Abstract
Neurotensin (NTS) is a neuropeptide neurotransmitter expressed in the central and peripheral nervous systems. Many studies over the years have revealed a number of roles for this neuropeptide in body temperature regulation, feeding, analgesia, ethanol sensitivity, psychosis, substance use, and pain. This review will provide a general survey of the role of neurotensin with a focus on modalities that we believe to be particularly relevant to the study of reward. We will focus on NTS signaling in the ventral tegmental area, nucleus accumbens, lateral hypothalamus, bed nucleus of the stria terminalis, and central amygdala. Studies on the role of NTS outside of the ventral tegmental area are still in their relative infancy, yet they reveal a complex role for neurotensinergic signaling in reward-related behaviors that merits further study.
1. Introduction
NTS is a 13-amino acid neuropeptide that was originally isolated from bovine hypothalami (Carraway and Leeman, 1973). It is expressed in the central and peripheral nervous systems as well as in the gastrointestinal tract. Early work investigating this neuropeptide found NTS-like immunoreactivity in the VTA (Uhl et al., 1977), an area implicated in reward processing, thus making NTS a promising target for reward modulation. Many studies over the years have revealed a variety of roles for this neuropeptide in body temperature regulation, feeding, analgesia, ethanol sensitivity, psychosis, substance use, and pain (Tyler-McMahon et al., 2000; Kinkead, 2002; Dobner, 2005, 2006; Ferraro et al., 2016; Schroeder and Leinninger, 2018; Tschumi and Beckstead, 2019). As may be expected from the breath of these phenotypes, NTS-containing cells have been identified in a number of different brain areas (Schroeder et al., 2019). While most of the work investigating the role of NTS in reward has historically focused on the VTA, the field has now expanded into additional brain regions that are crucial regulators of emotional valence and reward.
This review will provide a general survey of the role of neurotensin with a focus on modalities that we believe to be particularly relevant to the study of reward. More specifically, it will concentrate on rodent studies, as that is where the bulk of region-specific work has been performed. We will summarize work in brain regions where NTS signaling has been linked to reward, as well as regions that are traditionally linked to reward but where the study of NTS has focused on other aspects of behavior not specifically linked to reward, for example most of the work investigating NTS signaling in the nucleus accumbens has focused on its implications in schizophrenia.
We will discuss work that has examined NTS inputs to the VTA, as well as the role of NTS signaling in promoting dopamine release within this structure. More specifically, we will discuss two neurotensinergic projections to the VTA that have been shown to modulate reward-related behaviors: the medial preoptic area (mPOA), and the lateral hypothalamus (LH). The VTA exerts many of its effects on reward behavior through its dopaminergic projection to the nucleus accumbens (NA). NTS has been extensively studied in the nucleus accumbens (NA) with regard to dopamine release, including a particular interest in how NTS may impact dopaminergic signaling in schizophrenia. Additionally, we will discuss evidence supporting accumbal NTS involvement in both the stimulant effects of amphetamines, as well as adaptations following long-term antipsychotic regimens. In the LH, NTS signaling has been linked to important homeostatic mechanisms such as metabolic control, feeding behavior, and sleep. The bed nucleus of the stria terminalis (BNST) does not send a neurotensinergic projection to the VTA, but there is mounting evidence that NTS from the BNST may traffic retrogradely to the VTA, though the functional significance of this mechanism is unclear. In the BNST itself, neurotensinergic signaling appears to play a role in the intersection of drug intake and stress, and particularly in neuroadaptations following chronic stress or drug use. Lastly, in the central amygdala (CeA), NTS-containing cells (NTSCeA) and neurotensinergic signaling appear to promote positive valence, and also serve to improve performance on a variety of memory-related tasks.
2. Receptor Pharmacology
Neurotensin binds to four known receptors: the GPCRs NTSR1 and NTSR2 (Vincent et al., 1999), and the type 1 receptors Sortilin (Sort1) and SorLA (Sorl1; Jacobsen et al., 2001). More is known about the roles of NTSR1 and NTSR2 in behavior, as they were the first to be identified. These were originally referred to as the ‘high affinity’ (NTSR1) and ‘low affinity’ (NTSR2) NTS receptors respectively. These were also differentiated by their sensitivity to the antihistamine levocabastine, which competes for NTS binding to NTSR2 but not NTSR1 (Chalon et al., 1996; Schotte et al., 1986), and so are referred to as the as the levocabastine-sensitive (NTSR2) and insensitive (NTSR1) receptor in some of the literature. The cellular distribution of these receptors in the brain is contentious, but it appears that NTSR1 is expressed predominantly in neurons and NTSR2 abundantly expressed in glia, and weakly in neurons (Woodworth et al., 2018b; Yamauchi et al., 2007). NTSR1 is found on dendrites, soma, and presynaptic terminals (Boudin et al., 1998; Pickel et al., 2001). A number of important tools have been developed for manipulation of neurotensinergic signaling. NTS does not cross the blood-brain barrier, but a brain-penetrant NTSR1 agonist (PD 149163) has been developed (Wustrow et al., 1995). This truncated form of NTS allows for manipulation of NTSR1 signaling in the brain from peripheral injections. A nonpeptide NTSR1 antagonist has also been developed (SR 48692; Gully et al., 1993), which is orally bioavailable.
It is surprisingly unclear how these receptors signal in vivo, given the amount of work that has gone into outlining downstream signaling pathways in this system. This is largely due to conflicting evidence from different cell types, expression vectors, and brain regions. Recent work systematically exploring NTSR1 signaling has shown that this receptor is capable of signaling through various excitatory and inhibitory G alpha subunits (Besserer-Offroy et al., 2017) as well as dimerizing, which does not appear to be required for G-protein function (White et al., 2007). NTS-bound NTSR1 is also rapidly internalized (for review see Hermans and Maloteaux, 1998), a source of rapid desensitization that may also impact the signaling dynamics of NTSR1. Furthermore, co-expression of NTSR1 and NTSR2 in culture has shown that heterodimerization of these two receptors results in sequestration of NTSR1 and subsequent loss of signaling (Hwang et al., 2010). Lastly, there is evidence that NTSR1 may form heterodimers with the dopamine D2 receptor (D2R) which would also impact NTSR1 signaling (Borroto-Escuela et al., 2013). The complexity revealed by all of these different studies suggests that it would be difficult to translate findings from in vitro experiments to in vivo signaling, or even findings from one brain region to another. The data on the possible transduction mechanisms of NTSR2 and NTSR3 are equally as conflicting (Mazella and Vincent, 2006). See Tschumi and Beckstead, 2019 for a detailed summary of intracellular signaling and overall action of NTS-ergic signaling in different brain regions. Table 1 provides a summary of the pharmacological experiments cited in this review that probe the neurotensinergic system. Table 2 provides a short summary of manipulations cited here that result in changes in Nts mRNA expression or NTS tissue content.
Table 1.
Summary of experiments cited in this review that manipulate neurotensin and its receptors organized by injection method, target, drug/peptide, outcome, and species/sex. NTS: neurotensin. NTS 8–13: Neurotensin fragment aminoacids 8–13. SR48692: non-peptide NTSR1 antagonist. SR142948: non-peptide NTS receptor antagonist. JMV431: degradation-resistant NTS analog, NTSR2 agonist. PD14963: NTSR1 agonist. Quinpirole: D2 and D3 receptor agonist.
| Injection method/target | Drug/peptide | Outcome | Species/Sex |
|---|---|---|---|
| icv | NTS | ↓ feeding | Fasted Male Sprague-Dawley rats (Luttinger et al., 1982) |
| Fasted Male Wistar rats (Cooke et al., 2012) | |||
| NTS | ↑ Fos in dBNST | High maternal aggression female mice on Hsd:ICR background (Gammie et al., 2009) | |
| ↓ maternal aggression | |||
| SR48692 | ↑ maternal aggression | High maternal aggression female mice on Hsd:ICR background (Gammie et al., 2009) | |
| SR142948 | ↓ wakefulness during light phase | Male C57BL/6J mice (Furutani et al., 2013) | |
| NTS, JMV431, PD149163 | ↓ USVs in response to fear context | Male Wistar rats (Steele et al., 2017) | |
| s.c. | PD149163 | ↓ fear-potentiated startle response | Male Sprague Dawley rats (Shilling and Feifel, 2008) |
| PD149163 | ↓ USVs in response to fear context only if acute, not repeated |
Male Wistar rats (Prus et al., 2014) | |
| i.p. | NTS | ↓ feeding | Fasted male C57BL/6 mice (Cooke et al., 2012; Ratner et al., 2016) |
| Ad lib fed male C57BL/6 mice (Cooke et al., 2012) | |||
| Male Sprague Dawley rats (Ratner et al., 2016) | |||
| SR48692 | ↓ intestinal lipid absorption | Male C57BL/6 mice (Li et al., 2016) | |
| SR48692 | ↓cocaine-induced locomotion | Male Sprague Dawley rats (Felszeghy et al., 2007) | |
| ↓cocaine CPP when administered during withdrawal | |||
| PD149163 | ↑ paired-pulse inhibition | Male C57BL/6J mice (Vadnie et al., 2016) | |
| ↓ startle magnitude | |||
| quinpirole | ↑ NTS in the lateral caudate | Male Sprague-Dawley rats (Wagstaff et al., 1996) | |
| ↑ NTS in the NA | |||
| VTA | NTS | Supports conditioned place preference | Female Sprague-Dawley rats (Glimcher et al., 1984) |
| NTS | Supports lever-pressing for microinfusions | Sprague-Dawley rats (Glimcher et al., 1987) | |
| NTS | dopamine release in caudal NA | Male Sprague-Dawley rats (Kalivas et al., 1983; Sotty et al., 1998; Sotty et al., 2000) | |
| Male mice crossed onto C57BL/6 (Leonetti et al., 2004) | |||
| NTS (bath application in slice) | ↑ EPSCs in dopaminergic and non-dopaminergic VTA neurons | Long Evans pups (Bose et al., 2015) | |
| NTS 8–13 (bath application in slice) | ↑ EPSCs in VTA neurons (10 nM, can be blocked by 100 nM SR48692) | Male C57BL/6J mice (Kempadoo et al., 2013) | |
| ↓ EPSCs in VTA neurons (100 nM) | |||
| SR142948 | ↓ methamphetamine intake | Male DBA mice (Dominguez-Lopez et al., 2018) | |
| SR48692 | ↓ pressing for optical stimulation of LH→VTA pathway | Male C57BL/6J mice (Kempadoo et al., 2013) | |
| NA | NTS 8–13 (bath application in slice) | Enhanced evoked DA release in the NA following pulse trains | 4–6 week old Sprague Dawley rats (Fawaz et al., 2009) |
| NTS | ↓ Amphetamine-induced locomotion | Male Sprague Dawley rats (Feifel et al., 1997) | |
| NTS | Normalizes PPI following amphetamine (dose-rependent) |
Male Sprague Dawley rats (Feifel et al., 1997) | |
| NTS | Blocked psychostimulant effects of cocaine | Male Wistar rats (Robledo et al., 1993) | |
| = cocaine self-administration | |||
| NTS | Attenuated dopamine supersensitivity | Male Sprague Dawley rats (Servonnet et al., 2017) | |
| BNST | SR142984 | Normalizes open arm time in chronically-stressed rats | Male Wistar rats (Normandeau et al., 2018b) |
| NTS (bath application in slice) | ↑ evoked GABAA-IPSC | Male Long Evans rats (Krawczyk et al., 2013) | |
| SR14298 (bath application in slice) | Blunts D1-mediated GABA LTP | Cocaine maintenance male Long Evans rats (Krawczyk et al., 2013) | |
| CeA | NTS | Supports conditioned place preference | Male Wistar rats (László et al., 2018; László et al., 2010a) Blocked by pre-administration of SR48692 (László et al., 2010a) |
| NTS | = Elevated plus maze | Male Wistar rats (László et al., 2010a) | |
| NTS | Improved escape latency in Morris Water Maze | Male Wistar rats (László et al., 2010b) | |
| NTS | Improved escape performance in passive avoidance test | Male Wistar rats (László et al., 2012) | |
| SR48692 | No effect on conditioned place preference | Male Wistar rats (László et al., 2010a) | |
| pPVT | NTS | ↑ Fos in pPVT | Male Long Evans rats (Pandey et al., 2019) |
| ↓ drinking in high-drinking animals | |||
| ↓ rearing in a novel chamber | |||
| aPVT | NTS | ↑ Fos in aPVT, mPVT, pPVT | Male Long Evans rats (Pandey et al., 2019) |
| Striatum | NTS | ↑ TH mRNA in the ipsilateral substantia nigra | Male Sprague Dawley rats (Burgevin et al., 1992) |
| LH | NTS | No effect on feeding | Male Sprague Dawley rats (Hawkins et al., 1986) |
Table 2.
Summary of experiments cited in this review examining changes in Nts gene expression and NTS protein across manipulations. Nucleus accumbens (NA), bed nucleus of the stria terminalis (BNST), oval nucleus of the BNST (ovBNST), central nucleus of the amygdala (CeA), lateral hypothalamus (LH), medial preoptic area (mPOA).
| Behavioral manipulation | Outcome | Species/Sex |
|---|---|---|
| Cocaine (i.p.) | ↑ Nts mRNA in the NA shell | Chronic cocaine: C57BL/6J × 129Sv crossed mice (Betancur et al., 2001) |
| Wistar rats (Betancur et al., 1997) | ||
| Acute cocaine: C57BL/6J × 129Sv crossed mice (Betancur et al., 2001) | ||
| Chronic haloperidol | ↑ NTS tissue content in NA shell | Male Sprague Dawley rats (Kinkead et al., 2000) |
| Female Sprague Dawley rats (See et al., 1995) | ||
| Intermittent haloperidol | ↑ Nts mRNA in striatum (dorsomedial, dorsolateral, and ventrolateral) | Male Sprague Dawley rats (Servonnet et al., 2017) |
| Postpartum state | ↑ Nts mRNA in the BNST | Female outbred hsd:ICR mice (Driessen et al., 2014) |
| Chronic unpredictable stress | ↓ Ntsr1 mRNA in the BNST | Male Wistar and Long Evans rats (Normandeau et al., 2018b) |
| Hypertonic saline ingestion (Dehydration) | ↓Nts mRNA in the CeA | Male Sprague-Dawley rats (Watts et al., 1995) |
| ↑ Nts mRNA in the LH | ||
| = Nts mRNA in the ovBNST | ||
| High estrogen | ↑ Nts mRNA in the mPOA | Female C57BL/6 mice (exogenous estrogen; Watters and Dorsa, 1998) |
| Female Sprague Dawley rats (naturally-cycling proestrus phase; Smith and Wise, 2001). |
3. Circuitry
VTA
Neurotensinergic signaling in the VTA has been of interest since the discovery of NTS immunoreactivity in the VTA of rats and monkeys (Uhl et al., 1977; Kataoka et al., 1979; Uhl et al., 1979). Evidence of NTS receptors on dopamine neurons specifically, was reported as early as 1981 (Palacios and Kuhar, 1981), and has been validated by subsequent studies (Szigethy and Beaudet, 1989). This has made NTS receptors a promising target for the manipulation of dopaminergic VTA neurons and subsequently, the modulation of reward-related behavior.
Neurotensinergic signaling is rewarding in the VTA
It has been shown that rats will demonstrate conditioned place preference following NTS injections into the VTA (Glimcher et al., 1984), and that they will also lever-press for microinfusions of NTS into the VTA (Glimcher et al., 1987). Additionally, mice will nose-poke for optical stimulation of pathway specific inputs to the VTA (Kempadoo et al., 2013). Infusion of NTS into the VTA induces downstream dopamine efflux into the caudal NA (Kalivas et al., 1983; Leonetti et al., 2004; Sotty et al., 2000, 1998), mainly driven by NTSR-1 (Leonetti et al., 2004). As dopamine release in the caudal NA is implicated in many aspects of reinforcement, motivation, and reward (Wise, 2004; for a contrasting view see Salamone et al., 2005), NTS signaling in the VTA is well poised to exert influence over these processes. Furthermore, neurotensinergic inputs to the VTA are well situated to regulate an important output of this crucial reward node. In fact, it was recently shown that intra-VTA antagonism of neurotensinergic signaling using SR142948 reduced operant methamphetamine intake in male DBA mice, a phenomenon that is well-established to be mediated by dopamine in the NA (Dominguez-Lopez et al., 2018). This suggests that intra-VTA NTS can indeed impact reward-related behaviors, although whether this effect is mediated through downstream dopamine release in the NA has yet to be explicitly studied.
Neurotensinergic projections to the VTA
Reported sources of NTS input to the VTA vary between mice and rats. The main sources of neurotensinergic input to the VTA in rats appear to be the lateral and preoptic areas of the hypothalamus (LH and lPOA; Zahm et al., 2001). Work in mouse has revealed that the medial and lateral preoptic areas (mPOA, lPOA), LH, and NA shell send significant neurotensinergic input to the VTA (Woodworth et al., 2018a). The CeA, zona incerta, and peraquedictal gray also send neurotensinergic projections to the VTA in mouse, albeit less abundantly. While mice and rats both express NTS in the lateral septum, dorsal raphe and endopiriform nucleus, these neurons appear to project to the VTA only in rats (Geisler and Zahm, 2006). In general, the lateral septum and endopiriform nucleus project to the VTA more strongly in rats (Yetnikoff et al., 2015) than in mice (Faget et al., 2016), so the lack of VTA projecting NTS neurons from these regions may not be particularly surprising. It has been reported that dopaminergic neurons in the VTA of rats express NTS (Studler et al., 1988) and these NTS-containing VTA neurons (NTSVTA) do indeed project to the NA (Kalivas and Miller, 1984); however this co-expression has not been observed in mice (Woodworth et al., 2018a). In fact, there appear to be very few NTSVTA neurons at all in mice. These species differences indicate that NTS may perform different roles in mice and rats, and generalizations across rodents should be made cautiously. Interestingly, it has been shown that the Nts mRNA distribution in primates is closer to that of mice than that of rats (Smits et al., 2004). Additional work should explore the relative strength of NTS input to the VTA in primates to further that comparison.
NTSmPOA->VTA signaling is rewarding and promotes social interaction
The NTS-containing GABAergic projection from the mPOA to the VTA (NTSmPOA->VTA) has been identified as an important mediator of social reward in mice (McHenry et al., 2017). Stimulation of NTS neurons in the mPOA (NTSmPOA) is reinforcing in both sexes, and estrogen magnifies the reinforcing properties of the NTSmPOA->VTA circuit by enhancing the neuronal excitability of NTSmPOA neurons, many of which express the estrogen receptor (Herbison and Theodosis, 1991; McHenry et al., 2017). In vivo optogenetic manipulation of this circuit leads to bidirectional modulation of social interaction with an opposite sex conspecific or simply the odor of the conspecific (McHenry et al., 2017). Additionally, the magnitude of reinforcement induced by optogenetic stimulation of the NTSmPOA->VTA projection is dynamically modulated in naturally cycling mice, with a greater response in the high estrogen phases. It has been previously shown that estrogen induces an increase in Nts mRNA expression in the mPOA whether administered exogenously (Watters and Dorsa, 1998), or naturally during the proestrus phase (Smith and Wise, 2001). This suggests that the NTS in NTSmPOA neurons can dynamically modulate the rewarding value of social interaction. Many of the NTSmPOA neurons that were investigated in this paper failed to respond to these opposite sex odors, which is a common result in most calcium imaging/multiunit recording experiments. However, this leaves a number of NTSmPOA neurons which did not respond to opposite-sex mouse odor but may respond to other social cues and/or may be involved in other social behaviors such as parenting, grooming, nesting, or huddling. Indeed, NtsmPOA neurons overlap with the galanin-containing (GalmPOA) population (McHenry et al., 2017) that has been found to govern parental behaviors (Wu et al., 2014), suggesting that NTSmPOA neurons may be implicated in similar behaviors. Different GalmPOA projections to the peraqueductal gray (PAG), medial amygdala (MeA) and VTA govern various aspects of parental behaviors (Kohl et al., 2018). NTSmPOA projections to these other brain regions may also contribute to parental behaviors in response to sensory and hormonal cues. Studies investigating these other projections may further illuminate a role for NTS in a wider repertoire of social behaviors.
NTSLH→VTA signaling is rewarding and promotes consumption
The LH to VTA projection is heterogeneous (Godfrey and Borgland, 2019) and has been linked to reward (Kempadoo et al., 2013), feeding (Maracle et al., 2019), social interaction (Nieh et al., 2016), and object investigation (Nieh et al., 2016). For a review of the LH → VTA projection see Tyree and de Lecea, 2017. C57BL/6J NTS-Cre mice will nose-poke for optical stimulation of LH terminals in the VTA, and NTSR1 blockade blunts this behavior (Kempadoo et al., 2013). Interestingly, stimulation of NTS-containing LH neurons (NTSLH) supports conditioned place preference in NTSR1 global knockout mice (NTSR1−/−), but not those with intact NTSR1 (Woodworth et al., 2017b) suggesting that NTS may interact with other neurotransmitter systems within this circuit to mediate these effects. 30% of NTSLH neurons express LepRb (the long form leptin receptor; Leinninger et al., 2011), and stimulation of the GABAergic LepRbLH→VTA projection promotes consumption and motivation for food and water (Schiffino et al., 2019). Importantly, while these animals were trained under food or water restriction they were sated during the experiment. About 60% of LepRbLH neurons are co-localized with NTS-Cre (Leinninger et al., 2011), raising the possibility that NTS could modulate the rewarding properties of this projection. We discuss the behavioral role of these NTSLH neurons further in the LH-specific section below.
BNST as a non-traditional NTS input
Lastly, the BNST was originally hypothesized to be the major source of neurotensinergic signaling in the VTA, but this has not been borne out by tracing studies (Woodworth et al., 2018a; Zahm et al., 2001). There is, however, evidence of retrograde axonal transport of intact neurotensin from striatal terminals into the VTA through a mechanism involving, at least partly, NTSR1 (Castel et al., 1989, 1991; Steinberg et al., 1994). It has been hypothesized that NTS, possibly in combination with one of its receptors, may impact gene expression by being trafficked into the nucleus (Castel et al., 1994). Indeed, injection of NTS into the striatum resulted in upregulation of tyrosine hydroxylase mRNA in the ipsilateral substantia nigra (Burgevin et al., 1992), an effect which could serve to upregulate dopamine in this important reward node. Given the substantial role of dopamine in the substantia nigra, this suggests a functional role for this retrogradely-trafficked NTS. Future work examining the site of action of this retrogradely-trafficked NTS would be particularly illuminating, as NTSR-1 has been observed at the nuclear membrane (Pickel et al., 2001) and in association with nuclear pores (Delle Donne et al., 2004). Furthermore, NTSR1 contains a nuclear localization signal, which is necessary for the transport of such a large protein to the nucleus (Laduron, 1992). Future studies should examine a behavioral role for this nonconventional neurotensinergic VTA input, as well as behavioral and pharmacological modulators of this retrograde transport.
Mechanisms of neurotensinergic signaling in the VTA
Multiple mechanisms have been suggested as mediators of fast NTS signaling in the VTA, all or some of which may occur in the same neurons. NTS is thought to act on the VTA largely through volume transmission as opposed to synaptic transmission, as few NTS+ terminals in the VTA demonstrate synaptic specialization (Woulfe and Beaudet, 1989). There is some evidence in Long Evans rats that NTS increases glutamatergic excitatory post-synaptic currents (EPSCs) in dopaminergic and non-dopaminergic VTA neurons through both NTSR1 and non-NTSR1 mechanisms (Bose et al., 2015). In C57BL/6 mice, relatively low concentrations of NTS (10nM) increases NMDA-mediated EPSCs through an NTSR1-related mechanism, whereas higher concentrations (100nM) decrease NMDA-mediated EPSCs through a non-NTSR1 mechanism (Kempadoo et al., 2013). There is also mounting evidence that NTSR1 activity inhibits the dopamine D2 autoreceptor on postsynaptic dopaminergic terminals which induces a subsequent increase in excitability (Fawaz et al., 2009). For an extensive review on the interactions between dopamine and neurotensin see (Binder et al., 2001).
NTSR1 neurons in the VTA: projections and function
Transgenic mice have proven to be a useful tool for assessing the co-expression of TH and NTSR1 in the VTA. Studies seem to agree that most NTSR1 neurons in the VTA are dopaminergic (Szigethy and Beaudet, 1989; Woodworth et al., 2018b, 2017a), while the number of THVTA neurons expressing NTSR1 is likely close to 70% (Woodworth et al., 2018b). Interestingly, NTSR1 is more widely and generally expressed in the immature mouse, and is more restricted to THVTA neurons in the adult animal (Woodworth et al., 2018b). This suggests that neurotensinergic projections to the VTA outlined previously (Woodworth et al., 2018a) may have an outsized effect in the developing brain. Thus the NTS system might account for one of the mechanisms by which the adolescent brain is particularly sensitive to reward. The extent to which NTS may play different roles in developing and adult brains is an interesting and important question for future study.
Recent work has shown that dopaminergic VTA outputs, inputs, and genetic makeup are congruent across sex (Chung et al., 2017). As they are a large subpopulation of THVTA cells, this is also likely the case for NTSR1VTA neurons, but this has yet to be explored. NTSR1VTA neurons project strongly to ventral striatal areas including the NA core and shell, striatum, and interstitial nucleus of the posterior limb of the anterior commissure (IPAC; Woodworth et al., 2018b, 2017a). Notably, projections to prefrontal cortical areas have not been observed, suggesting that NTSR1VTA neurons are a subset of mesolimbic, as opposed to mesocortical VTA neurons. Lesioning NTSR1VTA neurons using a viral diphtheria toxin A construct resulted in increased ambulation and consumption, and resistance to the development of high fat diet (HFD)-induced obesity through increased energy expenditure (Woodworth et al., 2017a). This is in contrast to developmental NTSR1VTA knockout mice, which have increased susceptibility to HFD-induced weight gain (Opland et al., 2013). Interestingly, NTSR1VTA ablated mice also showed increased motivation for and consumption of sucrose (Woodworth et al., 2017a), similar to the developmental NTSR1VTA knock out animals which showed increased sucrose preference (Opland et al., 2013). In summary, NTSR1 VTA neurons appear to be a crucial in regulating feeding and future work should investigate downstream targets to further parse out their mechanism of action.
Nucleus Accumbens
The nucleus accumbens (NA) is the predominant target of VTA dopaminergic signaling, and thus, a crucial node impacting reward, reinforcement, and motivated behaviors (Volkow et al., 2017; Klawonn and Malenka, 2018). The NA consists of shell and core regions which have differences in cytoarchitecture as well as the distribution of a number of neuropeptides and receptors (Salgado and Kaplitt, 2015). The NA core has been traditionally considered a continuation of striatum, with NA shell forming part of the ‘extended amygdala’ (Heimer et al., 1997). Manipulations of the core and shell have been shown to differentially impact behavior across a wide range of measurements and paradigms (Di Chiara, 2002), with the shell having a more widespread role in reinforcement (Zahm, 1999), and the core having a more specialized role in cue-related reward learning and behavior (Ito et al., 2004).
NTS and dopamine signaling in the NA
NTSR1s are found in both the core and shell, with a higher density of NTSR1s on both terminals and spines in the core (Pickel et al., 2001). NTS-containing cells are also located within both the core and shell regions (Schroeder et al., 2019; Zahm, 1992). Dopamine receptor 2 (DRD2) signaling has been shown to modulate NTS release in the NA, as administration of a DRD2 antagonist reduces extracellular NTS (Wagstaff et al., 1996). NTS itself can enhance the release of dopamine in the NA through inhibition of DRD2 (Fawaz et al., 2009), tightly linking NTS neurotransmission with dopamine signaling in the NA. This interconnection between dopamine and NTS signaling (reviewed in Binder et al., 2001) has led to an interest in using NTS signaling as a target for impacting dopamine signaling, as mentioned previously. For this reason, there is a rich and extensive literature linking neurotensinergic signaling and schizophrenia (Binder et al., 2001), and in particular, linking NTS signaling to the effects of antipsychotic medications. Here, we are re-framing some of this rodent literature as it helps inform the role of NTS in the NA. Finally, while this section describes some effects of neurotensinergic signaling in the NA, and the NA contains NTS+ cells, the sources of NTS driving these phenotypes have not been elucidated.
NTS in the NA normalizes amphetamine-mediated disruption in sensorimotor gating
Prepulse inhibition (PPI) is a phenomenon in which a mild sensory stimulus reduces the startle response to a subsequent stimulus (Geyer et al., 2002), and is commonly used as a readout of sensorimotor gating. Sensorimotor gating is thought to mediate the ability of organisms to ignore irrelevant stimuli in favor of relevant stimuli. Deficiencies in PPI have been extensively described in studies of humans with schizophrenia and other psychoaffective disorders (Braff et al., 2001). Intra-accumbal administration of NTS has dose-dependent effects on pre-pulse inhibition (PPI), where low doses ameliorate disruptions caused by amphetamine administration (Feifel et al., 1997). In keeping with these findings, amphetamine administration fails to increase PPI in NTS knockout mice (Kinkead et al., 2005). Subcutaneous administration of an NTS mimetic, PD149163, also dose-dependently restores PPI to baseline levels following amphetamine administration in male Sprague-Dawley rats (Feifel et al., 1999). However, as PD149163 was later found to be selective for NTSR1 (Petrie et al., 2004), this work can be re-evaluated to support a role for NTSR1 signaling as the mediator of the anti-stimulant effects of NTS signaling. This makes particular sense, as PD149163 also increased PPI in both saline and amphetamine-treated C57BL/6J mice in a separate study (Vadnie et al., 2016). Virally-induced overexpression of NTSR1 in the NA restores amphetamine-induced disruption in PPI in rats, returning it to a saline baseline (Caceda et al., 2005). Crucially, this amelioration is then blocked by the NTSR1 antagonist SR 142948A, further supporting NTSR1 signaling in mediating the anti-stimulant effects of NTS signaling. Interestingly, NTS injected into the NA core blocks the psychostimulant effects of intraperitoneal (i.p.) cocaine and amphetamine, but fails to blunt cocaine operant selfadministration in male Wistar rats (Robledo et al., 1993). In summary it appears that accumbal NTS, through NTSR1, modulates the stimulant effects of amphetamines and cocaine without necessarily impacting drug intake. More broadly, NTSR1 signaling appears to be an important target in remediating drug-induced dopamine disruption in the NA.
NTS in the NA attenuates dopamine supersensitivity
A number of behavioral and pharmacological manipulations have been found to impact Nts expression in the NA, which as described above, has clear implications for dopamine signaling in this region. Acute (Betancur, 2001) and chronic (Betancur, 2001; Betancur et al., 1997) cocaine injections have been found to increase Nts mRNA in the NA shell. Chronic haloperidol administration has also been shown to increase tissue NTS content in the NA of male (Kinkead et al., 2000) and female (See et al., 1995) Sprague-Dawley rats. Long-term antypsychotic treatment regimens have been linked to aberrant reward processing in rats (Bédard et al., 2011), possibly through the development of dopamine supersensitivity (Servonnet et al., 2017). Dopamine supersensitivity is a phenomenon wherein a chronic antipsychotic regimen can then itself lead to a paradoxical increase in psychotic episodes and the requirement for a higher dose of antipsychotics (Yin et al., 2016). This phenomenon is thought to be driven by an increase in DRD2 number and sensitivity following treatment. There is mounting evidence that dopamine supersensitivity may underlie some aspects of treatment failure in schizophrenia (Samaha et al., 2007). Interestingly, a recent study found that intra-NA NTS administration attenuated this dopamine supersensitivity (Servonnet et al., 2017), suggesting that NTS and its receptors are promising targets for modulating NA dopaminergic signaling.
Lateral Hypothalamus
The lateral hypothalamus (LH), an important homeostatic regulator, is another source of neurotensinergic signaling in the brain. NTS neurons in the LH (NTSLH) are GABAergic, synapse within the LH, and project to the VTA (as previously discussed), as well as the substantia nigra pars compacta (Brown et al., 2019).
NTS is an anorectic peptide
A wealth of data supports the characterization of NTS as an anorectic neuropeptide. Intracerebroventricular (icv) administration of NTS has been shown to decrease feeding in fasted male Sprague-Dawley rats (Luttinger et al., 1982), as well as in both fasted and sated male Wistar rats (Cooke et al., 2012). I.p. administration of NTS has also been shown to suppress feeding in both fasted (Cooke et al., 2012; Ratner et al., 2016) and sated (Cooke et al., 2012) male C57Bl/6 mice. Interpretation of these global manipulations has been complicated by findings using an NTS knockout mouse (NTS−/−). Specifically, male and female NTS−/− mice were protected against the development of high-fat diet-induced obesity, and showed impaired fat absorption in the intestines (Li et al., 2016). This effect is presumably mediated by the actions of NTSR1 signaling in the periphery, as i.p. administration of SR 48692 was able to reduce intestinal lipid absorption in male C57BL/6 mice. In humans, levels of NTS precursor (pro-NTS) in fasting blood plasma are predictive of later development of obesity (Li et al., 2016). These findings, and others, make it imperative that investigations into the role of NTS in feeding are pursued in a circuit specific manner.
NTSLH neurons supress feeding through NTSR1
The LH is an important homeostatic regulator, composed of a number of neuronal populations which have been linked to feeding and metabolic regulation (for a review on LH cellular populations see Brown et al., 2015). While administration of NTS into the LH does not appear to modulate feeding (Hawkins et al., 1986), the NTSLH neurons themselves are poised to affect food consumption. Chemogenetic activation of NTSLH neurons in mice was able to increase locomotor activity and suppress food intake in both sated and food-deprived mice (Woodworth et al., 2017b). Pre-treatment with either a NTSR1 (SR48692) or DRD1 antagonist (SCH23390) blocked this NTSLH-induced suppression of feeding, and NTSLH stimulation failed to suppress feeding in NTSR1−/− animals. Taken together, this data attributes the role of NTSLH neurons in feeding suppression to signaling through NTSR1, presumably through the NTSLH->VTA projection. Supporting this hypothesis, it has been shown that the anorectic effect of central NTS administration can be potentiated by the concurrent administration of L-DOPA (catecholamine precursor) or bromocriptidine (dopamine receptor antagonist), again supporting the idea that the anorectic actions of NTS can be attributed to NTSR1-mediated increases in dopamine signaling (Hawkins et al., 1986). However, NTSLH-induced locomotion is largely intact NTSR1−/− mice, and was not impaired by pretreatment with either SR48692 or SCH23390, suggesting that this aspect of NTSLH activation is independent of NTSR1 signaling on downstream DA neurons (Woodworth et al., 2017b).
The role of NTSR1 in food intake more generally is complicated by data from different studies using NTSR1−/− mice. The first reported study using a NTSR1−/− mouse found no differences in growth curves in their animals (Pettibone et al., 2002). However, others have reported significantly increased weight and food intake in NTSR1−/− mice both using the same NTSR1−/− line (Kim et al., 2008), and a separately generated NTSR1−/− mouse line (Remaury et al., 2002). However, as icv administration of leptin has a decreased anorectic effect in C57BL/6 NTSR1−/− mice (Kim et al., 2008), it is likely that NTSR1 is at least partly involved in the role of NTS in mediating appetite.
What appears to be the main difference between these knockout studies is that those which found differences in feeding (Kim et al., 2008; Remaury et al., 2002) used mice that were maintained on a C57BL/6 background, as opposed to a mixed C57BL/6 129/SvJ background. While it is unclear which of the differences between these strains underlies this disparity, it is apparent that there is some salient difference that is relevant to studies of NTS. This is concerning as many of the NTSrelated mouse strains (NTSR1−/−, NTSR2−/−, NTS−/−, NTS-Cre) are being published and maintained on mixed genetic backgrounds, making it very difficult to draw conclusions across conflicting studies. Studies exploring differences in NTS, NTSR1, and NTSR2 expression and function in the commonly used rat and mouse strains would be very informative to the field.
NTSLH neurons regulate food intake in response to metabolic signals
A small subset of NTSLH neurons (30%; Leinninger et al., 2011) express the long form leptin receptor (LepRb) and inhibit nearby feeding-promoting orexin neurons thus affecting leptinmediated actions on the inhibition of feeding (Goforth et al., 2014; Leinninger et al., 2011). Loss of LepRb specifically in NTSLH neurons decreases spontaneous locomotor activity in mice and increases both weight and percent body fat in a general disruption of energy balance (Brown et al., 2017). Deletion of LepRb in NTSLH neurons also resulted in the dysregulation of other LH mRNA transcripts, specifically, increased expression of Dlk1 (delta-like 1 homologue; Villanueva et al., 2012; Meister et al., 2013) and LepRb, possibly as a result of compensation from non-NTScontaining LepRbLH neurons, as well as decreased expression of Nts. Interestingly, these animals also show more specific dysregulation of reward-related adaptive feeding and palatability. Specifically, LepRb knockout in NTSLH neurons results in animals that do not decrease feeding in response to leptin and do not increase sucrose preference in response to ghrelin, which are the normal responses observed in wildtype animals. Furthermore, these animals do not demonstrate shifts in breakpoint performance for a sucrose reward in response to leptin or ghrelin challenges, as would be observed in wild type mice. Thus, NTSLH neuronal signaling is crucial for the adaptive evaluation of a natural caloric reward in response to the animals internal metabolic state.
NTSLH neurons acutely drive fluid consumption through a non-NTSR1 mechanism
Hypertonic saline-induced dehydration has been shown to decrease Nts mRNA in the LH of rats, suggesting a role for NTS in fluid consumption (Watts et al., 1995). Chemogenetic activation of NTSLH neurons suppresses food consumption, as described above, but on a relatively long timescale in sated animals (a day or more; Woodworth et al., 2017b). On the other hand, this same stimulation can drive fluid consumption in the span of a few hours in euhydrated mice (Kurt et al., 2019; Woodworth et al., 2017b). Activation of NTSLH neurons promotes drinking of any available fluid, while maintaining the natural preferences of the animal (palatable fluids over unpalatable ones; Kurt et al., 2019). This phenomenon does not require NTSR1 signaling, as it is intact in NTSR1−/− animals and is not blocked by pretreatment with SR48692 or SCH23390 (Woodworth et al., 2017b).
A recent paper identified a fluid-responsive NTSLH population in mice that does not overlap with the NTS/LepRbLH population (Brown et al., 2019). Briefly, overnight dehydration induces Fos activation in some NTSLH neurons, but not LepRbLH neurons. These dehydration-activated NTSLH neurons do not project to mesolimbic dopaminergic regions, unlike LepRb/NTSLH neurons, suggesting that the role of these neurons in impacting different consumption behaviors is mediated through different downstream regions (Brown et al., 2019). Future work exploring the downstream projections of dehydration-responsive NTSLH neurons specifically is sure to be informative.
NTSLH neurons promote wakefulness
Sleep is a fundamental process that affects the overall wellbeing of an organism and has a significant impact on reward and learning processes (Byrne and Murray, 2017; Poe, 2017; Sara, 2017). Neurotensinergic signaling onto orexinLH neurons is also important for the control of sleep and wakefulness. Icv administration of a non-selective NTSR antagonist (SR142948) decreased wakefulness during the light period in wildtype but not orexin-ablated mice (Furutani et al., 2013). OrexinLH neurons appear to express NTSR2, as NTS (100nM) was able to depolarize these cells in slice, but pretreatment with the NTSR2 preferential agonist levocabastine blocked this effect. Furthermore, chemogenetic and brief optogenetic stimulation of NTSLH neurons promotes transition from NREM, but not REM, sleep to wakefulness (Naganuma et al., 2019). In the same study, chemogenetic and longer optogenetic activation of these neurons also induced hyperthermia. (Body temperature and wakefulness are tightly linked in mammals, see (Krauchi, 2010) for a review). This is in line with previous work which had shown that chemogenetic activation of both NTSLH (Kurt et al., 2019) and GABALH neurons more generally (de Vrind et al., 2019) results in increased body temperature. Interestingly, inhibition of NTSLH neurons failed to affect spontaneous sleep, but did both attenuate arousal in response to a novel environmental stress and increase arousal in response to a metabolic stress (Naganuma et al., 2019). Similarly to observations in the LepRb knockout described above (Brown et al., 2017), both of these responses are maladaptive. Taken together, this suggests that NTSLH neurons are an important nexus for the integration of different sources of stress and the selection of an appropriate behavioral response.
BNST
Part of the extended amygdala, the bed nucleus of the stria terminalis (BNST) is a hetereogenous structure that integrates reward and stress/anxiety processes (McElligott and Winder, 2009). The BNST contains a significant population of NTS producing neurons that have been implicated in these behaviors. Pharmacological blockade of NTS signaling in the BNST using SR142984 can normalize the increased open-arm avoidance observed in chronically stressed rats (Normandeau et al., 2018b). Chronic unpredictable stress can also decrease Ntsr1 mRNA detected in the dorsal BNST (Normandeau et al., 2018b), suggesting an important role for NTS modulation in response to stress.
NTS in the BNST induces inhibitory LTP
BNST NTS has also been implicated in cocaine self-administration. Cocaine self-administration induces a GABAergic LTP mediated by the D1 dopamine receptor (DRD1; Krawczyk et al., 2011). Blockade of DRD1 within the oval nucleus of the BNST (ovBNST) depresses cocaine responses from escalating beyond what is observed for sucrose self-administration, and the magnitude of this LTP is correlated with motivation for cocaine intake in individual rats as measured by the final level of lever pressing on a progressive ratio (Krawczyk et al., 2013). As ex vivo bath application of the NTSR antagonist SR-14298 blunts this LTP, it is presumably mediated through an increase in local NTS release. Because bath application of NTS increased the size of GABAA inhibitory post-synaptic currents (IPSCs) in both cocaine and control rats, the increase in local NTS release in cocaine rats could further increase local GABA. This work provides a link between NTS signaling in the BNST and the effects of drug-taking on natural reward systems.
Interestingly, in a model of binge-like sucrose consumption, bath application of dopamine produced a significant increase in GABA IPSCs in ovBNST neurons in animals that had intermittent access to sucrose, but not those with continuous access (Maracle et al., 2019). As might be expected, this effect developed over abstinence from sucrose. Following binge-like consumption these animals were insensitive to conditioned suppression, a phenomenon that was normalized following intra-BNST DRD1 blockade. It may be reasonably hypothesized that neurotensinergic signaling underlies this DRD1-mediated LTP as was shown with cocaine self-administration. If this were the case, it would suggest that the role of NTS in the BNST is related to the incubation of craving. Future work examining this possibility might illuminate an underlying neurobiological mechanism tying both drug and ‘natural’ reward.
Local NTS in the BNST enhances the strength of GABAergic CeA input
NTS is co-expressed with other neuropeptides in the BNST such as dynorphin (DYN) and corticotropin releasing factor (CRF; Ju and Han, 1989; Shimada et al., 1989). We have demonstrated that endogenous NTS and DYN in the BNST can bidirectionally modulate CeA to ovBNST inhibitory transmission through NTSR1 and the kappa opioid receptor (KOR), respectively (Normandeau et al., 2018a). NTSR1 signaling increases CeA GABAergic signaling, and KOR signaling decreases the strength of this input. Furthermore, chronic stress can enhance the ability of endogenous NTS to potentiate inhibitory synapses, and intra-BNST blockade of NTS receptors can attenuate the anxiety-like behavior induced by chronic stress (Normandeau et al., 2018b). These data suggest that NTS signaling in the BNST may provide a link between stress and drug intake, and possibly the stress of abstinence and withdrawal. Additionally, these data may suggest a means by which catecholamine signaling in the BNST, which has been shown to release neuropeptides, may regulate stress responses (McElligott et al., 2010; Nobis et al., 2011).
BNST NTS decreases maternal aggression
Nts mRNA is upregulated in the dorsal BNST of postpartum mice (Driessen et al., 2014), and icv administration of NTS enhances Fos expression in the dBNST (Gammie et al., 2009). Additionally, icv administration of either NTS or SR 48692 (NTSR1 antagonist) decreases or increases maternal aggression respectively (Gammie et al., 2009). In combination, this suggests that NTSR1 activity in postpartum mice is linked to maternal aggression and that NTS is dynamically regulated in order for the animal to engage the appropriate behavioral response. It remains to be seen whether NTS signaling in the BNST may impact aggression in other contexts, and whether other behavioral manipulations that impact NTS in the BNST may also impact aggression through a similar mechanism. These studies reveal a role for neurotensinergic signaling in the BNST beyond artificial drug manipulations, and show that it may be normally regulated in the brain throughout the life-cycle of the organism. Future studies examining the interaction between these sources of NTS regulation would broaden our understanding of NTS in this region.
CeA
The central amygdala (CeA) is a limbic subnucleus that functions as the main output of the amygdalar complex. While originally associated with aversive associative learning, more recent work has appreciated it as a region that encodes both positive and negative emotional valence (Janak and Tye, 2015). The CeA has also been identified as a crucial hub for both ethanol consumption, and the effect of ethanol consumption on the brain (Koob, 2003).
NTS in the CeA is reinforcing and strengthens learning
Intra-CeA administration of NTS has been shown to produce conditioned place preference in male Wistar rats, a phenomenon that is ablated by pre-administration of either an NTSR1 antagonist SR 48692 (László et al., 2010a), or the DRD1 antagonist SCH23390 (László et al., 2018). In the Morris Water Maze, intra-CeA NTS administration improves escape latency when injected during the memory consolidation phase following trials (László et al., 2010b), and intra-CeA administration of NTS in the consolidation phase of a passive avoidance test also improves performance; both of these effects are also curbed by administration of an NTSR1 antagonist (László et al., 2012). These studies would suggest that NTS signaling in the CeA is reinforcing and promotes learning across a variety of modalities and tasks.
Global NTS manipulations also support a role for NTSergic signaling in learning. Global NTSR1 knockout facilitated freezing in animals that received a single shock during contextual fear conditioning, but did not impact freezing when the animals received six or eight shocks (Yamada et al., 2010). This increase in freezing could be blocked by administration of either propranolol (βadrenergic antagonist) or the NMDA receptor antagonist MK-801 during the consolidation phase. If shock number were to be used as a proxy for memory strength, this would suggest that NTSR1 facilitates weaker contextual fear memories, but is not necessary for stronger memory expression. Interestingly, NTSR1−/− mice present an aberrant LTP in pyramidal cells in the basolateral amygdala (BLA; Amano et al., 2008). Acute pharmacological blockade of the NTSR1 receptor permitted the induction of this LTP in wildtype mice, showing that this phenomenon was not simply due to developmental compensation. The increased responsivity of BLA cells to electrophysiological input, and presumably sensory input in the intact animal, could explain the increased salience of a mild stressor observed in the conditioning experiment (Yamada et al., 2010), as the BLA is a main source of input to the CeA.
In contrast, systemic administration of an NTSR1 agonist (PD149163) has been shown to block fear-potentiated startle (Shilling and Feifel, 2008). Both PD149163 (Prus et al., 2014; Steele et al., 2017) and NTS (Steele et al., 2017) have also been shown to decrease ultra-sonic vocalizations (USVs) produced by rats in a context in which they had previously been shocked. Interestingly, this acute effect of NTSR1 agonism is blocked in animals that had received repeated administrations of PD 149163 (Prus et al., 2014). Taken in combination with the previous global knockout experiments, this suggests that the neurotensinergic system adapts to developmental knockout of NTSR1. To our knowledge, however, this yet to be directly examined.
NTSCeA neurons overlap with anxiety-related populations, but NTS in the CeA does not impact anxiety-like behavior
NTSCeA neurons are GABAergic and overlap with a number of other peptidergic populations in the CeA. They have extensive overlap with of corticotropin- releasing factor (Crf; Kim et al., 2017; Pomrenze et al., 2019; Torruella-Suárez et al., in press), crf receptor-1 (Crfr1; Torruella-Suárez et al., in press), and dynorphin (Dyn; Kim et al., 2017; Pomrenze et al., 2019; Torruella-Suárez et al., in press) neurons. They also share some some overlap with somatostatin (Sst) neurons (McCullough et al., 2018; Torruella-Suárez et al., in press) and minimal overlap with protein kinase-c delta (Prkcδ)- containing populations (Kim et al., 2017). It is important to note that quantification of degrees of overlap varies by amygdalar subregion, location on the anteriorposterior axis, and methodology. In contrast with the CeA CRF-containing neurons (CRFCeA) neurons with which they overlap, NTSCeA neurons (Kim et al., 2017; Torruella-Suárez et al., in press) and intra-CeA neurotensinergic signaling (László et al., 2010a; Pomrenze et al., 2019) do not appear to have a role in anxiety-like behaviors. In fact, optogenetic stimulation of NTSCeA cell bodies appears to be reinforcing (Kim et al., 2017), in line with intra-CeA NTS administration promoting conditioned place preference (László et al., 2018, 2010a).
Due to the overlap in CeA peptidergic populations described above, recent work has attempted to disentangle the role of specific neuropeptides within these populations. A recent paper performed systematic shRNA-mediated knockdown of Vgat (vesicular GABA transporter), Crf, Dyn, and lastly Nts, within the CRFCeA population in rats (Pomrenze et al., 2019). This study found that impairing NTS signaling in CRFCeA neurons left anxiety-like behaviors largely unaffected, with other neurotransmitters being responsible for baseline and evoked anxiety-related phenotypes in these neurons. Nts knockdown in CRFCeA neurons failed to affect time spent in the open arms of an elevated plus maze or time spent in the center of an open field, but did increase the number of entries into the open arms. When the authors activated CRFCeA neurons using the hM3Dq DREADD Nts knockdown again failed to impact these measures of anxiety-like behavior. These data indicate the NTS signaling in the CeA does not play a role in either baseline or acutely induced anxiety. Interestingly, Nts knock-down in the CeA results in heightened cue-induced freezing after fear memory extinction whereas vGAT knockdown had no effect. These data suggest that fear learning in the CeA is at least partly mediated by NTS signaling. It is yet to be seen whether Nts knockdown in the CeA would similarly impact learning related to a positive stimulus. Studies like this by Pomrenze et al. suggest that we may be able to disentangle the role of these overlapping neuropeptides using acute genetic manipulations.
NTSCeA neurons play a role in ethanol consumption
NTSCeA neurons project to the BNST (Arluison et al., 1994; Torruella-Suárez et al., in press) and PBN (Torruella-Suárez et al., in press) in mice. The NTSCeA->BNST pathway has yet to be explored, but as NTSCeA neurons are a subpopulation of CRFCeA neurons, work exploring the CRFCeA->BNST projection is relevant in this context. CRFCeA neurons have been of interest in ethanol consumption, particularly in the later stages that are thought to be driven by negative reinforcement (Koob, 2010). It was recently shown that inhibition of the CRFCeA->BNST pathway in ethanol-dependent rats decreases both consumption of ethanol and withdrawal symptomology (de Guglielmo et al., 2019). While CRF and CRF1 (CRF receptor 1) are important mediators of the increase in CeA GABAergic transmission observed in ethanol dependence (Herman et al., 2016; Roberto et al., 2010), the role of other neuropeptides in the development of ethanol dependence and ethanolrelated neuroadaptations has not been as extensively explored. In this vein, our recent study explored whether NTSCeA neurons were involved in ethanol consumption and found that viral ablation of these neurons reduce voluntary homecage ethanol consumption in non-dependent C57BL/6J background mice (Torruella-Suárez et al., in press). As mentioned previously, NTS in the BNST is capable of modulating the NTSR1CeA->BNST projection (Normandeau et al., 2018a) suggesting that other sources of NTS near these synapses, such as NTS from the CeA itself, may also modulate this input.
NTSCeA->PBN neurons promote rewarding fluid consumption
We also recently explored the role of the NTSCeA->PBN projection. We found that optogenetic stimulation of the NTSCeA->PBN projection promotes positive valence and is reinforcing (Torruella-Suárez et al., in press). This follows the pattern of other CeA->PBN projections that have been examined such as Htr2aCeA->PBN (serotonin receptor 2a; Douglass et al., 2017) and PnocCeA->PBN (nociceptin; Hardaway et al., 2019), which also promote positive valence and reinforcement. Optogenetic stimulation of the NTSCeA->PBN projection also promotes consumption of palatable and rewarding fluids, whereas stimulation of the Htr2aCeA population and the Htr2aCeA->PBN pathway specifically, drives consumption of chow. Interestingly, optogenetic inhibition of the Htr2aCeA population also decreases consumption of a palatable fluid, but this was not further explored in that study. This suggests that NTSCeA->PBN neurons have a unique role in fluid reward. Additionally, Nts mRNA in the CeA was significantly decreased after dehydration in rats (Watts et al., 1995), and these neurons showed increased Fos expression after rewatering in C57BL/6 mice (Kim et al., 2017). Inhibition of the lateral subpopulation of NTSCeA neurons decreased water drinking in water-deprived mice, but had no effect on feeding in food-deprived animals (Kim et al., 2017). This suggests that NTSCeA neurons play a role in rewarding fluid consumption in hydrated animals, but switch to a more general role in fluid consumption in dehydrated animals.
4. Conclusions
As described here, neurotensinergic signaling is involved in a number of different reward-related behaviors and plays a variety of contextually- and regionally-dependent roles. There has been interest in manipulating this system in the pursuit of novel treatments for schizophrenia (Kinkead and Nemeroff, 2006; Meltzer et al., 2004; Vadnie et al., 2016), pain (Dobner, 2006; Eiselt et al., 2019), and substance use disorders (Felszeghy et al., 2007; Ferraro et al., 2016). In the treatment of schizophrenia, agonism of the NTS system may serve as an antipsychotic on its own (Vadnie et al., 2016), or may serve to ameliorate the effects of a chronic antipsychotic regimen (Servonnet et al., 2017). As an analgesic, NTSR agonists appear to improve the analgesic effects of morphine, leading to a lower dose required to produce analgesia and therefore lowering adverse reactions (Eiselt et al., 2019). In the treatment of substance use disorders, NTSR1 antagonism has been shown to decrease some of the rewarding effects of cocaine when administered during withdrawal in rats (Felszeghy et al., 2007). There is also evidence that the actions of NTS in the paraventricular nucleus of the thalamus (PVT) are relevant to alcohol use disorder, with low endogenous NTS levels leading to a higher propensity to drink excessive amounts of ethanol, and intra-PVT administration of NTS reducing ethanol drinking (Pandey et al., 2019).
A more thorough understanding of the acute and long-term effects of NTS agonism and antagonism in the brain is crucial for both of these aims. It is particularly important to understand how neurotensinergic signaling acts in the typically functioning nervous system, and how it is impacted by neuropsychiatric illness as well as drug use. Studies on the role of NTS outside of the VTA are still in their relative infancy, yet they reveal a complex role for neurotensinergic signaling in reward-related behaviors that merits further study.
Supplementary Material
Figure 1.
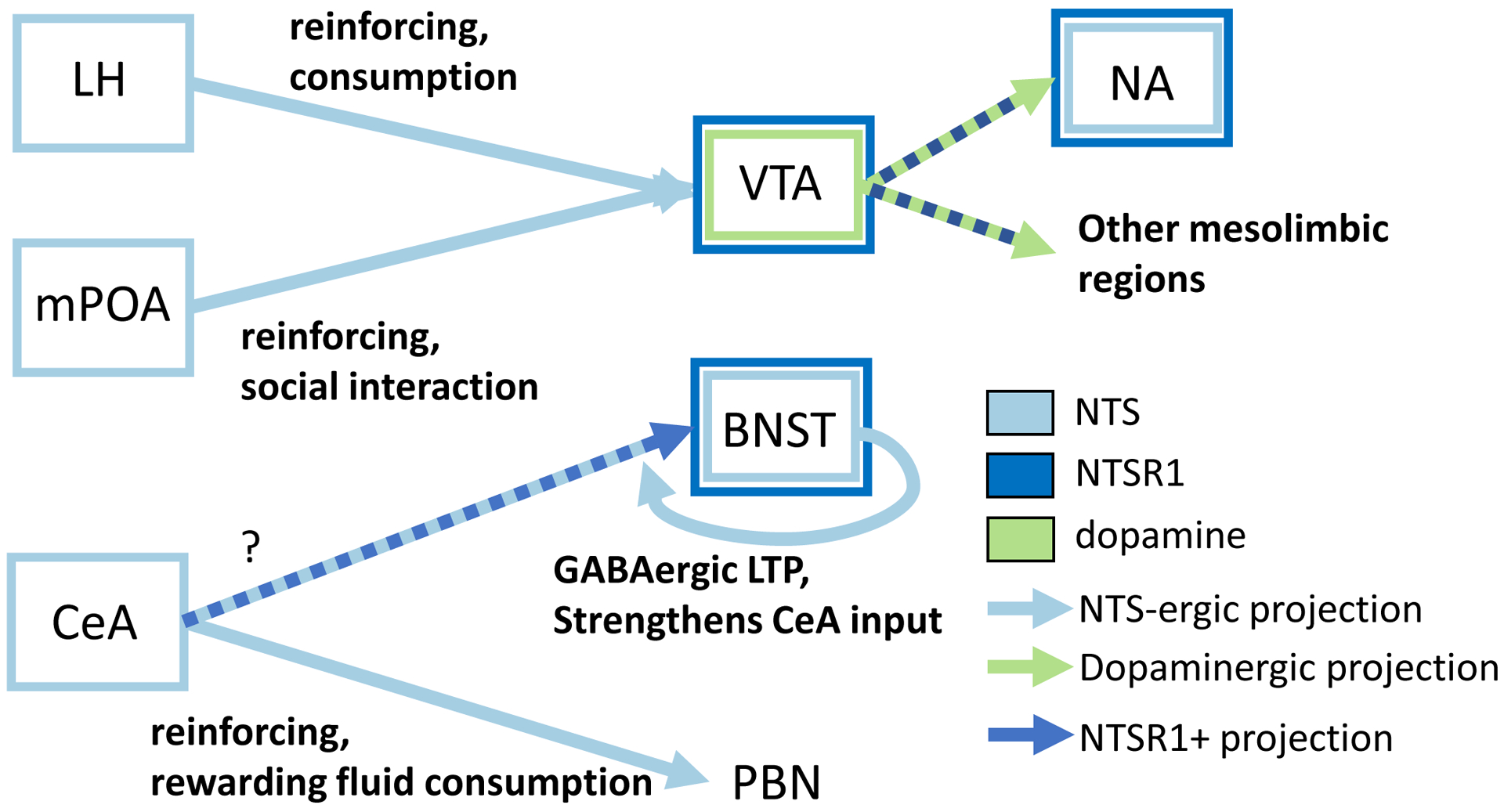
Circuit diagram outlining regions where NTS and NTSR1 are expressed, physiological ramifications of NTS signaling, and behaviors mediated by these circuits. Lateral hypothalamus (LH), medial preoptic area (mPOA), central nucleus of the amgydala (CeA), ventral tegmental area (VTA), bed nucleus of the stria terminalis (BNST), parabrachial nucleus (PBN), nucleus accumbens (NA). Light blue = NTS neurons and projections, dark blue = NTSR1 expressing neurons, green = dopamine neurons and projections.
Highlights.
Neurotensin (NTS) is involved in reward signaling beyond the VTA.
NTS signaling impacts neuronal signaling differently across brain regions.
Review of rodent literature with some primate and human highlights.
Citations
- Amano T, Wada E, Yamada D, Zushida K, Maeno H, Noda M, Wada K, Sekiguchi M, 2008. Heightened Amygdala Long-Term Potentiation in Neurotensin Receptor Type-1 Knockout Mice. Neuropsychopharmacology 33, 3135–3145. 10.1038/npp.2008.38 [DOI] [PubMed] [Google Scholar]
- Arluison M, Brochier G, Vankova M, Leviel V, Villalobos J, Tramu G, 1994. Demonstration of peptidergic afferents to the bed nucleus of the stria terminalis using local injections of colchicine. A combined immunohistochemical and retrograde tracing study. Brain Res. Bull 34, 319–337. 10.1016/0361-9230(94)90026-4 [DOI] [PubMed] [Google Scholar]
- Bédard A-M, Maheux J, Lévesque D, Samaha A-N, 2011. Continuous, but not Intermittent, Antipsychotic Drug Delivery Intensifies the Pursuit of Reward Cues. Neuropsychopharmacology 36, 1248–1259. 10.1038/npp.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer-Offroy É, Brouillette RL, Lavenus S, Froehlich U, Brumwell A, Murza A, Longpré J-M, Marsault É, Grandbois M, Sarret P, Leduc R, 2017. The signaling signature of the neurotensin type 1 receptor with endogenous ligands. Eur. J. Pharmacol 805, 1–13. 10.1016/j.ejphar.2017.03.046 [DOI] [PubMed] [Google Scholar]
- Betancur C, 2001. Neurotensin Gene Expression and Behavioral Responses Following Administration of Psychostimulants and Antipsychotic Drugs in Dopamine D3 Receptor Deficient Mice. Neuropsychopharmacology 24, 170–182. 10.1016/S0893133X(00)00179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C, Rostène W, Bérod A, 1997. Chronic cocaine increases neurotensin gene expression in the shell of the nucleus accumbens and in discrete regions of the striatum. Mol. Brain Res 44, 334–340. 10.1016/S0169-328X(96)00289-6 [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB, 2001. Neurotensin and Dopamine Interactions. Pharmacol. Rev 53, 453. [PubMed] [Google Scholar]
- Borroto-Escuela DO, Ravani A, Tarakanov AO, Brito I, Narvaez M, Romero-Fernandez W, Corrales F, Agnati LF, Tanganelli S, Ferraro L, Fuxe K, 2013. Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem. Biophys. Res. Commun 435, 140–146. 10.1016/j.bbrc.2013.04.058 [DOI] [PubMed] [Google Scholar]
- Bose P, Rompré P-P, Warren RA, 2015. Neurotensin enhances glutamatergic EPSCs in VTA neurons by acting on different neurotensin receptors. Peptides 73, 43–50. 10.1016/j.peptides.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Boudin H, Pélaprat D, Rostène W, Pickel VM, Beaudet A, 1998. Correlative Ultrastructural Distribution of Neurotensin Receptor Proteins and Binding Sites in the Rat Substantia Nigra. J. Neurosci 18, 8473–8484. 10.1523/JNEUROSCI.18-2008473.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR, 2001. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl.) 156, 234–258. 10.1007/s002130100810 [DOI] [PubMed] [Google Scholar]
- Brown JA, Bugescu R, Mayer TA, Gata-Garcia A, Kurt G, Woodworth HL, Leinninger GM, 2017. Loss of Action via Neurotensin-Leptin Receptor Neurons Disrupts Leptin and Ghrelin-Mediated Control of Energy Balance. Endocrinology 158, 1271–1288. 10.1210/en.2017-00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Woodworth HL, Leinninger GM, 2015. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front. Syst. Neurosci 9 10.3389/fnsys.2015.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Wright A, Bugescu R, Christensen L, Olson DP, Leinninger GM, 2019. Distinct Subsets of Lateral Hypothalamic Neurotensin Neurons are Activated by Leptin or Dehydration. Sci. Rep 9, 1873 10.1038/s41598-018-38143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgevin M-C, Castel M-N, Quarteronet D, Chevet T, Laduron, 1992. Neurotensin increases tyrosine hydroxylase messenger RNA-positive neurons in substantia nigra after retrograde axonal transport. Neuroscience 49, 627–633. 10.1016/03064522(92)90232-Q [DOI] [PubMed] [Google Scholar]
- Byrne JEM, Murray G, 2017. The sleep and circadian modulation of neural reward pathways: a protocol for a pair of systematic reviews. Syst. Rev 6, 237 10.1186/s13643-017-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceda R, Kinkead B, Owens MJ, Nemeroff CB, 2005. Virally Mediated Increased Neurotensin 1 Receptor in the Nucleus Accumbens Decreases Behavioral Effects of Mesolimbic System Activation. J. Neurosci 25, 11748–11756. 10.1523/JNEUROSCI.4282-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R, Leeman SE, 1973. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem 248, 6854–6861. [PubMed] [Google Scholar]
- Castel MN, Beaudet A, Laduron PM, 1994. Retrograde axonal transport of neurotensin in rat nigrostriatal dopaminergic neurons: Modulation during ageing and possible physiological role. Biochem. Pharmacol 47, 53–62. 10.1016/00062952(94)90437-5 [DOI] [PubMed] [Google Scholar]
- Castel MN, Faucher D, Cuiné F, Dubédat P, Boireau A, Laduron PM, 1991. Identification of Intact Neurotensin in the Substantia Nigra After Its Retrograde Axonal Transport in Dopaminergic Neurons. J. Neurochem 56, 1816–1818. 10.1111/j.1471-4159.1991.tb02086.x [DOI] [PubMed] [Google Scholar]
- Castel MN, Malgouris C, Blanchard J-C, Laduron PM, 1989. Retrograde axonal transprot of neurotensin in the rat brain. Eur. J. Pharmacol 166, 353–354. 10.1016/0014-2999(89)90083-6 [DOI] [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D, 1996. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett 386, 91–94. 10.1016/0014-5793(96)00397-3 [DOI] [PubMed] [Google Scholar]
- Chung AS, Miller SM, Sun Y, Xu X, Zweifel LS, 2017. Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci. Rep 7, 11120 10.1038/s41598-017-11478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG, 2012. Peripheral and Central Administration of Xenin and Neurotensin Suppress Food Intake in Rodents. Obesity 17 10.1038/oby.2008.652 [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, Koob GF, Messing RO, George O, 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat. Commun 10, 1238 10.1038/s41467-019-09183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrind VAJ, Rozeboom A, Wolterink-Donselaar IG, Luijendijk-Berg MCM, Adan RAH, 2019. Effects of GABA and Leptin Receptor-Expressing Neurons in the Lateral Hypothalamus on Feeding, Locomotion, and Thermogenesis. Obesity oby 22495 10.1002/oby.22495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Donne KT, Chan J, Boudin H, Pélaprat D, Rostène W, Pickel VM, 2004. Electron microscopic dual labeling of high-affinity neurotensin and dopamine D2 receptors in the rat nucleus accumbens shell: Neurotensin and D2- Dopamine Receptors. Synapse 52, 176–187. 10.1002/syn.20018 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, 2002. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res 137, 75–114. 10.1016/S01664328(02)00286-3 [DOI] [PubMed] [Google Scholar]
- Dobner PR, 2006. Neurotensin and pain modulation. Peptides 27, 2405–2414. 10.1016/j.peptides.2006.04.025 [DOI] [PubMed] [Google Scholar]
- Dobner PR, 2005. Multitasking with neurotensin in the central nervous system. Cell. Mol. Life Sci 62, 1946–1963. 10.1007/s00018-005-5128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Piccart E, Lynch WB, Wollet MB, Sharpe AL, Beckstead MJ, 2018. Antagonism of Neurotensin Receptors in the Ventral Tegmental Area Decreases Methamphetamine Self-Administration and Methamphetamine Seeking in Mice. Int. J. Neuropsychopharmacol 21, 361–370. 10.1093/ijnp/pyx117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Alcala Morales PL, Conzelmann K-K, Lüthi A, Klein R, 2017. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci 20, 1384–1394. 10.1038/nn.4623 [DOI] [PubMed] [Google Scholar]
- Driessen TM, Zhao C, Whittlinger A, Williams H, Gammie SC, 2014. Endogenous CNS Expression of Neurotensin and Neurotensin Receptors Is Altered during the Postpartum Period in Outbred Mice. PLoS ONE 9, e83098 10.1371/journal.pone.0083098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiselt E, Côté J, Longpré J-M, Blais V, Sarret P, Gendron L, 2019. The combination of opioid and neurotensin receptor agonists improves their analgesic/adverse effect ratio. Eur. J. Pharmacol 848, 80–87. 10.1016/j.ejphar.2019.01.048 [DOI] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, Yoo JH, Callaway EM, Hnasko TS, 2016. Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep. 15, 2796–2808. 10.1016/j.celrep.2016.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D, Trudeau L-E, 2009. Presynaptic action of neurotensin on dopamine release through inhibition of D2 receptor function. BMC Neurosci. 10, 96 10.1186/1471-2202-10-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR, 1997. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Res. 760, 80–84. 10.1016/S0006-8993(97)00306-5 [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD, 1999. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J. Pharmacol. Exp. Ther 288, 710–713. [PubMed] [Google Scholar]
- Felszeghy K, Espinosa JM, Scarna H, Bérod A, Rostène W, Pélaprat D, 2007. Neurotensin Receptor Antagonist Administered during Cocaine Withdrawal Decreases Locomotor Sensitization and Conditioned Place Preference. Neuropsychopharmacology 32, 2601–2610. 10.1038/sj.npp.1301382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tiozzo Fasiolo L, Beggiato S, Borelli AC, Pomierny-Chamiolo L, Frankowska M, Antonelli T, Tomasini MC, Fuxe K, Filip M, 2016. Neurotensin: A role in substance use disorder? J. Psychopharmacol. (Oxf.) 30, 112–127. 10.1177/0269881115622240 [DOI] [PubMed] [Google Scholar]
- Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M, Shioda S, Sakurai T, 2013. Neurotensin Co-Expressed in Orexin-Producing Neurons in the Lateral Hypothalamus Plays an Important Role in Regulation of Sleep/Wakefulness States. PLoS ONE 8, e62391 10.1371/journal.pone.0062391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, D’Anna KL, Gerstein H, Stevenson SA, 2009. Neurotensin inversely modulates maternal aggression. Neuroscience 158, 1215–1223. 10.1016/j.neuroscience.2008.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS, 2006. Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur. J. Neurosci 24, 116–134. 10.1111/j.1460-9568.2006.04928.x [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R, 2002. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry 7, 1039–1053. 10.1038/sj.mp.4001159 [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Giovino AA, Hoebel BG, 1987. Neurotensin self-injection in the ventral tegmental area. Brain Res. 403, 147–150. 10.1016/0006-8993(87)90134-X [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Margolin DH, Giovino AA, Hoebel BG, 1984. Neurotensin: A new ‘reward peptide.’ Brain Res. 291, 119–124. 10.1016/00068993(84)90657-7 [DOI] [PubMed] [Google Scholar]
- Godfrey N, Borgland SL, 2019. Diversity in the lateral hypothalamic input to the ventral tegmental area. Neuropharmacology 154, 4–12. 10.1016/j.neuropharm.2019.05.014 [DOI] [PubMed] [Google Scholar]
- Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG, 2014. Leptin Acts via Lateral Hypothalamic Area Neurotensin Neurons to Inhibit Orexin Neurons by Multiple GABA-Independent Mechanisms. J. Neurosci 34, 11405–11415. 10.1523/JNEUROSCI.5167-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard JC, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, 1993. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc. Natl. Acad. Sci 90, 65–69. 10.1073/pnas.90.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL, 2019. Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron 102, 1037–1052.e7. 10.1016/j.neuron.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MF, Barkemeyer CA, Tulley RT, 1986. Synergistic effects of dopamine agonists and centrally administered neurotensin on feeding. Pharmacol. Biochem. Behav 24, 1195–1201. 10.1016/0091-3057(86)90170-X [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS, 1997. The accumbens: beyond the core-shell dichotomy. J. Neuropsychiatry Clin. Neurosci 9, 354–381. 10.1176/jnp.9.3.354 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT, 1991. Neurotensin-lmmunoreactive Neurons in the Rat Medial Preoptic Area are Oestrogen-Receptive. J. Neuroendocrinol 3, 587–589. 10.1111/j.1365-2826.1991.tb00322.x [DOI] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M, 2016. A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J. Neurosci 36, 10729–10741. 10.1523/JNEUROSCI.1267-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Maloteaux J-M, 1998. Mechanisms of Regulation of Neurotensin Receptors. Pharmacol. Ther 79, 89–104. 10.1016/S0163-7258(98)00009-6 [DOI] [PubMed] [Google Scholar]
- Hwang JR, Baek MW, Sim J, Choi H-S, Han JM, Kim YL, Hwang J-I, Kwon HB, Beaudet N, Sarret P, Seong JY, 2010. Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem. Biophys. Res. Commun 391, 1007–1013. 10.1016/j.bbrc.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ, 2004. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat. Neurosci 7, 389–397. 10.1038/nn1217 [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM, 2001. Activation and Functional Characterization of the Mosaic Receptor SorLA/LR11. J. Biol. Chem 276, 22788–22796. 10.1074/jbc.M100857200 [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Han Z, 1989. Coexistence of corticotropin releasing factor and neurotensin within oval nucleus neurons in the bed nuclei of the stria terminalis in the rat. Neurosci. Lett 99, 246–250. 10.1016/0304-3940(89)90454-0 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJ, 1983. Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience 8, 495–505. 10.1016/0306-4522(83)90195-1 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Miller JS, 1984. Neurotensin neurons in the ventral tegmental area project to the medial nucleus accumbens. Brain Res. 300, 157–160. 10.1016/00068993(84)91351-9 [DOI] [PubMed] [Google Scholar]
- Kataoka K, Mizuno N, Frohman LA, 1979. Regional distribution of immunoreactive neurotensin in monkey brain. Brain Res. Bull 4, 57–60. 10.1016/03619230(79)90058-3 [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger G-M, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A, 2013. Hypothalamic Neurotensin Projections Promote Reward by Enhancing Glutamate Transmission in the VTA. J. Neurosci 33, 7618–7626. 10.1523/JNEUROSCI.2588-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ER, Leckstrom A, Mizuno TM, 2008. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav. Brain Res 194, 66–71. 10.1016/j.bbr.2008.06.024 [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, 2017. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479.e5. 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead B, 2002. Neurotensin: an endogenous antipsychotic? Curr. Opin. Pharmacol 2, 99–103. 10.1016/S1471-4892(01)00128-X [DOI] [PubMed] [Google Scholar]
- Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB, 2005. Neurotensin-Deficient Mice Have Deficits in Prepulse Inhibition: Restoration by Clozapine but Not Haloperidol, Olanzapine, or Quetiapine. J. Pharmacol. Exp. Ther 315, 256–264. 10.1124/jpet.105.087437 [DOI] [PubMed] [Google Scholar]
- Kinkead B, Nemeroff C, 2006. Novel Treatments of Schizophrenia: Targeting the Neurotensin System. CNS Neurol. Disord. - Drug Targets 5, 205–218. 10.2174/187152706776359655 [DOI] [PubMed] [Google Scholar]
- Kinkead B, Shahid S, Owens MJ, Nemeroff CB, 2000. Effects of acute and subchronic administration of typical and atypical antipsychotic drugs on the neurotensin system of the rat brain. J. Pharmacol. Exp. Ther 295, 67–73. [PubMed] [Google Scholar]
- Klawonn AM, Malenka RC, 2018. Nucleus Accumbens Modulation in Reward and Aversion. Cold Spring Harb. Symp. Quant. Biol 83, 119–129. 10.1101/sqb.2018.83.037457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, Dulac C, 2018. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. 10.1038/s41586-018-0027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2010. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 1314, 3–14. 10.1016/j.brainres.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2003. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. Neuropsychopharmacol 13, 442–452. 10.1016/j.euroneuro.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Krauchi K, 2010. The interrelationship between sleep regulation and thermoregulation. Front. Biosci 15, 604 10.2741/3636 [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, Di Prospero C, Chiang C, Martinez A, Jones AA, Doudnikoff E, Caille S, Bezard E, Georges F, Dumont EC, 2013. D1 Dopamine Receptor-Mediated LTP at GABA Synapses Encodes Motivation to Self-Administer Cocaine in Rats. J. Neurosci 33, 11960–11971. 10.1523/JNEUROSCI.1784-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Sharma R, Mason X, DeBacker J, Jones AA, Dumont EC, 2011. A Switch in the Neuromodulatory Effects of Dopamine in the Oval Bed Nucleus of the Stria Terminalis Associated with Cocaine Self-Administration in Rats. J. Neurosci 31, 8928–8935. 10.1523/JNEUROSCI.0377-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt G, Woodworth HL, Fowler S, Bugescu R, Leinninger GM, 2019. Activation of lateral hypothalamic area neurotensin-expressing neurons promotes drinking. Neuropharmacology 154, 13–21. 10.1016/j.neuropharm.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laduron PM, 1992. Genomic pharmacology: More intracellular sites for drug action. Biochem. Pharmacol 44, 1233–1242. 10.1016/0006-2952(92)90520-S [DOI] [PubMed] [Google Scholar]
- László K, Péczely L, Kovács A, Zagoracz O, Ollmann T, Kertes E, Kállai V, Csetényi B, Karádi Z, Lénárd L, 2018. The role of intraamygdaloid neurotensin and dopamine interaction in conditioned place preference. Behav. Brain Res 344, 85–90. 10.1016/j.bbr.2018.01.021 [DOI] [PubMed] [Google Scholar]
- László K, Tóth K, Kertes E, Péczely L, Lénárd L, 2010a. The role of neurotensin in positive reinforcement in the rat central nucleus of amygdala. Behav. Brain Res 208, 430–435. 10.1016/j.bbr.2009.12.022 [DOI] [PubMed] [Google Scholar]
- László K, Tóth K, Kertes E, Péczely L, Ollmann T, Lénárd L, 2010b. Effects of neurotensin in amygdaloid spatial learning mechanisms. Behav. Brain Res 210, 280–283. 10.1016/j.bbr.2010.02.038 [DOI] [PubMed] [Google Scholar]
- László K, Tóth K, Kertes E, Péczely L, Ollmann T, Madarassy-Szücs A, Lénárd L, 2012. The role of neurotensin in passive avoidance learning in the rat central nucleus of amygdala. Behav. Brain Res 226, 597–600. 10.1016/j.bbr.2011.08.041 [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo Y-H, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG, 2011. Leptin Action via Neurotensin Neurons Controls Orexin, the Mesolimbic Dopamine System and Energy Balance. Cell Metab. 14, 313–323. 10.1016/j.cmet.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti M, Brun P, Clerget M, Steinberg R, Soubrié P, Renaud B, Suaud-Chagny MF, 2004. Specific involvement of neurotensin type 1 receptor in the neurotensinmediated in vivo dopamine efflux using knock-out mice: NT1R induces in vivo dopamine efflux in KO mice. J. Neurochem 89, 1–6. 10.1046/j.14714159.2003.02231.x [DOI] [PubMed] [Google Scholar]
- Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Fan TW-M, Weiss HL, Dobner PR, Melander O, Jia J, Evers BM, 2016. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 533, 411–415. 10.1038/nature17662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ, 1982. The effect of neurotensin on food consumption in the rat. Eur. J. Pharmacol 81, 499–503. 10.1016/0014-2999(82)90116-9 [DOI] [PubMed] [Google Scholar]
- Maracle AC, Normandeau CP, Dumont ÉC, Olmstead MC, 2019. Dopamine in the oval bed nucleus of the stria terminalis contributes to compulsive responding for sucrose in rats. Neuropsychopharmacology 44, 381–389. 10.1038/s41386-018-0149y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Vincent J-P, 2006. Functional roles of the NTS2 and NTS3 receptors. Peptides 27, 2469–2475. 10.1016/j.peptides.2006.04.026 [DOI] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ, 2018. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eneuro 5, ENEURO.0010–18.2018 10.1523/ENEURO.0010-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG, 2010. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc. Natl. Acad. Sci 107, 2271–2276. 10.1073/pnas.0905568107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG, 2009. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1329–1335. 10.1016/j.pnpbp.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, Stuber GD, 2017. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci 20, 449–458. 10.1038/nn.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Perez-Manso M, Daraio T, 2013. Delta-like 1 Homologue is a HypothalamusEnriched Protein that is Present in Orexin-Containing Neurones of the Lateral Hypothalamic Area. J. Neuroendocrinol 25, 617–625. 10.1111/jne.12029 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study Group, 2004. PlaceboControlled Evaluation of Four Novel Compounds for the Treatment of Schizophrenia and Schizoaffective Disorder. Am. J. Psychiatry 161, 975–984. 10.1176/appi.ajp.161.6.975 [DOI] [PubMed] [Google Scholar]
- Naganuma F, Kroeger D, Bandaru SS, Absi G, Madara JC, Vetrivelan R, 2019. Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLOS Biol. 17, e3000172 10.1371/journal.pbio.3000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, Izadmehr EM, Tye KM, 2016. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron 90, 1286–1298. 10.1016/j.neuron.2016.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG, 2011. β-Adrenergic Receptors Enhance Excitatory Transmission in the Bed Nucleus of the Stria Terminalis Through a Corticotrophin-Releasing Factor Receptor–Dependent and Cocaine-Regulated Mechanism. Biol. Psychiatry 69, 1083–1090. 10.1016/j.biopsych.2010.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandeau CP, Torruella Suárez ML, Sarret P, McElligott ZA, Dumont EC, 2018a. Neurotensin and dynorphin Bi-Directionally modulate CeA inhibition of oval BNST neurons in male mice. Neuropharmacology 143, 113–121. 10.1016/j.neuropharm.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandeau CP, Ventura-Silva AP, Hawken ER, Angelis S, Sjaarda C, Liu X, Pêgo JM, Dumont ÉC, 2018b. A Key Role for Neurotensin in Chronic-Stress-Induced AnxietyLike Behavior in Rats. Neuropsychopharmacology 43, 285–293. 10.1038/npp.2017.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M, Leinninger G, 2013. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol. Metab 2, 423–434. 10.1016/j.molmet.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ, 1981. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294, 587–589. 10.1038/294587a0 [DOI] [PubMed] [Google Scholar]
- Pandey S, Badve PS, Curtis GR, Leibowitz SF, Barson JR, 2019. Neurotensin in the posterior thalamic paraventricular nucleus: inhibitor of pharmacologically relevant ethanol drinking: Neurotensin in PVT. Addict. Biol 24, 3–16. 10.1111/adb.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie KA, Bubser M, Casey CD, Davis MD, Roth BL, Deutch AY, 2004. The Neurotensin Agonist PD149163 Increases Fos Expression in the Prefrontal Cortex of the Rat. Neuropsychopharmacology 29, 1878–1888. 10.1038/sj.npp.1300494 [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Mallorga PJ, Pascarella DM, Snyder MA, Williams JB, Zeng Z, 2002. The Effects of Deleting the Mouse Neurotensin Receptor NTR1 on Central and Peripheral Responses to Neurotensin. J. Pharmacol. Exp. Ther 300, 305–313. 10.1124/jpet.300.1.305 [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Delle Donne KT, Boudin H, Pélaprat D, Rosténe W, 2001. Highaffinity neurotensin receptors in the rat nucleus accumbens: Subcellular targeting and relation to endogenous ligand: Neurotensin Receptors in Accumbens. J. Comp. Neurol 435, 142–155. 10.1002/cne.1198 [DOI] [PubMed] [Google Scholar]
- Poe GR, 2017. Sleep Is for Forgetting. J. Neurosci 37, 464–473. 10.1523/JNEUROSCI.0820-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO, 2019. Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning. Cell Rep. 29, 13–21.e4. 10.1016/j.celrep.2019.08.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, Hillhouse TM, LaCrosse AL, 2014. Acute, but not repeated, administration of the neurotensin NTS1 receptor agonist PD149163 decreases conditioned footshockinduced ultrasonic vocalizations in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 49, 78–84. 10.1016/j.pnpbp.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner C, Skov LJ, Raida Z, Bächler T, Bellmann-Sickert K, Le Foll C, Sivertsen B, Dalbøge LS, Hartmann B, Beck-Sickinger AG, Madsen AN, Jelsing J, Holst JJ, Lutz TA, Andrews ZB, Holst B, 2016. Effects of Peripheral Neurotensin on Appetite Regulation and Its Role in Gastric Bypass Surgery. Endocrinology 157, 3482–3492. 10.1210/en.2016-1329 [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou J-M, Le Fur G, Soubrié P, Caput D, Shire D, Kopf M, Ferrara P, 2002. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 953, 63–72. 10.1016/S0006-8993(02)03271-7 [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH, 2010. Corticotropin Releasing Factor–Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol. Psychiatry 67, 831–839. 10.1016/j.biopsych.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Maldonado R, Koob GF, 1993. Neurotensin injected into the nucleus accumbens blocks the psychostimulant effects of cocaine but does not attenuate cocaine self-administration in the rat. Brain Res. 622, 105–112. 10.1016/00068993(93)90808-Z [DOI] [PubMed] [Google Scholar]
- Salamone J, Correa M, Mingote S, Weber S, 2005. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr. Opin. Pharmacol 5, 34–41. 10.1016/j.coph.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG, 2015. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg 93, 75–93. 10.1159/000368279 [DOI] [PubMed] [Google Scholar]
- Samaha A-N, Seeman P, Stewart J, Rajabi H, Kapur S, 2007. “Breakthrough” Dopamine Supersensitivity during Ongoing Antipsychotic Treatment Leads to Treatment Failure over Time. J. Neurosci 27, 2979–2986. 10.1523/JNEUROSCI.5416-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, 2017. Sleep to Remember. J. Neurosci 37, 457–463. 10.1523/JNEUROSCI.0297-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Siemian JN, Petrella M, Laing BT, Sarsfield S, Borja CB, Gajendiran A, Zuccoli ML, Aponte Y, 2019. Activation of a lateral hypothalamic-ventral tegmental circuit gates motivation. PLOS ONE 14, e0219522 10.1371/journal.pone.0219522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A, Leysen JE, Laduron PM, 1986. Evidence for a displaceable non-specific [3H]neurotensin binding site in rat brain. Naunyn. Schmiedebergs Arch. Pharmacol 333, 400–405. 10.1007/BF00500016 [DOI] [PubMed] [Google Scholar]
- Schroeder LE, Furdock R, Quiles CR, Kurt G, Perez-Bonilla P, Garcia A, Colon-Ortiz C, Brown J, Bugescu R, Leinninger GM, 2019. Mapping the populations of neurotensin neurons in the male mouse brain. Neuropeptides 10.1016/j.npep.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder LE, Leinninger GM, 2018. Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders. Biochim. Biophys. Acta BBA - Mol. Basis Dis 1864, 900–916. 10.1016/j.bbadis.2017.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Lynch AM, Aravagiri M, Nemeroff CB, Owens MJ, 1995. Chronic haloperidolinduced changes in regional dopamine release and metabolism and neurotensin content in rats. Brain Res 704, 202–209. 10.1016/0006-8993(95)01114-5 [DOI] [PubMed] [Google Scholar]
- Servonnet A, Minogianis E-A, Bouchard C, Bédard A-M, Lévesque D, Rompré P-P, Samaha A-N, 2017. Neurotensin in the nucleus accumbens reverses dopamine supersensitivity evoked by antipsychotic treatment. Neuropharmacology 123, 10–21. 10.1016/j.neuropharm.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Shilling P, Feifel D, 2008. The neurotensin-1 receptor agonist PD149163 blocks fear-potentiated startle. Pharmacol. Biochem. Behav 90, 748–752. 10.1016/j.pbb.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, Ogawa N, Shibasaki T, Takagi H, 1989. Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience 30, 377–383. 10.1016/0306-4522(89)90259-5 [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wise PM, 2001. Neurotensin Gene Expression Increases during Proestrus in the Rostral Medial Preoptic Nucleus: Potential for Direct Communication with Gonadotropin-Releasing Hormone Neurons*. Endocrinology 142, 3006–3013. 10.1210/endo.142.7.8256 [DOI] [PubMed] [Google Scholar]
- Smits SM, Terwisscha van Scheltinga AF, van der Linden AJA, Burbach JPH, Smidt MP, 2004. Species differences in brain pre-pro-neurotensin/neuromedin N mRNA distribution: the expression pattern in mice resembles more closely that of primates than rats. Mol. Brain Res 125, 22–28. 10.1016/j.molbrainres.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Sotty F, Brun P, Leonetti M, Steinberg R, Soubrié P, Renaud B, Suaud-Chagny MF, 2000. Comparative effects of neurotensin, neurotensin(8–13) and [D-Tyr11]neurotensin applied into the ventral tegmental area on extracellular dopamine in the rat prefrontal cortex and nucleus accumbens. Neuroscience 98, 485–492. 10.1016/S0306-4522(00)90023-X [DOI] [PubMed] [Google Scholar]
- Sotty F, Soulière F, Brun P, Chouvet G, Steinberg R, Soubrié P, Renaud B, Suaud-Chagny MF, 1998. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: A combined in vivo electrochemical and electrophysiological study. Neuroscience 85, 1173–1182. 10.1016/S03064522(97)00691-X [DOI] [PubMed] [Google Scholar]
- Steele FF, Whitehouse SC, Aday JS, Prus AJ, 2017. Neurotensin NTS 1 and NTS 2 receptor agonists produce anxiolytic-like effects in the 22-kHz ultrasonic vocalization model in rats. Brain Res. 1658, 31–35. 10.1016/j.brainres.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Steinberg R, Bougault I, Souilhac J, Gully D, Fur GL, Soubrié P, 1994. Blockade of neurotensin receptors by the antagonist SR 48692 partially prevents retrograde axonal transport of neurotensin in rat nigrostriatal system. Neurosci. Lett 166, 106–108. 10.1016/0304-3940(94)90851-6 [DOI] [PubMed] [Google Scholar]
- Studler J-M, Kitabgi P, Tramu G, Herve D, Glowinski J, Tassin J-P, 1988. Extensive Colocalization of neurotensin with dopamine in rat meso-cortico-frontal dopaminergic neurons. Neuropeptides 11, 95–100. 10.1016/0143-4179(88)90076-5 [DOI] [PubMed] [Google Scholar]
- Szigethy E, Beaudet A, 1989. Correspondence between high affinity125I-neurotensin binding sites and dopaminergic neurons in the rat substantia nigra and ventral tegmental area: A combined radioautographic and immunohistochemical light microscopic study. J. Comp. Neurol 279, 128–137. 10.1002/cne.902790111 [DOI] [PubMed] [Google Scholar]
- Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, Patel GK, McHenry JA, Hardaway JA, Kantak PA, Crowley NA, DiBerto JF, Faccidomo SP, Hodge CW, Stuber GD, McElligott ZA, in press Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice (article). Journal of Neuroscience. 10.1101/245274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumi CW, Beckstead MJ, 2019. Diverse actions of the modulatory peptide neurotensin on central synaptic transmission. Eur. J. Neurosci 49, 784–793. 10.1111/ejn.13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E, 2000. Neurotensin: peptide for the next millennium. Regul. Pept 93, 125–136. 10.1016/S0167-0115(00)00183-X [DOI] [PubMed] [Google Scholar]
- Tyree SM, de Lecea L, 2017. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front. Syst. Neurosci 11, 50 10.3389/fnsys.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Goodman RR, Snyder SH, 1979. Neurotensin-containing cell bodies, fibers and nerve terminals in the brain stem of the rat: Immunohistochemical mapping. Brain Res. 167, 77–91. 10.1016/0006-8993(79)90264-6 [DOI] [PubMed] [Google Scholar]
- Uhl GR, Kuhar MJ, Snyder SH, 1977. Neurotensin: immunohistochemical localization in rat central nervous system. Proc. Natl. Acad. Sci 74, 4059–4063. 10.1073/pnas.74.9.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnie CA, Ayers-Ringler J, Oliveros A, Abulseoud OA, Choi S, Hitschfeld MJ, Choi D-S, 2016. Antipsychotic-like effects of a neurotensin receptor type 1 agonist. Behav. Brain Res 305, 8–17. 10.1016/j.bbr.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva C, Jacquier S, de Roux N, 2012. DLK1 Is a Somato-Dendritic Protein Expressed in Hypothalamic Arginine-Vasopressin and Oxytocin Neurons. PLoS ONE 7, e36134 10.1371/journal.pone.0036134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J-P, Mazella J, Kitabgi P, 1999. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci 20, 302–309. 10.1016/S0165-6147(99)01357-7 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R, 2017. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci 18, 741–752. 10.1038/nrn.2017.130 [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR, 1996. Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res 721, 196–203. 10.1016/0006-8993(96)00132-1 [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM, 1998. Transcriptional Effects of Estrogen on Neuronal Neurotensin Gene Expression Involve cAMP/Protein Kinase A-Dependent Signaling Mechanisms. J. Neurosci 18, 6672–6680. 10.1523/JNEUROSCI.18-17-06672.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Kelly AB, Sanchez-Watts G, 1995. Neuropeptides and thirst: The temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav. Neurosci 109, 1146–1157. 10.1037/0735-7044.109.6.1146 [DOI] [PubMed] [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R, 2007. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc. Natl. Acad. Sci 104, 12199–12204. 10.1073/pnas.0705312104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, 2004. Dopamine, learning and motivation. Nat. Rev. Neurosci 5, 483–494. 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]
- Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, Fuller PM, Leinninger GM, 2017a. Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance. Cell Rep 20, 1881–1892. 10.1016/j.celrep.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Beekly BG, Batchelor HM, Bugescu R, Perez-Bonilla P, Schroeder LE, Leinninger GM, 2017b. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep 21, 3116–3128. 10.1016/j.celrep.2017.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Brown JA, Batchelor HM, Bugescu R, Leinninger GM, 2018a. Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides 68, 57–74. 10.1016/j.npep.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Perez-Bonilla PA, Beekly BG, Lewis TJ, Leinninger GM, 2018b. Identification of Neurotensin Receptor Expressing Cells in the Ventral Tegmental Area across the Lifespan. eneuro 5, ENEURO.0191–17.2018 10.1523/ENEURO.0191-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woulfe J, Beaudet A, 1989. Immunocytochemical evidence for direct connections between neurotensin-containing axons and dopaminergic neurons in the rat ventral midbrain tegmentum. Brain Res 479, 402–406. 10.1016/0006-8993(89)91649-1 [DOI] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG, 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. 10.1038/nature13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wustrow DJ, Davis MD, Akunne HC, Corbin AE, Wiley JN, Wise LD, Heffner TG, 1995. Reduced amide bond neurotensin 8–13 mimetics with potent in vivo activity. Bioorg. Med. Chem. Lett 5, 997–1002. 10.1016/0960-894X(95)00155-M [DOI] [Google Scholar]
- Yamada D, Wada E, Amano T, Wada K, Sekiguchi M, 2010. Lack of neurotensin type 1 receptor facilitates contextual fear memory depending on the memory strength. Pharmacol. Biochem. Behav 96, 363–369. 10.1016/j.pbb.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Wada E, Kamichi S, Yamada D, Maeno H, Delawary M, Nakazawa T, Yamamoto T, Wada K, 2007. Neurotensin type 2 receptor is involved in fear memory in mice: Neurotensin receptor 2 modulates fear memory. J. Neurochem 102, 1669–1676. 10.1111/j.1471-4159.2007.04805.x [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Cheng AY, Lavezzi HN, Parsley KP, Zahm DS, 2015. Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: A study in rat: RMTg, VTA, and LHb afferents compared. J. Comp. Neurol 523, 2426–2456. 10.1002/cne.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Barr A, Ramos-Miguel A, Procyshyn R, 2016. Antipsychotic Induced Dopamine Supersensitivity Psychosis: A Comprehensive Review. Curr. Neuropharmacol 15, 174–183. 10.2174/1570159X14666160606093602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, 1999. Functional-anatomical Implications of the Nucleus Accumbens Core and Shell Subterritories. Ann. N. Y. Acad. Sci 877, 113–128. 10.1111/j.17496632.1999.tb09264.x [DOI] [PubMed] [Google Scholar]
- Zahm DS, 1992. Subsets of neurotensin-immunoreactive neurons revealed following antagonism of the dopamine-mediated suppression of neurotensin immunoreactivity in the rat striatum. Neuroscience 46, 335–350. 10.1016/03064522(92)90056-8 [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Bérod A, 2001. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience 104, 841–851. 10.1016/S0306-4522(01)00118-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


