Abstract
Ecotones can increase free-living species richness, but little is known about how parasites respond to ecotones. Here we use parasite communities in raccoons (Procyon lotor) to test the hypothesis that parasite communities can be divided into core and satellite species, each with fundamentally different responses to ecotones. We used published parasite surveys to classify parasites as common core or rare satellite species and then surveyed raccoons in coastal California to examine how proximity to two aquatic ecotones altered parasite communities. Raccoons near ecotones had more satellite and fewer core parasite species. Specifically, the marine ecotone increased parasite diversity by adding satellite species to a persistent core community, whereas the freshwater ecotone shifted the community from core to satellite species without a net change in parasite richness. We hypothesize that increased parasite richness at the marine ecotone resulted from increased diet diversity, but that raccoons were sinks for some parasites. Increased exposure to rare parasites at ecotones has implications for wildlife health and provides insight into observed associations between ecotones and emerging disease.
Keywords: ecotone, species richness, Procyon lotor, beach, California, parasite
INTRODUCTION
Whether field to forest, desert to river, or ocean to land, ecotones can be biodiversity hotspots because edge effects mix species from two systems and create opportunities for species that depend on habitat diversity (Odum 1971, Kark 2013). On the other hand, ecotones might be hard to adapt to, penalizing some species that straddle two systems (Kunin 1998, Kark and van Rensburg 2006). Because host diversity begets parasite diversity and ecotones likely foster novel host interactions (Hechinger and Lafferty 2005), these transitional habitats should also affect parasite communities (Lanfranchi et al. 2016). This could have implications for host health, particularly where changing climate and anthropogenic impacts shift ecotone boundaries (Despommier et al. 2006).
Although free-living community responses to ecotones have been extensively examined (Kark 2013), ecotone effects on parasite communities are less understood (Despommier et al. 2006). Edge habitats can alter infection risk. For example, forest edges are the preferred habitat for brood-parasitic Brown-headed Cowbirds in North America (Howell et al. 2007), and are also associated with increased coccidian infection in European rodents (Svobodova et al. 2004). Increased infection can also occur at the interface between aquatic and terrestrial habitats, as seen for human cholera and schistosomiasis (Despommier et al. 2006). Ecotones can increase individual parasite abundance, but it is unclear if the ecotone effects observed in free-living communities (i.e. increased richness and abundance) also extend to parasites.
Ecotones could alter parasite communities through multiple mechanisms, each with different implications for host health. Mobile hosts can bring adjacent parasite communities together to increase total (component community) richness without altering the within-host parasite community (infracommunity). Alternatively, ecotones might change exposure and transmission, altering infracommunities (Lanfranchi et al. 2016). Because novel parasites can impact host health (Daszak et al. 2000), the infracommunity is the most appropriate scale to evaluate how ecotones affect parasite communities. However, it is not necessarily obvious how parasite infracommunities will respond to ecotones. If one habitat has a subset of the other’s parasites, hosts in the ecotone should have intermediate parasite richness. If adjacent habitats each have distinct parasite communities, the ecotone can mix these two communities, increasing infracommunity richness (Lanfranchi et al. 2016), unless parasite species interact through competition, whereby the ecotone community might shift in composition rather than increase in richness. Furthermore, because parasites differ in life history, parasite responses to ecotones could vary by parasite species. For example, parasites transmitted by conspecific contact, persistent environmental infectious stages, or other broadly distributed hosts (or vectors) are likely to be common and present throughout a host’s range (Bush and Holmes 1986, Combes 2004). For free living organisms, species that are locally abundant, regionally common, and spread throughout available niche space are termed “core” species, in contrast to “satellite” species that display the opposite characteristics (Hanski 1982). This core and satellite framework can also be applied to parasites (Bush and Holmes 1986). Here we hypothesize that range-wide parasite communities can be divided into core and satellite species, each with different responses to ecotones. Specifically, we predict that ecotones should have less effect on widespread “core” parasites; but should increase exposure to infectious stages that require hosts or conditions found only at the boundary or in a neighboring habitat. As a result, we hypothesize that ecotones should increase parasite richness by adding satellite species to a core community.
The raccoon (Procyon lotor) is an ideal host with which to test these hypotheses as it thrives in many habitats and its well-studied parasites vary by location (e.g. Harkema and Miller 1964, Bafundo et al. 1980, Hoberg and McGee 1982) and landscape features (e.g. Samson et al. 2012). Although studies on ecotones often focus on forest/grassland or natural/urban transitions, here we examine the shoreline and riparian ecotones formed between terrestrial and aquatic systems (Zalewski et al. 2001, Heerhartz et al. 2014). To examine how these ecotones affect raccoon parasite communities, we first used published studies to identify core and satellite parasites. We then examined parasite infracommunities in a coastal raccoon population to test how ecotones affected core, satellite and total parasite richness.
METHODS
Core species occur at most sites and satellite species occur at few sites, producing bimodal species occurrence frequencies in which satellite species create a peak near one and core species create a peak farther out on the x-axis (Hanski 1982). To use this expected frequency distribution to classify raccoon parasites into core and satellite species, we constructed a database of published raccoon gastrointestinal parasite surveys where we could estimate the proportion of surveyed raccoon populations from which each parasite species had been reported (Weinstein et al. In Press). Because species-level taxonomy remains uncertain for many helminths, and 28% of studies included at least one parasite identified only to genus, we distinguish core and satellite parasites at the genus level. Although this could result in multiple satellite species being grouped into a core genus, our classification was not sensitive to this approach. For raccoons, all core parasite genera (except Physaloptera) included only a single species and P. rara remained a core parasite even when all other Physaloptera spp. records were excluded from analysis.
We used this database of host-parasite records from 256 raccoon populations to determine raccoon core and satellite parasites. To incorporate studies that only surveyed a subset of the parasite community, we calculated the proportion of populations infected (hereafter referred to as range-wide prevalence) separately for each parasite by dividing the number of infected populations by the number of populations surveyed with methods that could detect that parasite. We inspected the histogram of range-wide prevalence and used the low point in the bimodal distribution to distinguish core and satellite taxa (Hanski 1982).
We examined intestinal parasites from 180 raccoons collected from 2012–2015 in southern Santa Barbara County, California. For each raccoon, we recorded collection location, sex, and age (see Weinstein 2016, Weinstein et al. In Press). After removing and opening the small and large intestine, we collected all visible nematodes and acanthocephalans. We then scraped gut contents from the mucosa and washed these scrapings into saline. We heat-killed parasites with near-boiling water and then washed intestinal contents with repeated sedimentation (Justine et al. 2012). We fixed a subset of parasites in 95% ethanol or 10% formalin and then preserved all remaining intestinal contents in 70% ethanol.
We sorted gut contents under a stereomicroscope to collect and count parasites. Because we did not examine the mouth, esophagus or stomach, our survey underestimates the presence of esophageal and stomach specialists like Capillaria procyonis and Physaloptera rara, respectively. We counted nematodes and acanthocephalans, but only estimated counts for cestodes (which were often fragmented) and trematodes (which were often too numerous for accurate counts). To identify parasites, we cleared and mounted nematodes and acanthocephalans in either 80% phenol or lactophenol. Cestodes and trematodes were stained with aqueous alum carmine, cleared in clove oil and mounted in Damar gum or Canada balsam. We calculated prevalence for each parasite species, estimating Bayesian credible intervals using the “prevalence” package in R (Devleesschauwer et al. 2014, R Core Team 2016).
In this area, raccoons forage on the beach, upland and near freshwater habitats, each of which provides distinct prey types and physical habitat characteristics consistent with standard ecotone definitions (Odum 1971, Kark 2013). To examine how proximity to aquatic ecotones influenced parasite communities, we georeferenced collection sites (n=179, excluding one animal with no location data) in Google Earth 7.1.8.3036 and then calculated their distance in meters to the nearest marine and fresh water boundary in QGIS 2.18.2 (Google Earth, 2017; QGIS Development Team, 2017). For each host, we measured the distance to nearest mapped freshwater (e.g. stream, river, marsh, lake) and then used the QGIS distance function to calculate the shortest distance to the coast (see map in Weinstein et al. In Press). Location was not a significant predictor of raccoon age for either freshwater or marine ecotones (correlation test, Kendall’s rank correlation (freshwater: tau = −0.05, p = 0.34; marine: tau = 0.08, p = 0.12). However, because individuals that were further away from the marine ecotone tended to be closer to freshwater ecotones (correlation test, Kendall’s rank correlation tau = −0.56, p < 0.001), the distance to the other ecotone was included in analyses. Although the sampling area included multiple terrestrial habitat types (i.e. urban/suburban, coastal scrub, grassland, oak woodland) that might also create ecotones, here we focus on aquatic/terrestrial ecotones because they allow for clear differentiation of habitat boundaries at a scale that is relevant to raccoon range size.
Using host, parasite, and spatial data, we tested whether ecotone proximity affected parasite richness. For each host, we calculated richness as the number of gut helminth taxa. To examine how core and satellite parasites differed in their response to marine and freshwater ecotones we used the range-wide raccoon parasite data to classify sampled parasites as either core or satellite species. Because parasite richness is constrained by the parasites present in the local community, we used binomial regression models to examine how distance influenced total, core and satellite richness. In these models, the response variable, parasite richness, was treated as the number of “successes” drawn from the population of all (or core, or satellite) raccoon parasites detected in the local community (Faraway 2016). The more flexible quasibinomial distribution was used to accommodate underdispersion in core and total richness models and overdispersion in satellite richness models. We ran these models using the “MASS” package in R 3.3.0 (Venables and Ripley 2002, R Core Team 2016), log10 transforming distances. We include host age and distance to both ecotones in all models but did not include sex or season as F-tests indicated that they did not significantly improve model fits. We also do not include sampling year in the models as 2012 (the first year) and 2015 (the last year) do not include a full calendar year of sampling.
In addition to examining the parasite community as a whole, for parasites found in at least 10% of raccoons in Santa Barbara county, we also examined how proximity to marine and fresh water influenced individual parasite presence using binomial generalized linear models (Venables and Ripley 2002, R Core Team 2016). For nematodes and acanthocephalans, we also examined how proximity to ecotones impacted parasite abundance using negative binomial generalized linear models. We log10 transformed distances and included host age and sex in these models. We selected models using backwards stepwise regression, comparing models with a chi-squared test (Appendix S1: Table S1).
RESULTS
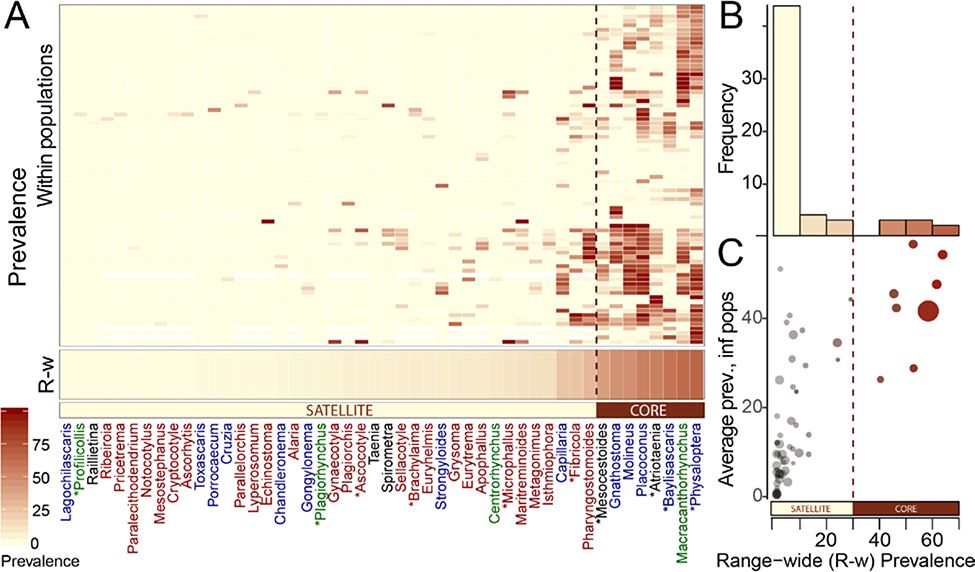
Across their range, raccoons host at least 100 gut helminth species from 65 genera. However, most raccoon parasites infect few raccoon populations (Figure 1A). The parasite community fit the bimodal frequency distribution assumed by the core-satellite hypothesis (Hanski 1982), with more than 80% of documented genera occurring in fewer than 25% of surveyed populations and only 8% occur in 50% of populations (Figure 1B). The most widespread parasites were also more prevalent in the populations they infected (Figure 1C). Based on the bimodal frequency distribution of parasite presence across the raccoon range, we conservatively defined core raccoon parasites as those present in at least 30% of surveyed populations.
Figure 1.
A. Heatmap of parasite prevalence, by taxa (x axis, ordered by increasing range-wide prevalence) across raccoon populations (y axis, ordered according to community similarity). For space constraints, the heatmap includes only parasites from the endemic raccoon range from studies that sampled at least 3 of the 4 parasite orders. Parasites detected in Santa Barbara raccoons are marked with asterisks and names are colored by order (Nematoda: blue, Acanthocephala: green, Cestoda: black, Trematoda: red). B. Histogram parasite frequency across raccoon populations. C. Scatter plot of parasite prevalence in occupied raccoon populations by range-wide prevalence. Point size is scaled to the number of surveyed populations. A dashed line indicates the 30% cut-off between core and satellite taxa.
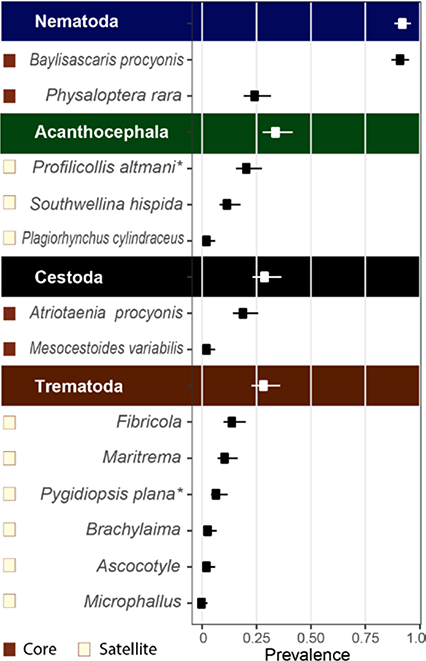
In Santa Barbara County, nearly all sampled raccoons (98%) hosted at least one intestinal helminth species (Figure 2), with some individuals hosting up to seven taxa (mean = 2.24, SD = 1.18). The most common parasites included the nematodes Baylisascaris procyonis and Physaloptera rara, acanthocephalan Profilicollis altmani, and cestode Atriotaenia procyonis. Of the 13 gastrointestinal helminths collected from Santa Barbara raccoons, four were core and nine were satellite species based on the range-wide data (Figure 2).
Figure 2.
Intestinal helminths from 180 raccoons in Santa Barbara County, California. Core parasites are marked with grey squares. Three adult Spirocamallanus sp. nematodes were also found in one raccoon; however, as these worms likely came from a recently eaten fish they were not included in analyses.*Parasites not previously reported in raccoons.
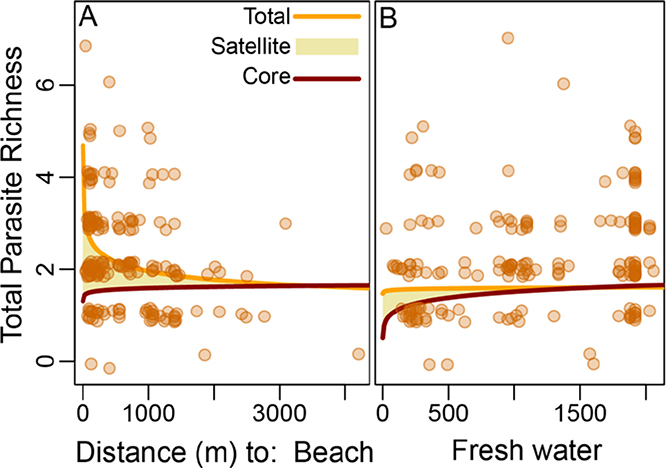
Ecotone proximity altered parasite community structure. Controlling for host age, raccoons nearer to the marine ecotone had more parasite species (Figure 3A, Table 1). This increase in parasite richness was due to a strong increase in satellite species. Nearer to the freshwater ecotone, core species declined and satellite species increased weakly, leading to no net change in parasite richness with distance from freshwater (Figure 3B, Table 1).
Figure 3.
Left panel (A). Total parasite richness in raccoons (orange line) decreases with distance from the beach ecotone (X axis), despite a slight increase in core parasites (red line). This ecotone effect is due to higher satellite parasite richness (yellow fill) near the beach ecotone. Right panel (B). Total parasite richness in raccoons does not change with distance from the freshwater ecotone, despite an increase in core parasites. This is due to higher satellite parasite richness cancelling the paucity of core parasites near the freshwater ecotone. Points represent measured total richness, lines represent predicted richness after controlling for age (set to the mean:13.7 months) and distance to the other ecotone (set to 2000 m for fresh water in the left panel, or 4000 m for marine in the right panel).
Table 1.
GLM regression results for modelsof raccoon intestinalparasite richness including coefficient estimate (β), standard error (SE), Wald’s Z score (Z value) and p-value. Note that the distance factors indicate how parasitism changes as one moves away from the ecotone.Significant factors are in bold.
| Factor | β | SE | Z value | P-value |
|---|---|---|---|---|
| Core Parasite Richness | ||||
| Log10(marine) | 0.101 | 0.164 | 0.614 | 0.540 |
| Age | 0.002 | 0.004 | 0.576 | 0.565 |
| Log10(freshwater) | 0.471 | 0.202 | 2.332 | 0.021 |
| Satellite Parasite Richness | ||||
| Log10(marine) | −1.117 | 0.273 | −4.097 | 6e-05 |
| Age | 0.015 | 0.006 | 2.665 | 0.008 |
| Log10(freshwater) | −0.518 | 0.341 | −1.517 | 0.131 |
| Total Parasite Richness | ||||
| Log10(marine) | −0.385 | 0.131 | −2.934 | 0.004 |
| Age | 0.008 | 0.003 | 2.973 | 0.003 |
| Log10(freshwater) | 0.030 | 0.164 | 0.183 | 0.855 |
Parasite species exhibited different responses to host and environmental factors (Table 2). All examined satellite parasites infected significantly more raccoons at either freshwater or marine ecotones. Fibricola infected more animals closer to freshwater and Profilicolis, Southwellina, and Maritrema infected more animals collected closer to the beach. In contrast, no examined core parasite (B. procyonis, P. rara, or A. procyonis), significantly increased in presence at an ecotone. Two satellite parasites (Profilicolis and Southwellina) and one core parasite (P. rara) were significantly more abundant at the marine ecotone, whereas proximity to an ecotone did not affect abundance of the core parasite B. procyonis (Appendix S1: Table S1).
Table 2.
GLM regression results for the best models for parasitepresence, by parasite, for the 7 taxa found in at least 10% of surveyed raccoons in Santa Barbara County, California. Results include the coefficient estimate, standard error (SE), Wald's Z score (Z value) and p-value. Significant factors are in bold.
| Factor | Estimate | SE | Z value | P-value |
|---|---|---|---|---|
| Core Parasites | ||||
| Baylisascarisprocyonis | ||||
| No significant predictors | ||||
| Physalopterarara | ||||
| Log10(fresh water) | 0.944 | 0.49 | 1.91 | 0.056 |
| Atriotaeniaprocyonis | ||||
| Age | 0.030 | 0.01 | 2.67 | 0.008 |
| Log10(fresh water) | 1.27 | 0.60 | 2.14 | 0.032 |
| Satellite Parasites | ||||
| Fibricolasp. | ||||
| Age | 0.06 | 0.01 | 4.35 | <0.0001 |
| Log10(fresh water) | −1.95 | 0.57 | −3.41 | 0.0007 |
| Maritrema sp. | ||||
| Log10(marine) | −1.48 | 0.56 | −2.65 | 0.008 |
| Profilicolisaltmani | ||||
| Log10(marine) | −2.28 | 0.49 | −4.64 | <0.0001 |
| Southwellinahispida | ||||
| Log10(marine) | −1.28 | 0.53 | −2.41 | 0.016 |
DISCUSSION
Like free-living species, parasites can be divided into core and satellite species, each with distinct but predictable responses to ecotones. Both marine and freshwater ecotones increased the proportion of satellite parasites in the raccoon infracommunity, suggesting that the link between ecotones and altered community composition extends to infectious agents. However, as with free-living species, parasite responses varied by taxa and habitat (Kark and van Rensburg 2006). In particular, the balance between more satellite and fewer core parasites differed between marine and freshwater ecotones. Satellite parasites increased at both ecotones, however, as freshwater ecotones had fewer core parasites, only marine ecotones increased in total richness.
Core parasites were relatively insensitive to ecotones. These globally common parasites were also among the most common parasites in this California raccoon population. Core parasites were present in raccoons throughout the sampling area and did not increase at ecotones. If anything, their presence declined with ecotone proximity. Although novel satellite parasites could out-compete core parasites, we suspect that observed declines in core parasites were not due to competition or reduced host density, but rather to host diet shifts (Valtonen et al. 2010). For example, a core cestode, Atriotaenia procyonis was more common in older raccoons and away from fresh water, perhaps due to these raccoons foraging more on the terrestrial insects that serve as intermediate hosts for this parasite (Gallati 1959). A similar spatial pattern also occurred for P. rara, which also requires terrestrial insects as intermediate hosts (Anderson 2000). Time spent foraging in ecotones likely reduces exposure to some parasites; however, most animals near ecotones also probably spent time in habitats where core parasites are transmitted.
Satellite richness increased at ecotones, with observed differences between marine and freshwater boundaries likely due to parasite life cycles and host diets. Although gut contents from our specimens were not amenable to diet analysis, available prey types differ dramatically across the ecotone at this site (J. P. McLaughlin unpublished food web studies). The marine-associated satellite species likely come from mollusks, crustaceans and fish that raccoons encounter only at the beach (Ching 1963, Nickol et al. 1999, García-Varela et al. 2012). As these parasites typically mature in birds, raccoons are probably dead-end hosts. For example, although acanthocephalans like P. altmani contribute to parasite richness, these worms cannot complete their life cycle in raccoons (and may cause mortality (Kreuder et al. 2003)). Other parasites, such as Maritrema sp., do mature in raccoons; however, raccoons likely act as sinks when they deposit parasite eggs away from where the life cycle is completed (but see Lafferty and Dunham 2005). Although ecotones are sinks for some parasites, the transition between aquatic and terrestrial habitats is an obligate part of other parasite life cycles. The satellite species Fibricola sp., for example, begins its lifecycle in an aquatic host, but matures in terrestrial mammals like raccoons (Cole and Shoop 1987). For free-living species, there has been debate as to whether increased diversity at ecotones is due to addition of ecotonal specialists or species spill-over from adjacent habitats (Kark and van Rensburg 2006); for parasites we see evidence of both processes.
Ecotones might affect parasite communities differently from free-living communities. For free-living communities, habitat heterogeneity derived from ecotones increases free-living species richness (Tews et al. 2004). For parasites, the main habitat is the host, which remains constant across the ecotone. However, ecotones alter food webs, and the observed parasite community changes suggest that moving across habitats alters host diets (Valtonen et al 2010). To that end, we suspect that while habitat differences across ecotones primarily affect differences in free-living communities, food-web differences across ecotones will have a greater effect on parasite communities.
Ecotones promote novel species interactions (Kark and van Rensburg 2006), and increased exposure to rare parasites at these sites has implications for parasite distributions, human health, and wildlife conservation. These habitat boundaries likely facilitate host-switching and ecotones may play an important role in generating novel host-parasite associations. While some new host-parasite interactions are benign, novel infectious agents often cause high pathology (Daszak et al. 2000). Many human emerging infectious diseases, including Lyme disease, Nipah virus, and yellow fever, are associated with ecotones (Despommier et al. 2006). For wildlife, similar patterns of disease spillover maybe widespread, but under examined. Natural ecotones play a critical role in parasite diversity; however, as natural habitats become increasingly fragmented and bounded by human modified environments, altered parasite communities at these boundaries could have important impacts on human and wildlife health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank C. Lake, M. Luo, R. Lee, S. Hannah, E. Yee, G. Dunn, E. Lum, T. Grigsby, J. Mendez, A. Tokuyama, M. Armijo, K. McKee, and J. Rydz for research assistance and M. Wilber, R. Crego, J. Buck, A. M. Kuris and H. Young for discussions and feedback. We also thank the UC Santa Barbara Coal Oil Point Reserve, Animal & Insect Pest Management, Inc., Santa Barbara City Animal Control, and the USDA for facilitating research and providing specimens. Specimens are archived at Santa Barbara Museum of Natural History (catalog numbers 9337–9522). Animal use was in accordance with a UCSB IACUC protocol and CA DFG permit #11188. Funding was provided by NSF grant numbers 1144085 and 1601362. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US Government.
LITERATURE CITED
- Anderson RC 2000. Nematode Parasites of Vertebrates: Their Development and Transmission. 2nd edition. CAB International, Wallingford, UK. [Google Scholar]
- Bafundo KW, Wilhelm WE, and Kennedy ML. 1980. Geographic variation in helminth parasites from the digestive tract of Tennessee raccoons, Procyon lotor. The Journal of Parasitology 66:134–139. [PubMed] [Google Scholar]
- Bush AO, and Holmes JC. 1986. Intestinal helminths of lesser scaup ducks: patterns of association. Canadian Journal of Zoology 64:132–141. [Google Scholar]
- Ching HL 1963. The description and life cycle of Maritrema laricola sp. N. (Trematoda: Microphallidae. Canadian Journal of Zoology 41:881–888. [Google Scholar]
- Cole RA, and Shoop WL. 1987. Helminths of the raccoon (Procyon lotor) in Western Kentucky. The Journal of Parasitology 73:762–768. [PubMed] [Google Scholar]
- Combes C 2004. Parasitism: The ecology and evolution of intimate interactions. University of Chicago Press, Chicago. [Google Scholar]
- Daszak P, Cunningham AA, and Hyatt AD. 2000. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 287:443–449. [DOI] [PubMed] [Google Scholar]
- Despommier D, Ellis BR, and Wilcox BA. 2006. The role of ecotones in emerging infectious diseases. EcoHealth 3:281–289. [Google Scholar]
- Devleesschauwer B, Torgerson P, Charlier J, Bruno L, Praet N, Roelandt S, Smit S, Dorny P, Berkvens D, and Speybroeck N. 2014. prevalence: Tools for prevalence assessment studies.
- Faraway JJ 2016. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. CRC Press. [Google Scholar]
- Gallati WW 1959. Life history, morphology and taxonomy of Atriotaenia (Ershovia) procyonis (Cestoda: Linstowiidae), a parasite of the raccoon. The Journal of Parasitology 45:363–377. [PubMed] [Google Scholar]
- García-Varela M, Aznar F, Pérez-Rodríguez R, and León G. 2012. Genetic and morphological characterization of Southwellina hispida Van Cleave, 1925 (Acanthocephala: Polymorphidae), a parasite of fish-eating birds. Comparative Parasitology 79:192–201. [Google Scholar]
- Hanski I 1982. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 38:210–221. [Google Scholar]
- Harkema R, and Miller GC. 1964. Helminth parasites of the raccoon, Procyon lotor in the southeastern United States. The Journal of Parasitology 50:60–66. [PubMed] [Google Scholar]
- Hechinger RF, and Lafferty KD. 2005. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proceedings of the Royal Society of London B: Biological Sciences 272:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerhartz SM, Dethier MN, Toft JD, Cordell JR, and Ogston AS. 2014. Effects of shoreline armoring on beach wrack subsidies to the nearshore ecotone in an estuarine fjord. Estuaries and Coasts 37:1256–1268. [Google Scholar]
- Hoberg EP, and McGee SG. 1982. Helminth parasitism in raccoons, Procyon lotor hirtus Nelson and Goldman, in Saskatchewan. Canadian Journal of Zoology 60:53–57. [Google Scholar]
- Howell CA, Dijak WD, and Thompson FR. 2007. Landscape context and selection for forest edge by breeding Brown-headed Cowbirds. Landscape Ecology 22:273–284. [Google Scholar]
- Justine J-L, Briand MJ, and Bray RA. 2012. A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitol Res 111:341–351. [DOI] [PubMed] [Google Scholar]
- Kark S 2013. Ecotones and Ecological Gradients Pages 147–160 in Meyers RA, editor. Encyclopedia of Sustainability Science andTechnology. Springer, New York. [Google Scholar]
- Kark S, and van Rensburg BJ. 2006. Ecotones: Marginal or central areas of transition? Israel Journal of Ecology & Evolution 52:29–53. [Google Scholar]
- Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, Carpenter TE, Conrad PA, and Mazet JAK. 2003. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. Journal of Wildlife Diseases 39:495–509. [DOI] [PubMed] [Google Scholar]
- Kunin WE 1998. Biodiversity at the edge: a test of the importance of spatial “mass effects” in the Rothamsted Park Grass experiments. Proceedings of the National Academy of Sciences 95:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, and Dunham EJ. 2005. Trematodes in snails near raccoon latrines suggest a final host role for this mammal in california salt marshes. The Journal of Parasitology 91:474–476. [DOI] [PubMed] [Google Scholar]
- Lanfranchi AL, Braicovich PE, Cantatore DMP, Alarcos AJ, Luque JL, and Timi JT. 2016. Ecotonal marine regions – ecotonal parasite communities: helminth assemblages in the convergence of masses of water in the southwestern Atlantic Ocean. Int J Parasitol 46:809–818. [DOI] [PubMed] [Google Scholar]
- Nickol BB, Crompton DWT, and Searle DW. 1999. Reintroduction of Profilicollis Meyer, 1931, as a genus in Acanthocephala: Significance of the intermediate host. The Journal of Parasitology 85:716–718. [PubMed] [Google Scholar]
- Odum EP 1971. Fundamentals of Ecology. 3rd edition. W.B. Saunders Company, Philadelphia, Pennsylvania, USA. [Google Scholar]
- R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Samson A, Dubay SA, Huspeni TC, and Cyr A. 2012. Influence of environmental variables on Baylisascaris procyonis infection in raccoons. The Journal of Parasitology 98:1279–1282. [DOI] [PubMed] [Google Scholar]
- Svobodova M, Voøíšek P, Votypka J, and Weidinger K. 2004. Heteroxenous coccidia (Apicomplexa: Sarcocystidae) in the populations of their final and intermediate hosts: European buzzard and small mammals. Acta protozoologica 43:251–260. [Google Scholar]
- Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, and Jeltsch F. 2004. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31:79–92. [Google Scholar]
- Valtonen ET, Marcogliese DJ, and Julkunen M. 2010. Vertebrate diets derived from trophically transmitted fish parasites in the Bothnian Bay. Oecologia 162:139–152. [DOI] [PubMed] [Google Scholar]
- Venables WN, and Ripley BD. 2002. Modern Applied Statistics with S. Springer, New York. [Google Scholar]
- Weinstein SB 2016. Baylisascaris procyonis demography and egg production in a California raccoon population. The Journal of Parasitology 102:622–628. [DOI] [PubMed] [Google Scholar]
- Weinstein SB, Van Wert JC, Kinsella M, Tkach VV, and Lafferty KD. In Press Southern California and range-wide raccoon gastrointestinal helminth database. Ecology xx:exxxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Schiemer F, and Thorpe J. 2001. Fish and land-inland water ecotones - Overview and synthesis. Ecohydrology and Hydrobiology 1:261–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.