions would have worse health status, including poorer quality of life and physical function, than those with fewer long-term health conditions.
Method
Design
This was a registry-based study from the Good Life with osteoArthritis in Denmark (GLA: D®) program—an ongoing nationwide initiative focusing on the implementation of treatment guidelines for knee and hip OA in clinical practice.11 GLA: D consists of two sessions of patient education followed by twice weekly 1-h sessions of neuromuscular exercise for 6 weeks supervised by primary care physiotherapists certified to deliver the treatment program. The treatment effects are evaluated using predefined and validated outcomes at baseline, immediately after the treatment program and at 12 months. A detailed description of GLA: D, including patient characteristics, treatment, and outcomes, is available elsewhere.11
The current report conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement for reporting observational studies. Ethical approval of GLA: D was not needed, according to the local ethics committee of the North Denmark Region. The GLA: D registry has previously been approved by the Danish Data Protection Agency and all patients consented to submit their data to the GLA: D registry.
Participants
Participants with pain or functional limitation from knee or hip joints that consult their primary care physiotherapist are eligible for GLA: D if they do not meet one or more of these exclusion criteria: (1) reason for the joint symptoms other than OA as evaluated by the physiotherapist, for example, inflammatory joint disease or patellar tendinopathy; (2) other symptoms that are more pronounced than the OA symptoms, for example, chronic, generalized pain, or fibromyalgia; and (3) do not understand Danish. Participants were assigned to the hip or knee group by whichever was their primary OA, but some participants had both.
For the current report, all participants in GLA: D with available data at baseline were included. All data were collected prior to participants taking part in the GLA: D program.
Outcomes
Grouping variable
Comorbidities that participants had along with OA were self-reported (via questionnaire) and included: hypertension, heart disease, diabetes, lung disease, rheumatoid arthritis, diseases in the stomach, neurological disease, depression, cancer, diseases in the kidney or liver, anemia or another blood disease, and miscellaneous medical conditions, defined as any conditions other than those mentioned above.11 Participants were asked whether they have any of the diseases above and were required to tick a yes/no box for each condition. For some conditions such as lung disease and heart disease, there were examples, such as chronic obstructive pulmonary disease (lung disease) and ischemic heart disease.
Health status variables
Health status variables were collected via a combination of patient form and physiotherapist forms. The majority of variables were collected via the patient forms with the exception of symptom duration, pain medication, and the functional test; 40-m walk test, use of walking aid, and sit to stand, which were all conducted under the supervision of a physiotherapist and entered by them into the database. Age and gender were retrieved from the personal identification number. Body mass index (BMI) was also calculated from weight and height collected by the therapist.
Demographics
Age, gender, BMI, and self-reported educational level (1 = primary school, 2 = secondary school, 3 = short-term education (under 3 years after secondary school), 4 = middle term education (3–4 years after secondary school), and 5 = long-term education (minimum 5 years after secondary school)) were recorded as demographic measures.
Symptoms
Symptom duration was recorded. Patients marked the total number of body areas (out of 56), where they had felt pain in the last 24 h on a pain mannequin to assess bodily pain.11 Mean pain intensity during the last month of the most affected joint was evaluated on a 100 mm visual analog scale (VAS), with a descriptor of “no pain” (0 mm) and “maximum pain” (100 mm). The VAS score is a widely used measure of pain for people with OA and is valid and reliable.12
Patients were asked about the frequency of their hip/knee pain using an item from the Knee Injury and Osteoarthritis Outcome Score (KOOS) or Hip disability and Osteoarthritis Outcome Score (HOOS) on a scale of 0–4 (0 = never, 1 = monthly, 2 = weekly, 3 = daily, 4 = always).13,14 Participants were asked whether they had problems walking due to their hip/knee (“yes” or “no”).
Pain medication
Patients were asked whether they had been using pain medication within the last 3 months and asked to indicate what kind of medication, with several options (paracetamol, nonsteroidal anti-inflammatory drug (NSAID) (oral or topical), morphine, or other opioids). The answers were recoded “yes” (any pain medication used) and “no” (no pain medication used). For analyzing each drug separately, the answers were recoded into a binary variable for the use of each type of drug.
Fear of movement
Patients were asked to indicate whether they were afraid that their joint would be damaged from physical activity and exercise (“yes” or “no”).
Quality of life
Joint-related quality of life was evaluated using the subscale quality of life from the self-reported questionnaires: KOOS and the HOOS, with scores ranging from 0 (worst) to 100 (best). KOOS and HOOS are valid, reliable, and responsive patient-reported outcome measures previously applied in studies of OA.13,14
Generic quality of life was evaluated using the EQ-5D-5 L questionnaire (scored using Danish crosswalk value set) and the EQ VAS. The EQ-5D-5 L has been shown to be valid and reliable when used within an OA population.15
Physical activity and function
Physical activity was assessed using the University of California, Los Angeles (UCLA) activity questionnaire, where participants were asked to rate their physical activity on a scale from 1 to 10 (10 = regularly participates in impact sports; 9 = sometimes participates in impact sports; 8 = regularly participates in active events; 7 = sometimes participates in active events; 6 = regularly participates in moderate activities; 5 = sometimes participates in moderate activities; 4 = regularly participates in mild activities; 3 = sometimes participates in mild activities; 2 = mostly inactive or restricted to minimum activities of daily living; 1 = wholly inactive, dependent on others, and cannot leave residence). The UCLA has been found to be a useful measure of physical activity in people with OA.16
Physical function was assessed using the 40-m walk test (converted to meters per second for the purposes of this report) and the 30-s sit to stand test. Both tests are recommended by the Osteoarthritis Research Society International as components of core sets of performance-based physical function tests for knee and hip.17 Physiotherapists were asked to indicate if participants used a walking aid during the 40-m walk test.11
Patient involvement
Patients and patient organizations have been involved in the development and the ongoing update of GLA: D and its materials.
Data analysis
For the present analysis, data collected in GLA: D between June 30, 2014 (start of data collection on comorbidities) and end of December 31, 2017, were used.
In line with the aims of this study, the analysis was designed to identify how comorbidities cluster in people with hip or knee OA and whether there is an association between the number of comorbidities and outcomes, such as symptom severity, usage of pain medication, and quality of life. In addition, patient characteristics across the comorbidity group are reported to help understand the demographics of these groups. Finally, hip and knee OA patients are compared to understand whether there are differences in the number of comorbidities present with the two different sites of OA.
Potential differences in the number of present comorbidities between patients with hip and knee OA were examined using a Mann–Whitney U test (as the outcome being tested is ordinal data), with the significance level set to 0.05.
Cross tabs of the number of participants with each condition were generated and used to establish the top five pairs and trios of conditions that cluster together. The percentage of participants within each of these clusters was then calculated against the entire cohort to ascertain the prevalence of each cluster.
Frequencies for the number of other conditions were calculated, and for the purposes of analysis, these were grouped with 0 representing those with OA only; 1, OA and one other condition; 2, OA and two other conditions; 3, OA and three other conditions; 4+, OA and four or more other conditions. Groups were compared on each of the health status variables using independent samples t-tests. Each group was tested in comparison with the next number of comorbidities, so zero comorbidities compared to one comorbidity, one to two, and so on. The significance level was set to 0.0125 between groups as four t-tests were conducted on each variable (0.05/4 = 0.0125). Education level, expressed as an ordinal variable, was compared between comorbidity groups using Mann–Whitney U test with the significance level set to 0.0125 (due to the Bonferroni adjustment). Gender, medication usage (including breakdown), problems walking, fear of walking, and use of walking aid in 40-m walk test were assessed using the χ 2 test (significance set to 0.0125). Confidence intervals were set to 98.75 due to the number of tests conducted. All analyses were conducted using SPSS (version 24).
The purpose of the article was to describe the differences between comorbidity groups; as the number of comorbidities is not an experimental variable and due to the cross-sectional design, the study did not attempt to explore a cause and effect relationship with health status. Hence, methods such as multivariable models designed to control for confounders were not used.
Results
Of the 27,513 patients who attended GLA: D, 24,029 (87.3%) completed the patient form containing information on comorbidities and 12.7% did not complete the form (69.8% females, mean [standard deviation (SD)] age = 65.9 [11.1], BMI = 28.4 [5.3]).
Participants with missing data on other conditions were also removed, leaving 23,892 (72.4% females, age = 64.9 [9.5], BMI = 28.3 [5.3]).
Participants were characterized as having hip or knee OA based on their primary concern. However, 2441 (40.44%) of 6036 hip OA participants also had knee OA, and 3620 (20.34%) of 17,795 knee OA participants also had hip OA.
Comorbidities and clusters
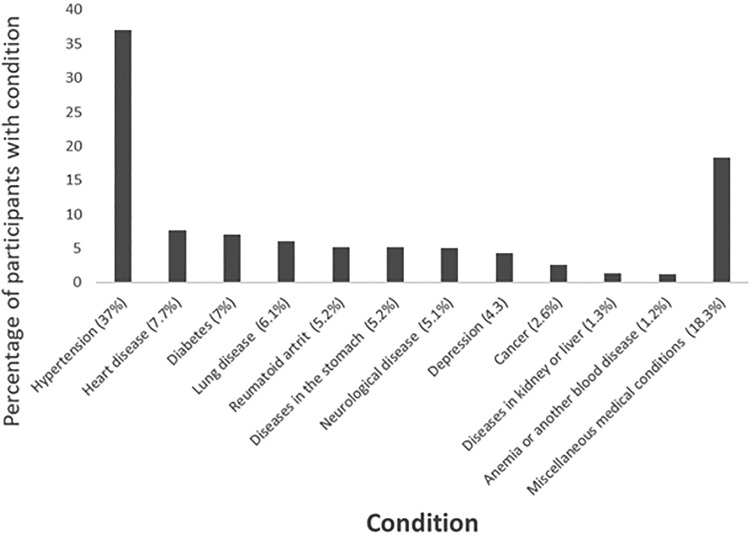
In all, 62.1% of the participants were affected by at least one condition other than OA (Table 1). The most common condition to present with OA was hypertension (37%; Figure 1).
Table 1.
Frequency of number of other conditions in people with knee and hip osteoarthritis.
| Number of other conditions | Frequency | Percentage |
|---|---|---|
| 0 | 9058 | 37.9 |
| 1 | 8518 | 35.7 |
| 2 | 4216 | 17.6 |
| 3 | 1506 | 6.3 |
| 4 | 409 | 1.7 |
| 5 | 134 | 0.6 |
| 6 | 37 | 0.2 |
| 7+ | 14 | 0.06 |
Figure 1.
Percentage of patients with osteoarthritis presenting with each other condition. Miscellaneous medical conditions were defined as any conditions other than those mentioned previously.
The most common two conditions to cluster together with OA were hypertension and diabetes (4.9% of participants, Table 2). The top five pairs of conditions that clustered together accounted for 16.2% of the participants, which is over half of all of those with two or more conditions.
Table 2.
Frequency of total participants for top five pairs and trios of other conditions in people with knee and hip osteoarthritis.a
| Name of conditions | Frequency | Percentage of participants |
|---|---|---|
| Top five pairs of conditions | ||
| Hypertension and diabetes | 1166 | 4.88 |
| Hypertension and heart disease | 1036 | 4.34 |
| Hypertension and lung disease | 611 | 2.56 |
| Hypertension and stomach diseases | 543 | 2.27 |
| Hypertension and rheumatoid arthritis | 523 | 2.19 |
| Top five trios of conditions | ||
| Hypertension, heart disease, and diabetes | 139 | 0.58 |
| Hypertension, heart disease, and lung disease | 96 | 0.40 |
| Hypertension, heart disease, and neurological diseases | 66 | 0.28 |
| Hypertension, diabetes, and rheumatoid arthritis | 55 | 0.23 |
| Hypertension, lung disease, diabetes | 50 | 0.21 |
a Miscellaneous medical conditions were removed from this grouping as it is unknown what conditions make up this group.
The most common trios of conditions that clustered together with OA were hypertension, heart disease, and diabetes (0.6%). The top five trios of conditions that clustered together accounted for 1.7% of the participants, which is about a fifth of all those with three or more conditions.
Knee versus hip OA
Participants with hip OA are slightly less likely to suffer from other comorbidities than those with knee OA. There is a statistically significant difference between the mean number of other conditions between groups (hip OA 0.97 vs. knee OA 1.01, mean difference 0.03, 98.75% confidence interval (CI) <0.01–0.06, p = 0.027). Thirty-nine percent of participants with hip OA have no other conditions, whereas 37.6% of those with knee OA have no other conditions (Table 3).
Table 3.
Frequency and percentage of participants with hip and knee osteoarthritis against number of conditions other than osteoarthritis.
| Number of other conditions | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4+ | |||||||||||||||
| Effected joint | n | % | N | % | n | % | n | % | n | % | |||||||||
| Hip (n = 6036) | 2355 | 39.00 | 2142 | 35.50 | 1026 | 17.00 | 377 | 6.20 | 137 | 2.30 | |||||||||
| Knee (n = 17795) | 6691 | 37.60 | 6354 | 35.70 | 3177 | 17.80 | 1125 | 6.30 | 591 | 2.60 | |||||||||
Link between comorbidities and health status
Demographics
As age increases, the number of comorbidities tends to increase, with participants only suffering from OA being the youngest (mean (98.75% CI) = 62.80 (62.53–63.06)), and participants suffering from four or more conditions in addition to OA being the eldest (mean (98.75% CI) = 67.18, (66.28–67.38)) (Table 4).
Table 4.
Health status in people with knee and hip osteoarthritis and one or more other long-term conditions.a
| Number of conditions other than osteoarthritis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4+ | ||||||
| Variable | Mean/percentage | CI (98.75%) | Mean/percentage | CI (98.75%) | Mean/percentage | CI (98.75%) | Mean/percentage | CI (98.75%) | Mean/percentage | CI (98.75%) |
| Demographics | ||||||||||
| Age (years) (n = 23,725) | 62.80b | 62.53–63.06 | 65.58b | 65.34–65.83 | 66.71 | 66.37–67.06 | 67.00 | 66.42–67.58 | 67.18 | 66.28–67.38 |
| Gender (percentage of females) (n = 23,809) | 73.30% | 72.1%–74.5% | 73.00% | 71.7%–74.2% | 71.10% | 69.3%–72.8% | 68.30% | 65.2%–71.2% | 68.40% | 63.3%–73.0% |
| BMI (n = 23,750) | 27.20b | 27.08–27.34 | 28.42b | 28.28–28.55 | 29.40b | 29.18–29.61 | 30.04b | 29.66–30.42 | 31.13 | 30.53–31.73 |
| Education level (n = 23,879) | 3.29b | 3.25–3.32 | 3.14b | 3.11–3.18 | 3.04b | 2.99–3.09 | 2.94b | 2.85–3.03 | 2.79 | 2.64–2.93 |
| Symptoms | ||||||||||
| Symptom duration (months) (n = 19,533) | 41.7 | 39.83–43.56 | 42.27 | 40.34–44.23 | 44.87 | 42.03–47.71 | 48.93 | 43.70–54.16 | 50.94 | 42.41–59.46 |
| Pain mannequin (max 56) (n = 23,892) | 3.65b | 3.56–3.73 | 3.91b | 3.82–4.01 | 4.39b | 4.24–4.55 | 4.91b | 4.61–5.21 | 6.29 | 5.68–6.90 |
| Frequency of knee or hip pain (n = 23,883) | 2.86b | 2.84–2.88 | 2.92b | 2.90–2.94 | 2.99b | 2.95–3.02 | 3.09 | 3.03–3.14 | 3.14 | 3.06–3.22 |
| Percentage who had walking problems (n = 23,876) | 73.10%b | 72.0%–74.3% | 77.40%b | 76.2%–78.5% | 81.80%b | 80.3%–83.3% | 85.00% | 82.5%–87.2% | 88.70% | 84.9%–91.6% |
| Mean pain in last month (n = 23,859) | 45.92b | 45.30–47.00 | 47.80b | 47.17–48.45 | 50.37b | 49.42–51.31 | 53.83b | 52.25–55.41 | 57.24 | 54.80–59.68 |
| Medication | ||||||||||
| Percentage who used pain medication in the last 3 months (n = 23,809) | 58.00%b | 56.7%–59.3% | 63.10%b | 61.7%–64.4% | 66.00% | 64.1%–67.8% | 68.70% | 66.3%–71.6% | 68.90% | 63.8%–73.5% |
| Paracetamol | 46.72%b | 45.41–48.04 | 52.54%b | 51.18–53.89 | 55.41% | 53.49–57.32 | 58.80% | 55.64–61.97 | 59.05% | 54.00–64.10 |
| NSAIDs | 37.88% | 36.61–39.17 | 36.54% | 35.23–37.84 | 35.64% | 33.79–37.48 | 32.23% | 29.22–35.23 | 28.60% | 23.95–33.24 |
| Opioids | 4.57%b | 4.02–5.12 | 6.98%b | 6.29–7.57 | 9.95%b | 8.80–11.11 | 14.55%b | 12.28–16.82 | 19.46% | 15.39–23.53 |
| Psychological | ||||||||||
| Percentage who had fear of movement (n = 23,885) | 14.80% | 13.9%–15.8% | 13.60% | 12.7%–14.6% | 13.10% | 11.9%–14.5% | 15.20% | 13.0%–17.7% | 19.20% | 15.4%–23.6% |
| Quality of life | ||||||||||
| EQ-5D-5 L questionnaire (n = 23,870) | 0.73b | 0.73–0.73 | 0.71b | 0.71–0.71 | 0.69b | 0.69–0.70 | 0.66b | 0.66–0.67 | 0.63 | 0.61–0.64 |
| EQ-5D-5 L VAS (n = 23,878) | 73.07b | 72.60–73.54 | 69.95b | 69.45–70.44 | 65.58b | 64.87–66.29 | 61.55b | 60.31–62.79 | 55.28 | 53.29–57.28 |
| KOOS QOL score (n = 17,795) | 46.83b | 46.38–47.29 | 45.11b | 44.64–45.57 | 43.18 | 42.52–43.84 | 42.02b | 40.89–43.16 | 38.11 | 36.38–39.82 |
| HOOS QOL score (n = 6036) | 48.81b | 48.02–49.59 | 47.34 | 46.51–48.17 | 45.96b | 44.74–47.17 | 42.74 | 40.71–44.77 | 41.01 | 37.57–44.45 |
| Physical function and activity | ||||||||||
| UCLA activity level (n = 23,871) | 6.08b | 6.03–6.12 | 5.76b | 5.71–5.81 | 5.43b | 5.36–5.50 | 5.23b | 5.11–5.35 | 4.82 | 4.62–5.02 |
| 40-m Walk test (m/s) (n = 22,509) | 1.57b | 1.56–1.58 | 1.47b | 1.46–1.48 | 1.40b | 1.39–1.42 | 1.35b | 1.33–1.37 | 1.24 | 1.21–1.27 |
| Percentage who used a walking aid in walk test (n = 22,923) | 1.40% | 1.1%–1.7% | 1.80%b | 1.5%–2.2% | 2.70% | 2.1%–3.4% | 3.40% | 2.3%–4.8% | 4.20% | 2.5%–6.9% |
| Sit to stand (n = 22,591) | 12.82b | 12.72–12.92 | 12.10b | 12.01–12.20 | 11.53b | 11.39–11.66 | 10.92b | 10.70–11.14 | 10.18 | 9.83–10.53 |
HOOS: Hip disability and Osteoarthritis Outcome Score; QOL: quality of life; KOOS: Knee Injury and Osteoarthritis Outcome Score; BMI: body mass index; CI: confidence interval; NSAID: nonsteroidal anti-inflammatory drug.
a For descriptions of variables and statistical methods, see “Method” section. In the view of clarity, it was decided to show mean and CI for education level instead of median and range, as this is easier to see and understand.
b Significant difference of 0.0125 or less to the group above (with more comorbidities) as calculated by the Bonferroni adjustment.
BMI significantly increases as the number of other conditions increases with participants with the lowest BMI having OA alone (mean (98.75% CI) = 27.20 (27.08–27.34)) and those with the highest BMI having four or more conditions (mean (98.75% CI) = 31.13 (30.53–31.73)). Education level appears to be linked with the number of comorbidities, with participants affected by OA alone being the highest educated people (mean (98.75% CI) = 3.29 (3 = under 3 years after secondary school), (3.25–3.31)), and participants affected by four or more conditions in addition to OA having the lowest education levels (mean (98.75% CI) 2.79 (2 = secondary school), (2.67–2.90)) (Table 4).
Symptoms
Significant differences were found in the number of bodily areas participants felt pain in the last 24 h between each group (OA alone, mean (98.75% CI) = 3.65, (3.56–3.73), 4+ conditions, mean (95% CI) = 6.29, (5.68–6.90)) (Table 4).
Problems with walking were more common in those with more comorbidities and were significantly higher as the number of additional conditions increased, with the exception of between 3 and 4 or other conditions (OA alone, percentage (98.75% CI) = 73.10%, (72.0–74.3), 3 conditions, percentage (98.75% CI) = 85.00%, (82.5–87.2)).
Participants had significantly higher pain severity in the last month the more conditions they were affected by OA alone, mean (98.75% CI) = 45.92, (45.30–47.00), 4+ conditions, mean (98.75% CI) = 57.24, (54.80–59.68) (Table 4).
Use of pain medication
Participants who are afflicted by one or two conditions in addition to OA are significantly more likely to use medication for pain management in the last 3 months, than those with OA alone (OA alone, percentage (98.75% CI) = 58.00%, (56.7–59.30), two conditions, percentage (98.75% CI) = 66.00%, (64.10–67.80)). However, once participants have two or more additional conditions, then pain medication analyzed as a binary variable does not significantly increase (Table 4). Paracetamol was the most used medication in OA only (percentage (98.75% CI) = 46.72%, (45.41–48.04)) and steadily increases to four or more conditions (percentage (98.75% CI) = 59.05, (54–64.10)). NSAID’s usage starts high (percentage (98.75% CI) = 37.88, (36.61–39.17)) and decreases steadily to four or more conditions (percentage (98.75% CI) = 28.60, (23.95–33.24)). Opioids usage starts low (percentage (98.75% CI) = 4.57, (4.02–5.12)) and increases considerably (percentage (98.75% CI) = 19.46, (15.39–23.53)) (Table 4).
Quality of life
Quality of life scores from the EQ-5D-5 L questionnaire worsen significantly as the number of comorbidities increases (OA alone, mean (98.75% CI) = 0.73, (0.73–0.73), 4+ conditions, mean (98.75% CI) = 0.63, [0.61–0.64]). This is matched by a significant drop in perceived health levels measured on EQ VAS between groups (OA alone, mean (98.75% CI) = 73.07, (72.60–73.54), 4+ conditions, mean (98.75% CI) = 55.28, [53.29–57.28]) (Table 4).
Similarly, KOOS QOL score worsens as the number of conditions increases (OA only, mean (98.75% CI) = 46.83, (46.38–47.29), one other condition, mean (98.75% CI) = 38.11, (36.38–39.82). However, there were no significant differences between two other conditions and three other conditions. HOOS QOL score also worsens as the number of conditions increases, however, only two of these changes are statistically significant (0–1 mean (98.75% CI) 48.81 (48.02–49.59) to 47.34 (46.51–48.17); and 2–3 mean (98.75% CI) 45.96 (44.74–44–7.17) to 42.74 [40.71–44.71]) (Table 4).
Physical activity and function
The highest physical activity levels were found in the OA only group (mean (98.75% CI) = 6.08, [6.03–6.12]) and the lowest levels in the four or more conditions group (mean (95% CI) 4.82, (4.62–5.02); Table 4).
There is a significant decrease in the walking speed achieved during the 40-m walk test between each group, with participants with more conditions walking significantly slower (OA alone, mean (98.75% CI) = 1.57m/s, (1.56–1.58), 4+ conditions, mean (98.75% CI) = 1.24 m/s, (1.21–1.27); Table 4).
With increasing number of conditions, there is a small increase in the percentage of participants using a walking aid to complete the test between each group. However, there is only a significant increase in the use of walking aids between those with one (percentage (98.75% CI) = 1.8%, [1.5%–2.2%]) and two (percentage (98.75% CI) = 2.7%, [2.1%–3.4%]) other conditions (Table 4).
The amount of sit to stands that participants achieved during the 30-s sit to stand test decreases significantly as the number of other conditions increases. Those with OA only average 12.82 (CI 98.75%= 12.72–12.92) sit to stands and those with 4+ additional conditions averaging 10.18 (CI 98.75% = 9.83–10.53; Table 4).
Three hundred and twelve (1.4%) participants were unable to perform the sit to stand test, 86 with OA alone (1% of group), 108 with OA and one other condition (1.3% of group), 63 with OA and two other conditions (1.6% of group, 35 with OA and three other conditions (2.4% of group), and 20 with OA, and four or more other conditions (3.5% of the group).
Discussion
Our large-scale study of people in primary care confirms and extends findings from previous similar cohort studies from secondary care2–4,7 by demonstrating that most people (62%) with knee and hip OA studied in primary care have at least one other long-term condition with the most common coexisting conditions being hypertension, heart disease, diabetes mellitus, and lung disease. We found that symptom severity, usage of pain medication, widespread pain, quality of life, physical activity level, and walking speed gradually worsen with more comorbidities. The current single-disease framework is associated with an increased burden from consultations and treatments in people with more long-term conditions. Our study highlights the importance of accounting for all coexisting conditions and points to including treatments,18 such as exercise therapy,19 that are able to improve individual health status across conditions.
Education and health
The positive association between education level and health is well established4,20,21; well-educated people experience better health than poorly educated people. The current study adds to this evidence, as those with OA alone had a significantly higher education level than those with more conditions.
Medication usage
Pain medication to help cope with OA is common22 and is also common in the treatment of chronic pain. Unsurprisingly, in the current study, medication usage is high with over half the participants with OA alone taking medication (58%), the most commonly used is paracetamol. As the number of comorbidities increases so does the use of pain medication, this itself is not surprising, there is, however, a potential cause for concern in the rapid increase in opioid usage from 4.6% to 19.5% going from 0 to 4+ comorbidities, considering the recent focus of opioid overuse,23 with further suggestion that there is considerable use in an OA setting.24 This raises long-term concerns especially when the analgesic effects of physical activity are so widely known.25,26
Physical activity and function with long-term health conditions
The present study found that the more long-term conditions a person had, the lower physical activity level and physical function. Physical inactivity, excessive fat levels, and pain, all of which increase in this cohort as the number of conditions increases, are connected with systemic low-grade inflammation, independent of BMI,27 which is part of the pathogenesis of a range of long-term, painful musculoskeletal and nonmusculoskeletal conditions, which might explain their co-occurrence.28,29 The long-term conditions could lead to reduced physical function and decreased physical activity levels, which could worsen the inflammation and lead to the development of other long-term conditions, thereby creating a “vicious cycle” of long-term low-grade inflammation.26,30 Maintaining or improving physical function is of particular importance for improving functional independence in later life.31
Patient education is essential to ensure that people increase and maintain their physical function, activity level, and to reduce risk of secondary conditions especially in a younger cohort, who can implement longer term lifestyle changes. Education should include causes and the disease process of OA,26 importance of physical activity, and self-help tools in order for them to self-manage their conditions. It is also crucial to reassure patients that the belief that exercise therapy will damage their joints is a myth.26
OA and comorbidity
It is well established that low physical activity levels can contribute to the development of long-term conditions31 and slower walking pace is a predictor of mortality.32 OA is an important factor in this, as it can cause pain in the joints, making movement and therefore activity painful and difficult for those who need it,26 coupled with a fear of movement itself, seen as a risk of causing more damage.26 This, in turn, increases the risk of further development of other long-term conditions, which can make pain worsen and thereby even further reduce physical activity.33 Even in the OA only group, problems walking were reported by 73% of the participants. This problem increases as the number of conditions increases but suggests that OA is one of the key barriers to walking.34 With walking as one of the easiest ways of increasing physical activity, this can be very detrimental, although it must be noted that in this study, it is unknown if OA or other morbidities came first. A recent study by Pereira et al.35 found that around 35% of people with one long-term condition had problems walking compared to 77.4% in the present study. They further found that 68% of people with three long-term health conditions had problems walking compared to the 80% found in this study. OA not only includes physical problems but also is often linked with psychological effect, with psychological distress more frequently experienced in patients with OA when compared to other chronic conditions.36 This is seen in the present study with increased fear of movement and lower quality-of-life scores.
Prevalence within the general population
It is important to understand the present results in some context against the general population. OA is thought to be one of the most prevalent of all musculoskeletal diseases, affecting an estimated 10% of the world’s population over the age of 60.35 Hypertension is also very prevalent within the population, and therefore, it was not surprising that it was the most common disease found with OA in this study. It is important to note, however, that hypertension prevalence is considerably higher in the current study (37%) than previous studies of the general population that found a prevalence of around 14%.37 When split by age groups, prevalence in the general population was 13% in 60- to69-year-olds and 16% in 70- to 79-year-olds. Diabetes has a prevalence in the general population of 6.8%,37 8% in 60- to 69-year-olds, and 10% in 70- to 79-year-olds,38 which are similar to the levels found in the same age-group in the present study. Heart disease has a prevalence in the general population of 3.137 and 10% prevalence in 60- to 69-year-olds and 13% in 70- to 79-year-olds,38 which is consistent with the present study. The prevalence of depression in the present study (4.3%) appears to be smaller than in the general population (9.9) aged 18 or over.38
Clinical implications
Most research so far has excluded people with multimorbidity,39,40 and most of the health care sector applies a single-disease framework focusing on the individual disease instead of focusing on the person most often having more than one long-term condition.41,42 This has an extensive negative impact on the quality of care in clinical practice and is inconvenient, inefficient, and unsatisfactory for the individual with the long-term conditions and their health care provider.43–45
In this large-scale study in primary care, we found that most people with OA have at least one other long-term condition and that the number of comorbidities is linked with the individual patient’s health status. Therefore, patients with more than one long-term condition may require a more individualized treatment approach, for example, require further supervision or lower intensity levels of the exercise due to cardiorespiratory conditions,46,47 as well as needing additional time and support to overcome higher levels of pain, fear of pain, and disability. Hence, clinicians are required to offer care adapted to the goals, needs, and preferences of the individual patient and address the complexity and interaction of all the long-term conditions and treatments of the patient with multiple conditions. It is important to note that physical activity is an important part of this individualized care of patients with OA and comorbidities, as physical inactivity is a risk factor for at least 35 long-term conditions31 and as exercise therapy is an effective treatment of at least 26 long-term conditions,29 including those commonly found in patients with OA. This highlights exercise therapy, supported by self-management and weight loss (if needed), as a treatment that might be particularly feasible and effective in patients with more than one chronic condition as it is able to improve health across different long-term conditions. Although this reflects current OA treatment recommendations,48,49 it is important to recognize that the presence of other conditions may lower a patient’s baseline levels of physical function and require adaptions of the treatment, which would therefore potentially change the initial goals and expectation they should be setting.
Limitations
Some limitations of the study must be mentioned. First, all of the participants in this study are part of a preselected group; they are the population with OA who are actively seeking patient education and exercise therapy (GLA: D) as treatment for OA (after referral from their general practitioner). Those who could not/would not seek this treatment may be different in some of the health status measures. It could well be that some of those who did not take part were worse off and had more long-term conditions. Second, 18% of participants reported having miscellaneous medical conditions and details on the individual conditions are unknown. Participants were asked whether they had any other conditions not already included. Therefore, participants could have thought that this included conditions that may not have been diagnosed or were unrelated to the ongoing study, such as eczema. This feeds into another limitation, which is that all the comorbidities were self-reported, which may have led to inaccuracies in actual presence of long-term conditions.50 This may have been the case with rheumatoid arthritis; before a supplementary text was added to explain that rheumatoid arthritis is different from OA in July 10, 2015, the prevalence was 10.43% (437 of 4191), once the supplementary text was in place, this dropped to 4.09% (805 out of 19661). It can, therefore, be questioned if those who filled in the questionnaire before this time understood the difference.
Conclusion
This is the first large-scale study of comorbidities in people with OA in primary care, demonstrating that most people (62%) have at least one other long-term condition, with the most common being hypertension and the most common to cluster together being hypertension and diabetes. In patients with OA, more comorbidities are linked with lower education levels, higher pain frequency, and severity, more widespread pain, fear of pain, lower quality of life, higher use of pain medication (particularly opioids), physical activity levels, and physical function. In educating and treating patients with OA, screening for comorbidities is important as these may affect the individual patient health status and treatment choices. Along with this, it is important for clinicians to consider and give information on how the treatments for OA will interact and help other common comorbidities, for example, exercise which is an important treatment and prevention of many other long-term health conditions.
Acknowledgements
The authors would like to thank the clinicians and patients involved in collecting data for GLA: D®.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EMR is the deputy editor of Osteoarthritis and Cartilage, the developer of the Knee injury and Osteoarthritis Outcome Score (KOOS) and several other freely available patient-reported outcome measures, and cofounder of Good Life with Osteoarthritis in Denmark (GLA: D®), a not-for-profit initiative hosted at the University of Southern Denmark aimed at implementing clinical guidelines for OA in clinical practice. STS, an associate editor of the Journal of Orthopaedic & Sports Physical Therapy, has received grants from The Lundbeck Foundation, personal fees from Munksgaard, all of which are outside the submitted work. He is the cofounder of GLA: D®.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GLA: D® has received funding from the Danish Physiotherapy Association’s fund for research, education, and practice development; the Danish Rheumatism Association; and the Physiotherapy Practice Foundation. PEM is funded by the Arthritis Research UK Centre for Sport, Exercise and Osteoarthritis [Grant Reference No. 21595]. STS is currently funded by a grant from Region Zealand and a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program [Grant Agreement No. 801790].
ORCID iD: Paul Edward Muckelt  https://orcid.org/0000-0001-5995-881X
https://orcid.org/0000-0001-5995-881X
References
- 1. Farmer C, Fenu E, O’Flynn N, et al. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ 2016; 354: 4843. [DOI] [PubMed] [Google Scholar]
- 2. Valderas JM, Starfield B, Sibbald B, et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009; 7(4): 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arokiasamy P, Uttamacharya U, Jain K, et al. The impact of multimorbidity on adult physical and mental health in low-and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med 2015; 13(1): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380(9836): 37–43. [DOI] [PubMed] [Google Scholar]
- 5. Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61(582): e12–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Academy of Medical Science. Multimorbidity: a priority for global health research. London: The Academy of Medical Sciences, 2018. [Google Scholar]
- 7. Schellevis FG, van der Velden J, van de Lisdonk E, et al. Comorbidity of chronic diseases in general practice. J Clin Epidemiol 1993; 46(5): 469–473. [DOI] [PubMed] [Google Scholar]
- 8. Wesseling J, Welsing PM, Bierma-Zeinstra SM, et al. Impact of self-reported comorbidity on physical and mental health status in early symptomatic osteoarthritis: the CHECK (Cohort Hip and Cohort Knee) study. Rheumatology 2013; 52(1): 180–188. [DOI] [PubMed] [Google Scholar]
- 9. van Dijk GM, Veenhof C, Schellevis F, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord 2008; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reeuwijk KG, de Rooij M, van Dijk GM, et al. Osteoarthritis of the hip or knee: which coexisting disorders are disabling? Clin Rheumatol 2010; 29(7): 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skou ST, Roos EM. Good Life with osteoarthritis in Denmark (GLA: D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord 2017; 18(1): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (sf-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (sf-36 bps), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011; 63(S11): S240–S252. [DOI] [PubMed] [Google Scholar]
- 13. Nilsdotter AK, Lohmander LS, Klässbo M, et al. Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003; 4(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roos EM, Roos HP, Lohmander LS, et al. Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998; 28(2): 88–96. [DOI] [PubMed] [Google Scholar]
- 15. Conner-Spady BL, Marshall DA, Bohm E, et al. Reliability and validity of the EQ-5D-5 L compared to the EQ-5D-3 L in patients with osteoarthritis referred for hip and knee replacement. Qual Life Res 2015; 24(7): 1775–1784. [DOI] [PubMed] [Google Scholar]
- 16. Terwee C, Bouwmeester W, van Elsland SL, et al. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthritis Cartilage 2011; 19(6): 620–633. [DOI] [PubMed] [Google Scholar]
- 17. Dobson F, Hinman RS, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage 2013; 21(8): 1042–1052. [DOI] [PubMed] [Google Scholar]
- 18. Arthritis Research, Musculoskeletal conditions and multimorbidity. London: Arthritis Research, 2017. [Google Scholar]
- 19. Perruccio AV, Katz JN, Losina E. Health burden in chronic disease: multimorbidity is associated with self-rated health more than medical comorbidity alone. J Clin Epidemiol 2012; 65(1): 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ross CE, Wu C-L. The links between education and health. Am Sociol Rev 1995; 60: 719–745. [Google Scholar]
- 21. Reyes C, Garcia-Gil M, Elorza JM, et al. Socio-economic status and the risk of developing hand, hip or knee osteoarthritis: a region-wide ecological study. Osteoarthritis Cartilage 2015; 23(8): 1323–1329. [DOI] [PubMed] [Google Scholar]
- 22. Kingsbury SR, Hensor EM, Walsh CA, et al. How do people with knee osteoarthritis use osteoarthritis pain medications and does this change over time? Data from the Osteoarthritis Initiative. Arthritis Res Ther 2013; 15(5): R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehendale AW, Goldman MP, Mehendale RP. Opioid overuse pain syndrome (OOPS): the story of opioids, prometheus unbound. J Opioid Manage 2013; 9(6): 421–438. [DOI] [PubMed] [Google Scholar]
- 24. Thorlund J, Turkiewicz A, Prieto-Alhambra D, et al. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage 2019; 27(6): 871–877. [DOI] [PubMed] [Google Scholar]
- 25. Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol 2015; 29(1): 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skou ST, Pedersen BK, Abbott JH, et al. Physical activity and exercise therapy benefit more than just symptoms and impairments in people with hip and knee osteoarthritis. J Orthop Sports Phys Ther 2018; 48(6): 439–447. [DOI] [PubMed] [Google Scholar]
- 27. Wedell-Neergaard A-S, Eriksen L, Grønbæk M, et al. Low fitness is associated with abdominal adiposity and low-grade inflammation independent of BMI. PLoS One 2018; 13(1): e0190645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol 2009; 587(23): 5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedersen BK, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015; 25: 1–72. [DOI] [PubMed] [Google Scholar]
- 30. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol 2015; 11(2): 86–97. [DOI] [PubMed] [Google Scholar]
- 31. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012; 2(2): 1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305(1): 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stubbs B, Hurley M, Smith T. What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? a systematic review of physical activity correlates. Clin Rehabil 2015; 29(1): 80–94. [DOI] [PubMed] [Google Scholar]
- 34. King LK, Kendzerska T, Waugh EJ, et al. Impact of osteoarthritis on difficulty walking: a population-based study. Arthritis Care Res (Hoboken) 2018; 70(1): 71–79. [DOI] [PubMed] [Google Scholar]
- 35. Pereira D, Peleteiro B, Araújo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011; 19(11): 1270–1285. [DOI] [PubMed] [Google Scholar]
- 36. Penninx BW, Beekman AT, Ormel J, et al. Psychological status among elderly people with chronic diseases: does type of disease play a part? J Psychosom Res 1996; 40(5): 521–534. [DOI] [PubMed] [Google Scholar]
- 37. England N. Quality and outcomes framework, achievement, prevalence and exceptions data - 2017-18 [PAS]—NHS Digital, https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2017-18 (accessed 8 December 2019).
- 38. Health DO. Long term conditions compendium of information. 3rd ed London: Department of Health and Social Care, 2012. [Google Scholar]
- 39. National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management. NG56. London: NICE, 2016. [Google Scholar]
- 40. Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007; 297(11): 1233–1240. [DOI] [PubMed] [Google Scholar]
- 41. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012; 380(9836): 7–9. [DOI] [PubMed] [Google Scholar]
- 42. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380(9836): 37–43. [DOI] [PubMed] [Google Scholar]
- 43. Noel PH, Frueh BC, Larme AC, et al. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect 2005; 8(1): 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bower P, Macdonald W, Harkness E, et al. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Fam Pract 2011; 28(5): 579–587. [DOI] [PubMed] [Google Scholar]
- 45. Smith SM, O’Kelly S, O’Dowd T. GPs’ and pharmacists’ experiences of managing multimorbidity: a ‘Pandora’s box’. Br J Gen Pract 2010; 60(576): 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Rooij M, van der Leeden M, Avezaat E, et al. Development of comorbidity-adapted exercise protocols for patients with knee osteoarthritis. Clin Interv Aging 2014; 9: 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dekker J, de Rooij M, van der Leeden M. Exercise and comorbidity: the i3-S strategy for developing comorbidity-related adaptations to exercise therapy. Disabil Rehabil 2016; 38(9): 905–909. [DOI] [PubMed] [Google Scholar]
- 48. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64(4): 465–474. [DOI] [PubMed] [Google Scholar]
- 49. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22(3): 363–388. [DOI] [PubMed] [Google Scholar]
- 50. Raina P, Torrance-Rynard V, Wong M, et al. Agreement between self-reported and routinely collected health-care utilization data among seniors. Health Serv Res 2002; 37(3): 751–774. [DOI] [PMC free article] [PubMed] [Google Scholar]