Abstract
Background
The use of autologous tissue for breast reconstructive surgery following mastectomy has become routine and allows for excellent symmetry and aesthetic results. However, in some cases, the amount of tissue available from the utilized flaps is not enough to achieve the desired outcome. The use of autologous fat grafting, as well as other techniques, has been described to deal with such problems. However, though well-established, these techniques may also fail to achieve the desired results. The aim of this study was to highlight the opportunity to improve aesthetic results using a secondary prosthesis underneath the previously used free flap and to examine whether this is a safe and reasonable procedure.
Material/Methods
In our study we included patients with unsatisfied aesthetic results after free flap procedures (DIEP, S-GAP, TMG, and FCI) between 2011 and 2018. In each case described, a secondary prosthesis was placed underneath the original flap in order to improve symmetry, shape and projection. Patient age, indication for surgery, adjuvant therapy, complications and outcomes have been registered. A 12-point scale was established to analyze patient satisfaction and aesthetic outcome.
Results
Overall “operative success” was achieved in all 13 patients (14 flaps) evaluated. At 12 months after reconstruction, all aesthetic scores collected were between good and excellent. In contrast to other studies, we chose a secondary approach for the flap augmentation and we used the epipectoral pocket for the placement of the implant. In our series, low rates of early and late post-operative complications were observed, with a high overall rate of satisfaction.
Conclusions
The demonstrated “Hybrid Breast Reconstruction” approach, using an implant underneath a free flap autologous breast reconstruction, provided a safe and reliable option to optimize breast reconstruction outcomes.
MeSH Keywords: Breast Implantation, Free Tissue Flaps, Mammaplasty
Background
For breast cancer patients, individualized, reconstructive breast surgery has become a standard and integral part of the treatment options offered in order to optimize both the radicality of treatment and the patient’s subsequent quality of life. The use of autologous tissue improves both the natural look and feel of the reconstructed breast [1]. Increasingly sophisticated techniques serve to ensure the viability of the grafted tissue, as well as reduce post-operative complications and enhance long-term outcome. In this context, the use of perforator flaps has become a routine technique that allows for high expectations with regard not only to safety and reliability but also symmetry and aesthetics [2,3]. However, there are situations where the amount of tissue derived from the flap is inadequate to achieve aesthetically desirable outcomes in terms of symmetry, shape and projection. This may be due to the inaccurate estimation of the tissue volume of the flap compared to the corresponding contralateral site, secondary fat necrosis or partial flap loss. Use of autologous fat grafting techniques or the use of a second flap have been described to deal with such problems [4,5].
However, since there are patients in which even these techniques fail to achieve the desired results, additional strategies are needed and preferable.
The combination of free tissue transfer combined with simultaneous implant placement represents an additional promising concept. It is well known that pedicled latissimus dorsi (LD) and transverse rectus abdominis myocutaneous (TRAM)) flaps can be used in combination with prostheses for breast reconstruction approaches [6,7]. Moreover, this technique has already been used as a primary augmentation of deep inferior epigastric perforator (DIEP) flaps in slender patients with larger breasts who wish to use their abdominal tissue for reconstruction of the breast, but the amount of tissue from the DIEP flap was not enough to achieve symmetrical breast [8].
Moreover, the transfer of well-vascularized soft tissue allows reconstruction of natural breast ptosis, the addition of an implant in a 1-step procedure provides the desired projection, without being associated with complications such as rippling or animation deformity [9].
This article highlights the opportunity to improve aesthetic results by placing a secondary prosthesis underneath the free flap for the purpose of a “Secondary Hybrid Breast Reconstruction”. In this context the aim of our study was to evaluate and to verify, if the correction approach by placing an implant right under a previously performed free flap is a safe and reasonable concept.
We analyze indication and results in order to outline and examine an additional choice in optimizing the quality of breast reconstruction with autologous tissue
Material and Methods
In our Department, a total of 339 autologous breast reconstructions were performed in 315 women (mean age, 48.8 years, range 21–76 years) between 2011 and 2018. Of these, 265 procedures (78%) were free DIEP flap procedures, 31 procedures (9%) were S-GAP (superior gluteal artery perforator) flap procedures, 6 procedures (2%) were FCI (fasciocutaneous infragluteal) flap procedures, and 37 procedures (11%) were TMG (transverse myocutaneous gracilis) flap procedures.
Of the 315 patients, 13 patients demonstrated, at a later time, inadequate symmetry and aesthetic unsatisfying results with the wish for improvement. They were included in a retrospective chart review. Of these 13 patients, 14 reconstructions were performed (8 DIEPs, 1 S-GAP, 1 FCI, and 4 TMGs). Typical secondary touch-up procedures to improve shape and symmetry, including autologous fat grafting techniques, failed or were unfeasible from the beginning due to a lack of donor site. In our series, fat grafting was used in 6 patients prior to the implant reconstruction to improve the aesthetic result, however, without achieving the desired outcome (Table 1).
Table 1.
Clinical Data of the 13 Patients Included in the Study.
| Patient | Age (years) | Diagnosis | Oncologic surgery | Radiation | Reconstructed by | Time between flap and implant in months | Lipofilling prior to implant insertion (yes/no) | Indication for implant asymmetry | Implant size, form, profile | Implant location |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Breast cancer | Mastectomy | Yes | DIEP | 6 | No | Primary | 100 cc, round, mpp | Epipectoral |
| 2 | 75 | Breast cancer | Nipple-sparing mastectomy | Yes | DIEP | 16 | Yes | Primary | 100 cc, round, mpp | Epipectoral |
| 3 | 57 | Ductal carcinoma in situ | Mastectomy | Yes | DIEP | 16 | No | Primary | 150 cc, round, mpp | Epipectoral |
| 4 | 52 | Ductal carcinoma in situ | Nipple-sparing mastectomy | Yes | TMG | 22 | No | Secondary | 200 cc, round, hp | Epipectoral |
| 5 | 46 | Breast cancer | Skin-sparing mastectomy | No | TMG | 53 | Yes | Secondary | 150 cc, round, mpp | Epipectoral |
| 6 | 50 | Ductal carcinoma in situ | Mastectomy | Yes | TMG | 19 | No | Primary | 145 cc, round, hp | Epipectoral |
| 7 | 59 | Breast cancer | Mastectomy | Yes | DIEP | 7 | Yes | Primary | 300 cc round, mpp | Epipectoral |
| 7 | 59 | Mastopathia | Skin-sparing mastectomy | No | S-GAP | 10 | Yes | Primary | 300 cc, round, mpp | Epipectoral |
| 8 | 42 | Breast cancer | Nipple-sparing mastectomy | Yes | FCI | 31 | Yes | Secondary | 100 cc, round, mpp | Epipectoral |
| 9 | 54 | Breast cancer | Nipple-sparing mastectomy | Yes | TMG | 87 | Yes | Primary | 150 cc, round, mpp | Epipectoral |
| 10 | 52 | Breast cancer | Nipple-sparing mastectomy | Yes | DIEP | 10 | No | Primary | 150 cc, round, mpp | Epipectoral |
| 11 | 52 | Breast cancer | Mastectomy | No | DIEP | 12 | No | Primary | 150 cc, round, mpp | Epipectoral |
| 12 | 51 | Breast cancer | Nipple-sparing mastectomy | Yes | DIEP | 10 | No | Primary (necrosis of the flap) | 125 cc, round, mpp | Epipectoral |
| 13 | 41 | Breast cancer | Nipple-sparing mastectomy | No | DIEP | 6 | No | Primary | 125 cc, round, mpp | Epipectoral |
DIEP – deep inferior epigastric perforator; TMG – transverse musculus gracilis; S-GAP – superior gluteal artery; FCI – fasciocutaneous infragluteal; mpp – moderate plus profile; hp – high profile.
Therefore, in all the presented cases, a secondary implant was placed underneath the previously used flap. Thus, we aimed to correct symmetry as well as the shape and projection of the breast. Patient’s age, indication for surgery, adjuvant therapy (radiotherapy), complications, outcomes, and follow-up were registered.
Indications for this secondary “Hybrid Breast Reconstruction” concept included inadequate breast volume, breast mound deficiencies, and lack of projection caused by primary asymmetry of the reconstructed breast or secondary loss of volume of the flap with a displeasing aesthetic outcome.
In all cases, textured round silicone gel implants were used, and in all cases, the implant volume was determined intraoperatively using sizers.
In all cases, the implant was placed in an epimuscular, “sub-flap” pocket (Figure 1). This approach was performed in order to control the implant pocket accurately. In addition, we wanted to maintain as much impact on the projection and the form of the breast as possible (Figure 2). Care was taken to preserve the flap pedicle during the operative procedure, since in all cases recipient vessels for the flaps were the internal mammary vessels. Therefore, in all cases the vascular pedicle of the flap was visualized and dissected carefully (Figure 1). All surgical procedures were performed by 2 experienced plastic and breast surgeons, which included the head of department and senior plastic surgeon.
Figure 1.
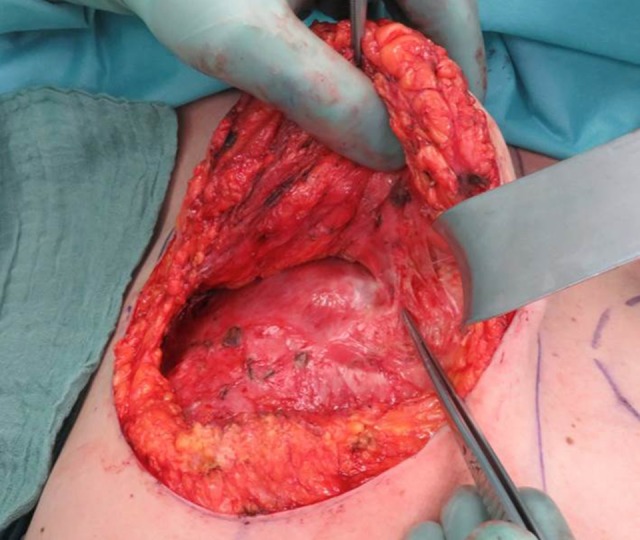
Patient number 4: Intraoperative situs 22 months after TMG flap, epipectoral pocket for the implant, tip of the forceps points to the flap perforator vessels. Abbreviations: TMG, transverse myocutaneous gracilis.
Figure 2.
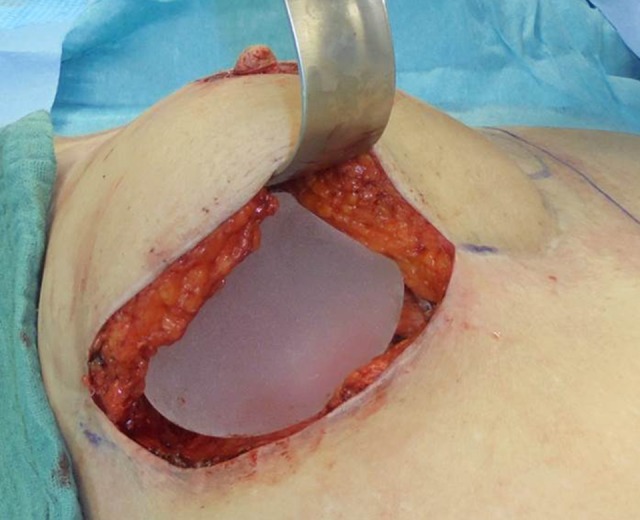
Patient number 2: Intraoperative situs: 100 cc silicone implant placed in the epipectoral pocket, 16 months after DIEP flap. Abbreviations: DIEP, deep inferior epigastric perforator.
Approximately 12 months after implantation of the prothesis, aesthetic scores were collected. Patients were asked to assess their satisfaction using a 12-point scale. This 12-point scale was prepared for this study by the authors. The range was from 0–3 (poor), 4–6 (fairly satisfied), 7–9 (good), and 10–12 (excellent). At the same time, an experienced consultant plastic surgeon, who did not participate in the reconstruction procedures itself, used the same 12-point scale to evaluate the aesthetic outcome, especially with regard to breast volume, shape, projection, and symmetry. He scored all the patients blindly as part of a personal examination to get a real 3-dimensional impression of the reconstructed breasts. In our opinion, this was necessary to evaluate the surgical outcome realistically, because pictures have obvious limitations in meaningfulness.
The case series was designed as a retrospective, single center study. It was conducted in accordance with the principles of the Declaration of Helsinki. Written consent was obtained from all patients to participate in this study. An ethical approval by the independent ethics committee of the hospital was obtained.
Results
Between 2011 and 2018 we performed 14 secondary Hybrid Breast Reconstructions in our department in the aforementioned manner (Table 1). Of these, 13 patients underwent unilateral reconstruction with a free flap and a secondarily placed implant. One patient underwent a bilateral surgery (DIEP flap on one side and S-GAP on the other side)
In 10 cases, the indication for the primary breast surgery was breast cancer, in 3 cases ductal carcinoma, and in 1 case mastopathia. The oncological surgery was radical-modified mastectomy in 5 cases, nipple-sparing mastectomy in 7 cases, and skin-sparing mastectomy in 2 cases.
Ten breasts received radiation therapy in advance of the reconstruction by the flap procedure. Most breasts were reconstructed with a DIEP flap (n=8), the others were TMG (n=4) S-GAP (n=1), and FCI (n=1) flaps, respectively. In all cases, the internal mammary vessels were used as the recipient vessels for the flaps.
The average time between flap reconstruction and implant placement was 22 months with a range of 6 to 87 months.
In 6 cases, we used partly repeated fat grafting prior to the insertion of the implant to provide for increased symmetry. This, however, failed to produce pleasing results.
In 3 cases, the indication for the implant was secondary shrinkage of the flap that resulted in an asymmetry. In these 3 cases, the reconstruction was performed twice with a TMG flap and once with an FCI flap. In the other cases (n=11), the asymmetry was present shortly after flap reconstruction.
Time between flap and implant reconstruction was longer in patients with secondary asymmetry (22, 31, and 53 months; average 35 months) compared to the rest of the patients (average 18 months).
We inserted round implants in all cases and placed them in an epipectoral pocket, directly underneath the flap. The average size of the implant was 160 cc with a range of 100–300 cc. In 12 cases we used a moderate plus profile and in 2 patients we used a high-profile implant (Table 1).
Follow-up period was at least 12 months after implant surgery and in 2 patients at least 12 months after the last implant exchange. The range of the follow up was 13 months to 7 years. No patient suffered from major complications after implant procedure. We had no early revision surgery and no hematoma, and no secondary flap loss was noted. During the implant surgery, the flap vessels and anastomosis of the flap were explored in order to prevent damage to the blood supply of the flap.
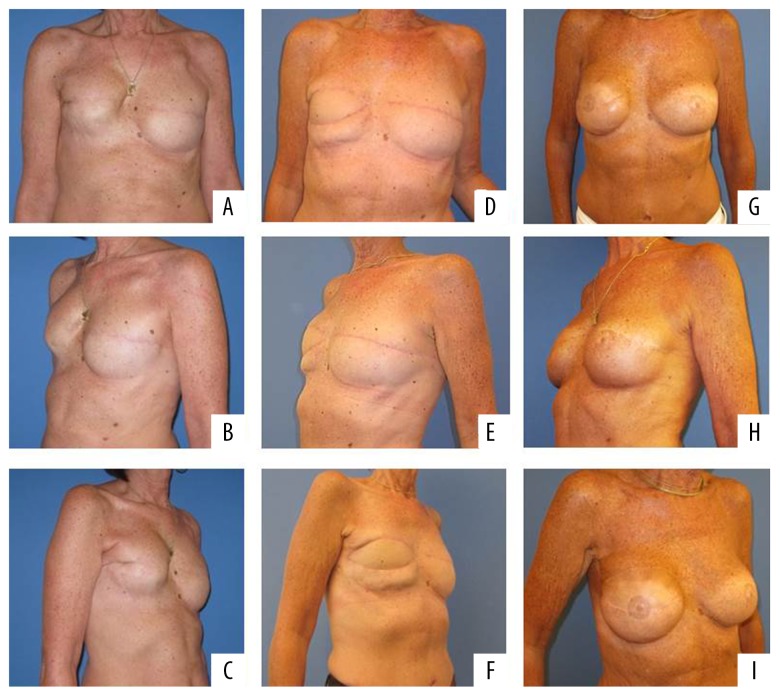
Generally, no considerable symptomatic capsular contracture or implant dislocation was noted during the study period. Only 1 patient (Patient No. 7, Figure 3) presented 5 years after bilateral flap reconstruction and secondary implants with capsular contracture on the “DIEP“ side (Table 1). On the affected side, the patient had received radiation therapy in advance of the reconstruction.
Figure 3.
Patient number 7 after mastectomy right breast and skin-sparing mastectomy left breast and temporal expander breast augmentation (A–C), reconstruction right breast with DIEP and left breast with S-GAP flap (D–F) and after augmentation with implant (left breast 300 cc round, right breast 300 cc round implants) (G–I). Abbreviations: DIEP, deep inferior epigastric perforator; S-GAP, superior gluteal artery perforator.
We performed a capsulectomy and an exchange of the implant. Again, we placed the implant into the epipectoral pocket. During the next 2 years of follow-up, the patient was free of symptoms.
Patient No. 8 developed a secondary malposition of the implant (bottoming out) after 1 year. In the revision surgery, we reconstructed the inframammary fold and replaced the implant with an equally sized one (100 cc moderate plus profile) (Table 1). Figures 3–6 show some clinical results after our hybrid surgery.
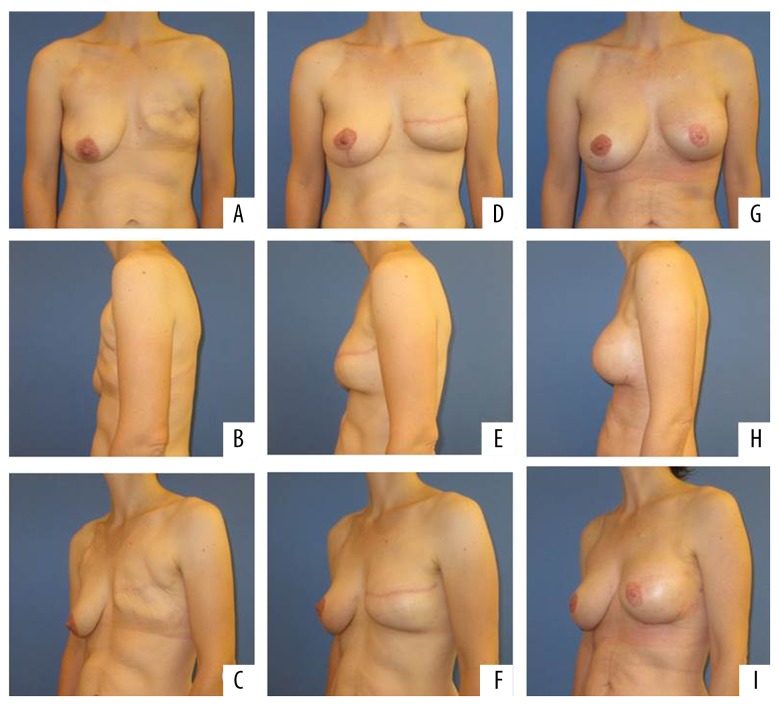
Figure 4.
Patient number 5 after skin sparing mastectomy (A–C), reconstruction with TMG flap left breast and mastopexy right breast (D–F) and after augmentation left breast with 150 cc implant (G–I).
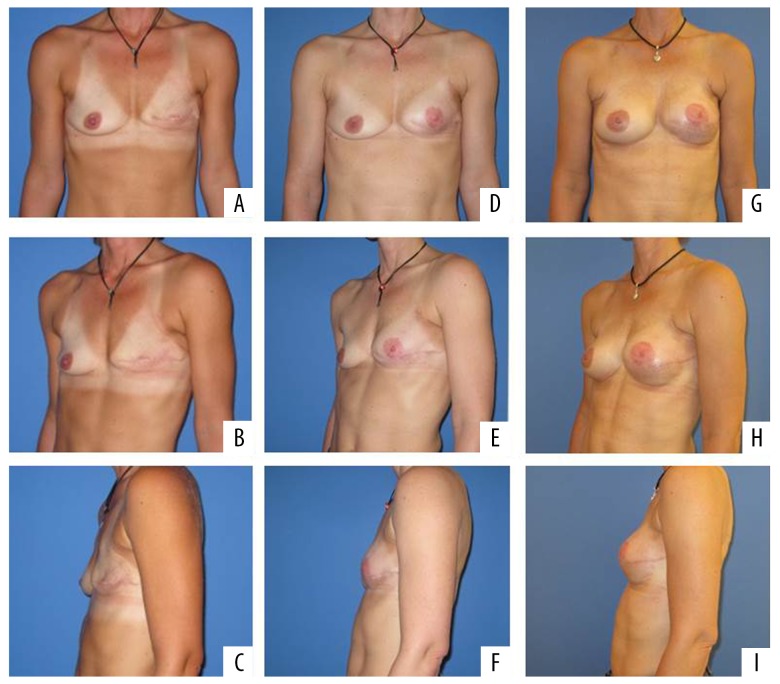
Figure 5.
Patient number 6 after mastectomy left breast (A–C), reconstruction with TMG flap (D–F) and after implant augmentation (left 145 cc, right 175 cc implant) (G–I). TMG – transverse myocutaneous gracilis.
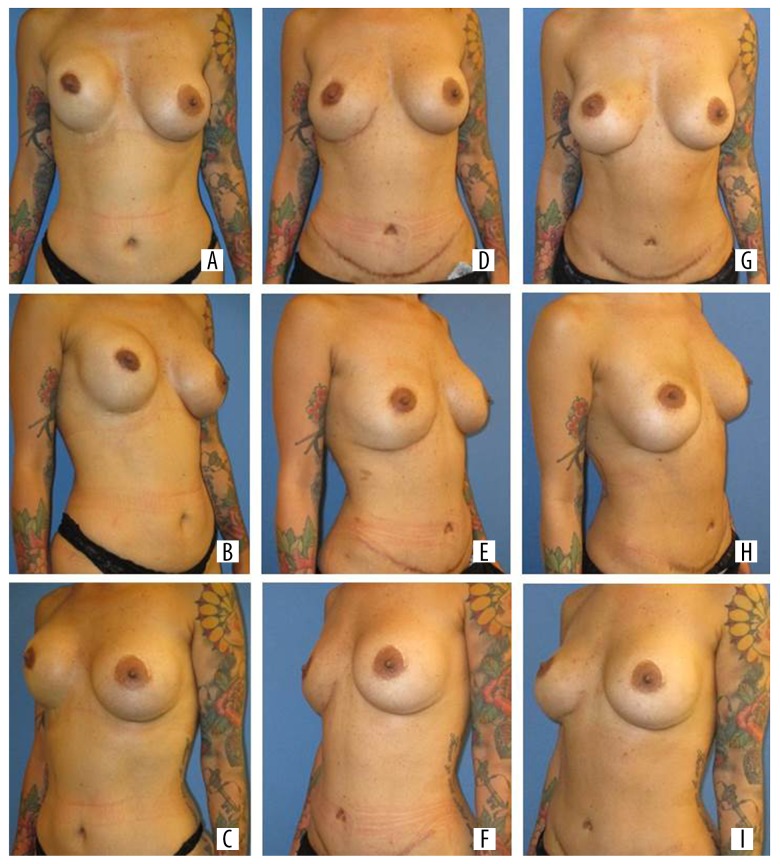
Figure 6.
Patient number 13 after nipple-sparing mastectomy right breast (A-C), reconstruction with DIEP flap (D-F) and after implant augmentation (125 cc implant) (G-I). DIEP – deep inferior epigastric perforator.
In order to estimate the success of our concept, we performed an evaluation of the achieved aesthetic results. All operated patients and a plastic surgeon who was not part of the prior surgery used the established 12-point scale (0–3 poor, 4–6 fairly satisfied, 7–9 good, 10–12 excellent). Time between last surgery and evaluation was at least 12 months.
The average evaluation of the patients was 10.5 points with a range from 8–12 points regarding their reconstruction. The average evaluation of the surgeon was 10.2 points with a range from 8–12 points (Table 2).
Table 2.
Patients’ outcome and complications, satisfaction scale of patient and surgeon: 0–3 poor, 4–6 sairly satisfied, 7–9 good, 10–12 excellent.
| Patient | Complications | Evaluation of patient | Evaluation of surgeon |
|---|---|---|---|
| 1 | None | 11 | 11 |
| 2 | None | 9 | 10 |
| 3 | None | 11 | 11 |
| 4 | None | 12 | 10 |
| 5 | None | 11 | 11 |
| 6 | None | 12 | 11 |
| 7 | Capsular contracture with resulting implant exchange after 5 years | 12 | 11 |
| 7 | None | 12 | 11 |
| 8 | Secondary malposition of the implant with resulting implant exchange after 1 year | 10 | 9 |
| 9 | None | 11 | 11 |
| 10 | None | 9 | 10 |
| 11 | None | 8 | 8 |
| 12 | None | 8 | 9 |
| 13 | None | 11 | 10 |
Discussion
The deep inferior epigastric perforator (DIEP) flap among other perforator flaps has become the gold standard in autologous breast reconstruction [10–13]. However, in some cases, the amount of tissue from the abdomen is limited and there is not sufficient abdominal tissue to achieve adequate breast volume and consequently symmetry. Moreover, prior surgeries leading to scarring can also limit the size of the flap. In other cases, extensive skin defect creates a significant problem to the surgeon. In these situations, the available abdominal tissue is primarily required in resurfacing the skin of the breast and is not available to provide adequate volume and projection for the new breast.
Although other flaps from other body areas are available, such as S-GAP, TMG, or FCI, these too are often limited with regard to the volume they can provide in cases where patients wish to receive a medium to large breast [14]. Moreover, these flaps are generally even more limited regarding the skin surface and flap volume made available.
Furthermore, it is well-known that sometimes all the aforementioned flaps can lose volume secondary after reconstruction (i.e., by fat necrosis or muscle atrophy) [15], which we also observed in our patients, especially with TMG flaps.
Therefore, some patients present with inadequate aesthetic results after autologous breast reconstruction following different free flaps. Such unfavorable outcomes include inadequate breast volume and mound deficiencies, superior pole deficiencies, asymmetry, and lack of projection.
Techniques such as fat grafting have been shown to be beneficial in order to augment flap-based breast reconstruction [16]. Articles underline the safety of fat grafting regarding breast cancer recurrence [4] and especially the effect of lipofilling after implant-based breast reconstruction [5,17]. In this context, Maxwell and Gabriel presented the “bioengineered breast approach” with repeated autologous fat grafting covering implants [18].
However, like every technique, fat grafting has also its limitations. One main disadvantage of this procedure is that it often it must be performed repeatedly to achieve the desired results due to an uncertain volume retention of up to 50%.
From our perspective, a significant improvement in projection with lipofilling is rarely possible, and a notable size augmentation is seldom possible. Moreover, an international consensus regarding lipofilling after cancer does not yet exist [19].
On the other hand, there are encouraging publications where the use of breast implants in combination with free perforator flaps as a one-step-procedure have been proposed [9,20].
From the breast reconstruction concept with the combination of latissimus dorsi (LD) flap and an implant, we know that the combination of both procedures is, in principle, safe and reasonable. However, by using a free flap procedure, especially with perforator flaps, compared to the LD flap, we can minimize donor site morbidity, animation deformity, and shrinkage of the flap caused by muscular atrophy. Moreover, the limitation of the skin area provided by the LD flap makes it difficult to create a natural breast ptosis.
The combined use of an implant with perforator flaps is particularly different compared to the LD flap/implant reconstruction concept. In the latter, in patients with large or medium-sized breasts, the implant influences mainly the shape and the volume of the reconstructed breast. In cases with perforator flap/implant breast reconstruction, the implant is basically used to augment the already shaped breast and define projection, because of the greater amount of tissue being recruited for reconstruction. Therefore, the shape of the reconstructed breast is essentially defined by the free flap and one can reconstruct a naturally ptotic breast.
Furthermore, our study demonstrated that the average size of the implants used with the perforator flaps was small and the majority of the breast volume and shape depended on the flap tissue and hence volume changes occur, in keeping with the rest of the body. Therefore, with the implant an improvement in projection and volume is possible and achievable.
This method was performed not only with DIEP flaps, as described by others [9,20], but we expanded the approach to other perforator flaps such as S-GAP, FCI, and TMG flaps.
In contrast to the sub-pectoral approach described by Figus et al. [20], we placed all implants in the pre-pectoral (sub-flap) pocket as also described by Momeni et al. [9]. We think that the ability to control the implant pocket and improve projection of the reconstructed breasts minimizes the risk of malposition of the implant and contributes to the protection of the vascular pedicle on one hand while improving aesthetic outcomes on the other hand.
We only used textured, round silicone gel implants for volume augmentation and not to improve the shape of the new breast which was, from our point of view, determined by the flap. Thereby, we combined the advantages of both procedures without using large size implants. In our case series, we did not note relevant symptomatic capsular contracture problems, although the majority of the patients had radiation therapy prior to reconstruction.
Risk for capsular contracture in patients after radiation and mastectomy has been reported to be high [21–26]. However, in our study, only 1 patient presented with a late capsular contracture after hybrid reconstruction. We attribute this observation to the fact that the non-radiated free flap improves the quality and vascularity of the soft tissue cover over the implant, comparable to a situation without radiation which minimizes the risk of developing a capsular contracture. However, since our post-implant observation period was relatively short in most of the cases and the number of patients was limited, further longitudinal observation will be necessary to address the question of capsular contracture rate among patients with the concept of hybrid breast reconstruction.
Momeni et al. have suggested performing the procedure of DIEP flap augmentation as a primary approach [9]. However, the authors believe that immediate flap/implant augmentation may interfere with the flap size and shape and has the risk of secondary asymmetry because of flap shrinkage in progress.
In contrast to that approach, all our cases of hybrid breast reconstruction were performed as a secondary procedure. Time between autologous and implant surgery was at least 6 months with the result that the final size and shape of the autologous reconstructed breast had developed and was stable. In this manner, we could exactly estimate the need for a secondary augmentation and the consequently required implant size. We were able to minimize the risks of partial flap necrosis and infection as well as avoid primary asymmetry. The need for implant change due to asymmetry was thereby minimized.
For all these reasons, we do not recommend an immediate augmentation strategy and favor our concept of secondary flap augmentation in order to increase predictability of final postoperative outcome.
In contrast to the aforementioned publications (Momeni et al. [9] and Figus et al. [20]) we have chosen a secondary approach for the implant augmentation and we used the epipectoral pocket. Also, with these different approaches we could underline that the hybrid reconstruction is safe and reasonable, knowing that limitations of our study were the relatively short follow up, the retrospective study design and the small group of patients.
Despite these existing limitations, we think that in selected patients, secondary hybrid breast reconstruction combines many advantages in terms of implant and autologous reconstruction options in order to optimize the aesthetic outcome of breast reconstruction. We demonstrated that, in cases where autologous tissue is insufficient to reconstruct the desired breast size or shape, we can achieve the desired results in combination with an implant. Our self-developed 12-point evaluation scale revealed a high satisfaction rate of the patients related to long-term outcomes following our strategy. This questionnaire was established to get a gross impression on how patients rated their individual aesthetic outcome following the aforementioned procedure. For further in-depth analyses, assessment using validated and more detailed outcome measurement questionnaires, such as the BreastQ, should be performed.
In summary, the presented approach was an effective, reasonable and safe tool for a selected group of patients after autologous breast reconstruction in order to improve aesthetic outcome.
Conclusions
In conclusion, secondary augmentation following autologous breast reconstruction offers pleasing breast volume and symmetry while attempting to preserve the natural appearance of the prior reconstructed breast. We believe that the presented concept of secondary augmentation of different flaps after breast reconstruction allows a reliable approach for optimizing unfavorable reconstructions in selected patients.
Footnotes
Sources of support: Departmental sources
References
- 1.Busic V, Das-Gupta R, Mesic H, et al. The deep inferior epigastric perforator flap for breast reconstruction, the learning curve explored. J Plast Reconstr Aesthetic Surg. 2006;59(6):580–84. doi: 10.1016/j.bjps.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 2.MacAdam SA, Bovill ES, Buchel EW, et al. Evidence-based medicine: autologous breast reconstruction. Plast Reconstr Surg. 2017;139(1):204e–29e. doi: 10.1097/PRS.0000000000002855. [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY, Momen B, Galdino G, et al. Breast reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110(2):466–75. doi: 10.1097/00006534-200208000-00015. discussion 476–67. [DOI] [PubMed] [Google Scholar]
- 4.Batista BN, Fraga MFP, Sampaio MMC, et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer. Plast Reconstr Surg. 2016;138(6):1068e–69e. doi: 10.1097/PRS.0000000000002794. [DOI] [PubMed] [Google Scholar]
- 5.Cogliandro A, Barone M, Tenna S, et al. The role of lipofilling after breast reconstruction: evaluation of outcomes and patient satisfaction with BREAST-Q. Aesthetic Plast Surg. 2017;41(6):1325–31. doi: 10.1007/s00266-017-0912-1. [DOI] [PubMed] [Google Scholar]
- 6.Bittar SM, Sisto J, Gill K. Single-stage breast reconstruction with the anterior approach latissimus dorsi flap and permanent implants. Plastic and Reconstructive Surgery. 2012;129(5):1062–70. doi: 10.1097/PRS.0b013e31824a2bbd. [DOI] [PubMed] [Google Scholar]
- 7.Hammond DC. Latissimus dorsi flap breast reconstruction. Plast Reconstr Surg. 2009;124(4):1055–63. doi: 10.1097/PRS.0b013e3181b6bf05. [DOI] [PubMed] [Google Scholar]
- 8.Chia HL, Breitenfeldt N, Canal ACE, et al. Implant augmentation after perforator flap breast reconstruction. J Plast Reconstr Aesthetic Surg. 2010;63(2):e172–73. doi: 10.1016/j.bjps.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 9.Momeni A, Kanchwala S. Hybrid prepectoral breast reconstruction: A surgical approach that combines the benefits of autologous and implant-based reconstruction. Plast Reconstr Surg. 2018;142(5):1109–15. doi: 10.1097/PRS.0000000000004858. [DOI] [PubMed] [Google Scholar]
- 10.Blondeel PN. One hundred free DIEP flap breast reconstructions: A personal experience. Br J Plast Surg. 1999 Mar;52(2):104–11. doi: 10.1054/bjps.1998.3033. [DOI] [PubMed] [Google Scholar]
- 11.Granzow JW, Levine JL, Chiu ES, et al. Breast reconstruction with the deep inferior epigastric perforator flap: History and an update on current technique. J Plast Reconstr Aesthetic Surg. 2006;59(6):571–79. doi: 10.1016/j.bjps.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Hamdi M, Weiler-Mithoff EM, Webster MHC, et al. Deep inferior epigastric perforator flap in breast reconstruction: experience with the first 50 flaps. Plast Reconstr Surg. 1999;103(1):86–95. doi: 10.1097/00006534-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Blondeel PN, Boeckx WD. Refinements in free flap breast reconstruction: The free bilateral deep inferior epigastric perforator flap anastomosed to the internal mammary artery. Br J Plast Surg. 1994;47(7):495–501. doi: 10.1016/0007-1226(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 14.Wechselberger G, Traintinger H, Larcher L, et al. Clinical applications of the transverse musculocutaneous gracilis flap for secondary breast reconstruction after simple mastectomy. Plast Reconstr Surg. 2016;137(1):19–28. doi: 10.1097/PRS.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 15.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106(3):576–83. doi: 10.1097/00006534-200009030-00008. [DOI] [PubMed] [Google Scholar]
- 16.Katzel EB, Bucky LP. Fat grafting to the breast: Clinical applications and outcomes for reconstructive surgery. Plast Reconstr Surg. 2017;140(5 Advances in Breast Reconstruction):69S–76S. doi: 10.1097/PRS.0000000000003945. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese S, Zingaretti N, De Francesco F, et al. Long-term impact of lipofilling in hybrid breast reconstruction: retrospective analysis of two cohorts. Eur J Plast Surg. 2019 [Google Scholar]
- 18.Maxwell GP, Gabriel A. Bioengineered breast. Plast Reconstr Surg. 2016;137(2):415–21. doi: 10.1097/01.prs.0000475750.40838.53. [DOI] [PubMed] [Google Scholar]
- 19.Petit JY, Lohsiriwat V, Clough KB, et al. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: A multicenter study – Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128(2):341–46. doi: 10.1097/PRS.0b013e31821e713c. [DOI] [PubMed] [Google Scholar]
- 20.Figus A, Canu V, Iwuagwu FC, et al. DIEP flap with implant: a further option in optimising breast reconstruction. J Plast Reconstr Aesthetic Surg. 2009;62(9):1118–26. doi: 10.1016/j.bjps.2007.12.089. [DOI] [PubMed] [Google Scholar]
- 21.Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg. 2015;135(4):959–66. doi: 10.1097/PRS.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 22.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128(2):353–59. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JA. An update. Plast Reconstr Surg. 2017;140(5 Advances in Breast Reconstruction):60S–68S. doi: 10.1097/PRS.0000000000003943. [DOI] [PubMed] [Google Scholar]
- 24.Marques M, Brown SA, Oliveira I, et al. Long-term follow-up of breast capsule contracture rates in cosmetic and reconstructive cases. Plast Reconstr Surg. 2010;126(3):769–78. doi: 10.1097/PRS.0b013e3181e5f7bf. [DOI] [PubMed] [Google Scholar]
- 25.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: A critical review of the literature. Plast Reconstr Surg. 2009;124(2):395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
- 26.Tadiparthi S, Staley H, Collis N, et al. An analysis of the motivating and risk factors for conversion from implant-based to total autologous breast reconstruction. Plast Reconstr Surg. 2013;132(1):23–33. doi: 10.1097/PRS.0b013e318290f83e. [DOI] [PubMed] [Google Scholar]