ABSTRACT
Huanglongbing (HLB) is a highly destructive disease to the citrus industry in Florida caused by the bacterium, Candidatus Liberibacter asiaticus(CLas) and is transmitted by Diaphorina citri. It is hypothesized that plants with high phenolic contents show higher tolerance to certain plant pathogens. In this regard, different citrus varieties and plants of genera related to Citrus were analyzed for their total phenolic and flavonoids contents, and their antioxidant capacities. In addition, the secondary metabolites in the leaves of seven citrus species were analyzed using high performance liquid chromatography-mass spectrometry (HPLC-MS). Colorimetric assays showed that curry leaf contained the highest total phenolic content and free radical scavenging activity (DPPH). Curry leaf also contained high concentrations of an unusual class of carbazole alkaloids. Tolerant Citrus species contained high levels of phenolics and flavonoids and showed high antioxidant capacities. Our results suggest that high phenolic and flavonoid leaf contents correlate with increased citrus tolerance to CLas bacterium. The results also suggest that the high level of carbazole alkaloids, known for their strong antimicrobial properties in curry leaf, could make it immune to the CLas bacteria. Understanding the mechanisms underpinning citrus tolerance to HLB will contribute to the development of commercially tolerant citrus cultivars.
KEYWORDS: Citrus, Huanglongbing, phenolics, flavonoids, antioxidants
Introduction
In North America, Huanglongbing (HLB) is caused by the bacterium Candidatus Liberibacter asiaticus (CLas) and is transmitted by the Asian citrus psyllid, Diaphorina citri. Currently, there is no cure for the HLB disease and its management mainly depends on insecticides to control the insect vector (D. citri).1 Several other management strategies including enhanced nutritional programs, thermotherapy, and removal of infected trees have been tested for the control of HLB; however, these strategies have usually shown poor results in the field.2 For these reasons, the development of CLas-resistant citrus cultivars might be the best means for the control of the HLB disease.
Field observations showed thatMurraya paniculata (L.) Jack (orange jasmine) and sweet oranges were the most preferred host for D. citri, while Poncirus trifoliata was a poor host.3 A greenhouse-controlled study showed that grapefruit was a more preferable host than Citrus jambhiri Lushington (rough lemon), Murraya paniculata, and Citrus aurantium L. (sour orange).4 Similarly, it was found that D. citri preferred Citrus macrophylla Wester (Alemow) more than accessions of Poncirus trifoliata x Citroncirus spp.5 Fewer eggs were laid on P. trifoliata accessions than C. macrophylla.5 The resistance of P. trifoliateto D. citri was attributed to the effect of antixenosis (deterrents) and antibiosis (antagonistic association).5
In a similar manner, field observations have shown that some citrus cultivars are more tolerant to the HLB pathogen than others.3 Using graft inoculation, Folimonova et al. (2009) studied the responses of several citrus species to CLas, and in agreement with the previous reports, certain tested species showed different degrees of tolerance to CLas. For example, Poncirus trifoliata, Eureka lemon (C. limonia Osbeck) and Citrus latipes were tolerant to the CLas bacterium, whereas sweet oranges and grapefruits were very sensitive.6 The sensitive species showed severe leaf chlorosis, decreased growth and death, whereas tolerant cultivars showed little or no symptoms. In a similar study, Malaysian citrus species also showed different degrees of tolerance to CLas pathogen.7
Unfortunately, it is not clear why are some citrus species are more tolerant to CLas than others. In order to examine possible mechanisms underpinning the tolerance of some cultivars, we investigated the metabolite profiles of several cultivars of citrus.8,9 Our results showed that tolerant cultivars contained higher levels of metabolites that possess strong antimicrobial activities and compounds implicated in plant defense.8,9 In another study, we analyzed the metabolite profiles of ‘Sugar Belle’ mandarin and four of its parents in order to understand why ‘Sugar Belle’ was more tolerant to HLB.10 Our metabolomic analysis showed that “Sugar Belle” mandarin was high in thymol, which has a strong anti-fungal and antimicrobial activity.10 ‘Sugar Belle’ was also high in several other polar compounds including benzoic acid, ferulic acid, caffeic acid, synephrine, and inositol, which may protect it from biotic and abiotic stress.10 We also showed that the metabolomic profile of Australian finger lime, a highly tolerant Citrus relative, was different from that of ‘Sugar Belle’, indicating that they could have different tolerance mechanisms.11
Plants produce a wide variety of secondary metabolites that can act as antimicrobial substances such as alkaloids, flavonoids, and phenols. Phenolics are a group of secondary metabolites, which are produced via the shikimic acid pathway through the phenylpropanoid pathways.12 It is believed that accumulation of phenolic compounds at the infection site could result in isolation of the attacking pathogens and prevent their spread to other plant’s tissues.13 In addition, crosslinking of phenylpropanoid esters resultsin the formation of lignin-like polymers, which leads to the stiffening of the plant cell wall.13 Previous reports also showed that apple resistance to Venturia inaequalis depends on the regulatory genes of phenol synthesis.14 The total phenolics and shikimic acid contents in Venturia inaequalis-tolerant cultivars are higher than susceptible apple cultivars.15
Flavonoids are widely distributed in plants and they are synthesized in the cytosol through the phenylpropanoid pathway by a set of enzymes.16,17 Previous reports showed that flavonoids were implicated in plant resistance to biotic (herbivory and nematodes) and abiotic stresses including drought, cold, UV-radiation, and toxic metals such as aluminum.16 Flavonoids are also implicated in plant resistance to fungal and microbial attacks. Flavonoids could exhibit their resistance to pathogens by inhibition and crosslinking of the microbial enzymes, chelation of metals necessary for enzyme activity, and formation of physical barrier.16
The total phenolic content is normally measured using the Folin-Ciocalteau method, which is based on the reaction between Folin-Ciocalteau reagent with reductants such as phenols, resulting in blue color formation.18 Yet a major limitation to this assay is its lack of specificity and the results of this assay cannot be unequivocally attributed to a single class of reductants (i.e., phenolics) in biological tissues. Flavonoids are generally estimated using aluminum chloride, which forms acid stable complexes with the C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonol.19 Yet, in complex plant extracts, even this method can show responses to compounds unrelated to flavones and flavonoids. The antioxidant activity is normally estimated using the DPPH assay, which is based on the ability of antioxidants to transfer an electron or a hydrogen to the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH).20,21
In this study, we investigated the total phenolic and flavonoid contents as well as the antioxidant and reducing activity of 21 citrus species and twoCitrus relatives. We hypothesize that CLas-tolerant cultivars contain higher amounts of phenolic and flavonoid compounds and that they will have higher antioxidant and/or reducing activity compared to susceptible cultivars. Furthermore, we investigated the secondary metabolites of a representative subclass of these species covering a wide range of CLas tolerance. This was done with the intention to better understand the mechanisms behind citrus tolerance to HLB and to help citrus breeders to develop commercially tolerant citrus cultivars.
Materials and methods
Plant materials
Healthy seedlings from 24 citrus cultivars and Citrus relatives were used in this study (Table 1). Seeds were purchased from Lyn Citrus Seed, Inc. (Arvin, CA, USA) and individually potted in square plastic pots (30 × 10 × 10 cm) containing Sungro professional growing mix (Sungro Horticulture, Agawam, MA). Germinated seedlings were kept in a greenhouse (28 ± 1ºC, 60 ± 5% relative humidity, L16:D8 h photoperiod) at the Citrus Research and Education Center (CREC), University of Florida, Lake Alfred, Florida. Seedlings were watered twice weekly. All seedlings were about one-year old. Five plants (about one-year-old) were sampled from each variety by collecting three mature leaves from three different locations (top, middle, and bottom).
Table 1.
Citrus species and citrus relatives used in this study and their degree of tolerance to CLas.
| Species used in this study | Degree of tolerance to CLas | |
|---|---|---|
| 1 | Valencia sweet orange (C. sinensis (L.) Osbeck) | 1a |
| 2 | Madam Vinous sweet orange (C. sinensis (L.) Osbeck) | 1a |
| 3 | Hamlin sweet orange (C. sinensis L. Osbeck) | 1a |
| 4 | Duncan grapefruit (C. paradisi MacFadyen) | 1a |
| 5 | Ruby red grapefruit (C. paradisi MacFadyen) | 1a |
| 6 | Sour orange (C. aurantium L.) | 2a |
| 7 | C. macrophylla Wester (Alemow) | 2a |
| 8 | Mexican lime (C. aurantifolia (Christm.) | 2a |
| 9 | Carrizo citrange (X Citroncirus webberi) | 3a |
| 10 | Citrus latipes | 3a |
| 11 | Minneola tangelo | 1a |
| 12 | Citron (Citrus medica) | 2a |
| 13 | Cleopatra mandarin | 1a |
| 14 | Sun Chu Sha Kat mandarin (C. reticulata Blanco RUTACEAE) | 2b |
| 15 | Orange jasmine (Murraya paniculata) | 1b |
| 16 | Pineapple sweet orange | 1b |
| 17 | Sugar Belle tangerine | 2b |
| 18 | Finger lime (Citrus australasica) | 2b |
| 19 | Curry leaf (Murraya koenigii (L.) Spreng) | 4b |
| 20 | Bearss lime (C. latifolia) | 2b |
| 21 | Frost Marsh grapefruit (Citrus paradisi Macfadyen) | 2b |
| 22 | Bingo tangerine ‘LB8-9’ | 2b |
| 23 | Dancy Tangerine | 3b |
aFolimonova et al.6
bField and greenhouse observations.
Extraction of phenolics and flavonoids from plant tissues
Leaves collected from each variety were pooled and ground in liquid nitrogen using a mortar and pestle, and 100 mg of the homogenous sample was transferred to a 2-mL centrifuge tube. One ml of methanol was added to each tube and the sample was vortexed at 750 rpm for 15 min at 20ºC using Eppendorf Thermomixer followed by sonication for 15 min. The samples were centrifuged at 12,000 rpm for 10 min at 20ºC. The supernatant was transferred into a new tube and kept at −20ºC until analysis.
Total Phenolic Content (TPC)
Total phenolics were determined using Folin-Ciocalteau reagents according to Singleton & Rossi (1965).22 Plant extracts or gallic acid standard (20 µl) were mixed with 0.9 mL of Folin-Ciocalteu reagent (10% in water), and then 0.6 mL of sodium carbonate (7.5%, w/v) was added to the mixture. After standing for 60 min at room temperature, absorbance was measured at 760 nm. Aqueous solutions of known gallic acid concentrations in the range of 100–600 ppm were used for calibration. Results were expressed as mg gallic acid equivalents (GAE) g−1fresh weight (FW).
Total Flavonoid Content (TFC)
Determination of total flavonoids was performed according to the colorimetric assay of Aktumsek et al. (2013) using aluminum chloride.23 Distilled water (200 µL) was added to 50 µL of the extract in a test tube. A 15 µL portion of 5% sodium nitrite solution was added, followed by 15 µL of 10% aluminum chloride solution. Test tubes were incubated at ambient temperature for 5 min, and then 100 µL of 1 M sodium hydroxide and 1.2 mL of distilled water were added to the mixture. The mixture was vortexed and the absorbance of the resulting pink color was measured at 510 nm. Aqueous solutions of known catechin concentrations in the range of 10–200 ppm were used for calibration and the results were expressed as mg catechin equivalents (CEQ) g−1FW sample.
Free radical scavenging activity (DPPH assay)
DPPH assay was performed according to Brand-Williams et al.24 Briefly, a 1000 µL aliquot of a 0.1 mM of DPPH solution in methanol was added to 25 µl of each extract or Trolox standard (prepared in the range of 20–200 ppm, dissolved in methanol). The mixture was vortexed for 5–10 sec and incubated at room temperature in dark for 30 min and then the absorbance was measured at 515 nm. The results were expressed as mg Trolox g−1 FW.
Chromatographic conditions
The HPLC-MSanalysis of the extracts were run on eclipse C18 column (Eclipse 150 mm, 5 μm). The mobile phase was a mixture of 0.5% acetic acid solution (A) and acetonitrile (B) and was run in a linear gradient mode. The start was a 95% (A) that descended to 70% (A) in 40 min. Then to 40% (A) in 20 min and finally to 10% (A) in 2 min and stayed there for 6 min and then back to the initial conditions in 2 min. The HPLC system was equilibrated for 5 min with the initial acidic water mobile phase (95% A) before injecting next sample. All the samples were filtered with a 0.45 μm PTFE filter. The column temperature was set at 25°C.
A 20-µL aliquot of the final leaf extract was analyzed by HPLC–MS using a Waters Alliance 2695 HPLC coupled with a 996 photodiode array detector and a Waters ZQ single quad mass spectrometer. A flow splitter (10-to-1) was used to simultaneously monitor UV and mass spectra of the eluting analytes. UV spectra were monitored between 600 and 240 nm. ZQ parameters were as follows: MS parameters were as follows: ionization mode, ES+; capillary voltage 3.0 kV; extractor voltage 5 V; source temperature 100°C; desolvation temperature 225°C; desolvation N2 flow 465 L/h, cone N2 flow 70 L/h; scan range m/z 50–1000; scan rate 1 scan/s; and cone voltages 20 and 40 V. Analysis of the chromatograms was performed using ZQ calculated mass-extracted total ion chromatograms (TIC) obtained in scanning mode, or in the single ion response mode. To normalize the mass spectrometer response during sequential runs, an internal standard, mangiferin, was used. Data handling was done with the MassLynx software version 4.1.
Phenolics in methanol extract were tentatively identified/classified by their UV and mass spectra. The numbers of compounds in particular classes of phenols can were estimated with reasonable accuracy using the high peak resolution afforded by analytical-reversed-phase of the HPLC. Estimates of the levels of these compounds were obtained by using peak area-to-microgram conversion factors of authentic standards identified in each group. The measured conversion factors for ferulic acid, nobiletin, hesperidin, diosmin, vitexin, marmin, and mahanimbine were used for hydroxycinnamates, polymethoxylated flavones, flavanone glycosides, flavone-O-glycosides, flavone-C-glycosides, coumarins, and carbazole alkaloids, respectively.
Saponification of hydroxycinnamates in citrus leaf extracts
Portions (150 uL of each leaf extract) were combined with 50 uL 4 N KOH and 200 uL 2 N KOH. The samples were vortexed for 3 min and stored at room temperature for 1 h. The saponified samples were then mixed with 50uL glacial acid and concentrated HCL until the resulting solutions were adjusted to pH 4.5–6. The final volumes of the samples were brought to 1.0 mL with deionized water. Analytical HPLC using a Waters XBridge C8 column (150 x 4.6 mm), using the same solvent gradients as described above, was used to measure the amounts of liberated hydroxycinnamic acids.
Statistical analysis
Data were analyzed using JMP 9.0 software (SAS, Cary, NC). Analysis of variance (ANOVA) followed by post hoc pairwise comparisons using Tukey–Kramer honestly significant different test (Tukey HSD) were used to compare the studied parameters (TPC, TFC, DPPH, and hydroxycinnamates) among the selected varieties.
Results
Total phenolic content
The colorimetricFolin-Ciocalteauassay showed the highest total phenolic content (13.68 ± 1.71 mg GA/g) in the leaves of Murraya koenigii (curry) followed by Dancy tangerine (12.55 ± 0.93 mg GA g−1 FW), Carrizo citrange (11.15 ± 2.00 mg GAg−1 FW), and ‘LB8-9ʹ, Bingo (10.55 ± 1.94 mg GA g−1 FW) (Figure 1(a)). The Tukey’s test showed that the level of total phenolic content in curry leaf was significantly higher than all other cultivars (Figure 1(a)). The lowest phenolic content occurred in Duncan grapefruit (4.11 ± 0.57 mg GA g−1 FW), ruby red grapefruit (4.62 ± 0.26 mg GA g−1 FW), and C. macrophylla (4.79 ± 0.53 mg GA g−1 FW) leaves (Figure 1(a)). The Tukey’s test showed that the level of total phenolic content in Duncan grapefruit was significantly lower than all other cultivars. The rest of cultivars contained moderate levels of phenolic content between 5.31 and 9.37 mg GA g−1 FW (Figure 1(a)).
Figure 1.
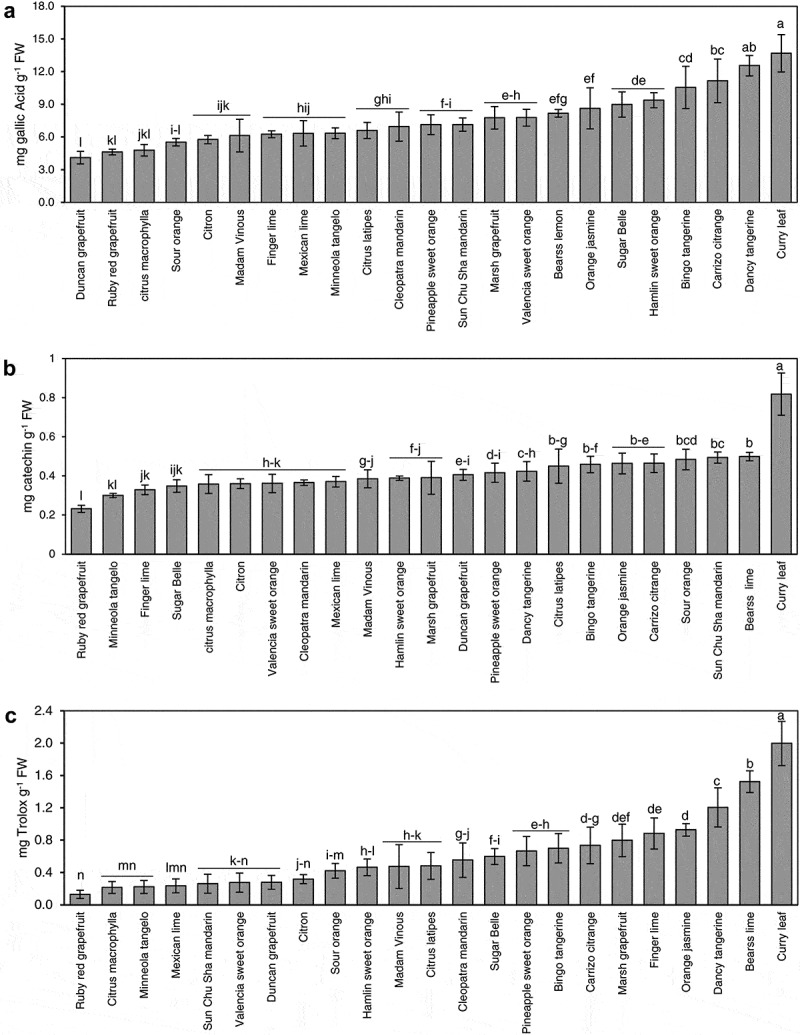
Total phenolics (a), flavonoids (b) contents, and antioxidant activity measured as (DPPH) (c) in selected citrus and citrus relative genotypes. Error bars represent standard deviation (n = 5). Varieties with different letters are statistically different using Tukey’s HSD test (P-value < 0.05).
Total flavonoid content
The highest level of flavonoids as measured by the aluminum chloride assay occurred in curry leaf (0.81 ± 0.10 mg g−1 FW), followed byBearss lime, Sunchu mandarin, sour orange, Carrizo citrange, orange jasmine, Bingo, C.latipes, and Carrizo citrange (0.49–0.43mgg−1 FW) (Figure 1(b)). The level of total flavonoids in curry leaf measured by the aluminum chloride assay was significantly higher than the rest of other plants (Figure 1(b)). The lowest level of flavonoids occurred in Ruby red (0.23 ± 0.018 mg g−1 FW) and Minneola tangelo (0.30 ± 0.010 mg g−1 FW) (Figure 1(b)). The rest of the cultivars contained moderate levels of flavonoids (with the range of 0.32–0.42 mg g−1 FW) (Figure 1(b)).
Total antioxidant activity
In agreement with the total phenolic and flavonoids contents, curry leaf was the highest free radical scavenging antioxidant activity DPPH (2.00 ± 0.27mgg−1 FW) (Figure 1(c)). Bearss lime was the second highest plant in DPPH (1.52 ± 0.13 mg g−1 FW), followed by Dancy tangerine (1.20 ± 0.24 mg/g), orange jasmine (0.92 ± 0.078 mg g−1 FW) and finger lime (0.88 ± 0.19 mg g−1 FW) (Figure 1(c)). Ruby red grapefruit had the lowest free radical scavenging activity (0.13 ± 0.051 mg g−1 FW) (Figure 1(c)). The rest of the cultivars showed moderate free radical scavenging activity (0.21–0.8 mg g−1 FW) (Figure 1(c)).
HPLC-MS results
Results of the HPLC-MS analyses of the phenolics in seven of the varieties in this study are summarized in Table 2. The main groups of compounds detected in each variety are shown in Figure 2. The number of compounds detected in each class of plant phenols and the calculated estimates of the sum total amounts in each class are given in Table 2 for leaves of curry, Duncan, and Ruby Red grapefruits, Bearss lime, Carrizo citrange, Valencia orange, and Dancy tangerine leaves. The results in Table 2 show that Dancy tangerine and Bears lime contained the highest summed values for flavone-O-glycosides and flavone-C-glycosides. Dancy tangerine contained the highest amounts of polymethoxylated flavonoids (PMFs, and was also the highest in hydroxycinnamates (Table 2). Unique to curry leaf were the high concentration of carbazole alkaloids. Calculations based on the known compound mahanimbine show an average carbazole alkaloid concentration of about 14 mg/g.
Table 2.
Concentrations (mgg−1 FW) of main classes of metabolites detected in seven citrus genotypes (n = 5).
| Species | Chemical group |
||||||
|---|---|---|---|---|---|---|---|
| FN-C-glyc | FN-O-glyc | Flavanone | HCA | PMF | Psoralen | Carbazole | |
| Bearss lime | 4.73 (10) | 11.09 (7) | 0.35 (1) | 0.19 (2) | 0 | 0.095 (2) | 0 |
| Valencia orange | 0.91 (7) | 1.87 (7) | 3.65 (2) | 0.10 (1) | 1.03 (11) | 0 | 0 |
| Ruby red grapefruit | 0.93 (4) | 4.17 (6) | 2.13 (7) | 0.15 (2) | 0.04 (3) | 0.13(2) | 0 |
| Duncan grapefruit | 0.11 (2) | 1.16 (9) | 0.28 (9) | 0.031 (2) | 0.10 (5) | 0.05 (1) | 0 |
| Dancy tangerine | 4.88 (7) | 1.92 (2) | 5.46 (2) | 4.88 (9) | 7.18 (12) | 0 | 0 |
| Carrizo citrange | 2.39 (8) | 2.27 (4) | 3.73 (2) | 0.55 (15) | 2.66 (6) | 0 | 0 |
| Curry leaf | 0 | 0.59 (4) | 0 | 0.75 (15) | 0 | 0 | 14.3 (14) |
FN-C-glyc: Flavone-C-glycosides, FN-O-glyc: flavone-O-glycosides.
Figure 2.
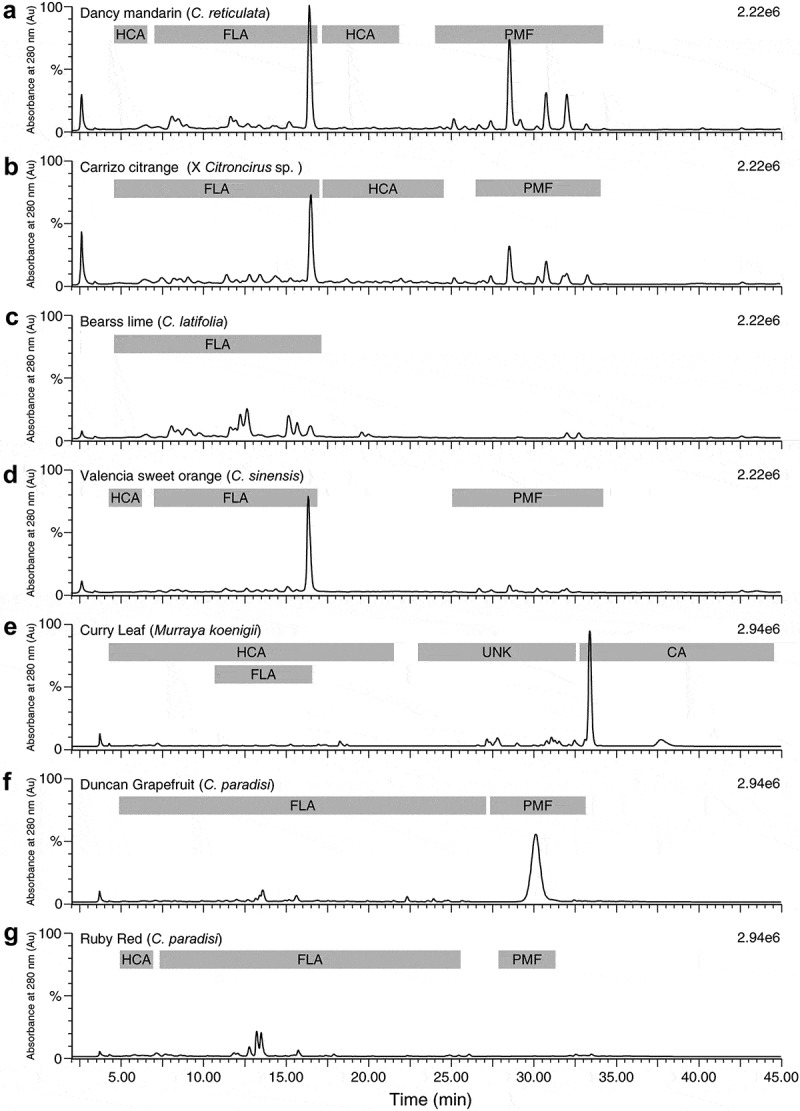
Representative HPLC chromatograms of selected citrus and citrus relative genotypes (a: Dancy mandarin, b; Carrizo citrange, c; Bearss lime, d; Valencia sweet orange, e; Curry leaf, f; Duncan grapefruit, and g; Ruby red) showing main peaks at 280 nm.
Accurate measurements of the total concentrations of ferulic, sinapinic, and p-coumaric acid-bound hydroxycinnamates were obtained by HPLC analysis of saponified leaf extracts. Dancy tangerine was shown to contain the highest levels of total bound hydroxycinnamic acids (4.30mgg−1 FW) followed by Carrizo citrange (3.6 mg/g FW), Bearss lime (1.90mgg−1 FW) and curry leaf (1.50mgg−1 FW) (Table 3). Curry leaf was the highest in P-coumaric acid followed by Bears lime (Table 3). Dancy tangerine was the highest in ferulic acid followed by Carrizo citrange (Table 3). The two grapefruit varieties, Ruby Red and Duncan contained the lowest levels of bound hydroxycinnamic acids. Curry leaves differed from the other citrus varieties by having a significantly higher p-coumaric acid content (1.30mgg−1 FW) than the other leaf sources.
Table 3.
Concentrations (mg g−1 FW) of hydroxycinnamic acids existing as hydrolysable hydroxycinnamates in seven citrus leaf extracts. Hydroxycinnamic acids measured after leaf extract saponification (n = 5).
| Species | P-Coumaric | Sinapic | Ferulic |
|---|---|---|---|
| Bearss lime | 0.62 ± 0.08b | 0.84 ± 0.05a | 0.49 ± 0.04b |
| Valencia orange | 0.11 ± 0.01d | 0.45 ± 0.04 c | 0.66 ± 0.38b |
| Ruby Red grapefruit | 0.12 ± 0.01d | 0.06 ± 0.01e | 0.61 ± 0.05b |
| Duncan grapefruit | 0.11 ± 0.01d | 0.03 ± 0.02e | 0.60 ± 0.41b |
| Dancy tangerine | 0.28 ± 0.03 c | 0.57 ± 0.02b | 3.44 ± 0.16a |
| Carrizo citrange | 0.26 ± 0.30 cd | 0.32 ± 0.01d | 2.98 ± 2.66ab |
| Curry leaf | 1.29 ± 0.30a | 0.03 ± 0.01e | 0.18 ± 0.04 c |
Varieties with different superscript letters are statistically different using Tukey’s HSD test (P-value < 0.05).
Discussion
Although field observations and greenhouse studies showed that certain citrus genotypes are more tolerant to HLB than other genotypes, the mechanisms behind this tolerance remained largely unknown. In the broad view, citrus tolerance to HLB could result from resistance to the insect vector or to the plant pathogen. The resistance to the vector insect could result from several reasons including antibiosis or antixenosis, whereas as resistance to the CLas bacterium could result from many different factors, primarily which is the presence of bacteriostatic and bactericidal compounds. In our current investigation, we measured the total phenolic and flavonoid contents as well as the DPPH activity of 23 citrus genotypes to test if any of these criteria correlated closely with citrus tolerance to the CLas bacterium.
Total phenolics, total flavonoids, and antioxidant activities can be measured using several methods with different mechanisms. For example, total flavonoids can be measured by colorimetric methods using aluminum chloride and 2,4-dinitrophenylhydrazine. Among the different classes of flavonoids, the aluminum chloride assay is specific for flavones and flavonols, while the 2,4-dinitrophenylhydrazine method is specific for flavanones.19 In the same manner, several methods with different mechanisms have been developed to determine the antioxidant activities in plants and foods.25 Consequently, it is recommended to use more than one method to measure the total antioxidant activities of a biological tissue.25 In this investigation, we measured the total phenolic and flavonoid contents as well as the DPPH activity in the leaves of a wide range of citrus varieties using colorimetric assays, and further analyzed the methanol extract of seven of the selected genotypes by HPLC-MS. To gain an accurate measure of the bound hydroxycinnamic acids in the leaf tissues, the leaf extracts were also saponified and analyzed by HPLC-MS.
The colorimetric assays showed that curry leaf was the highest in total phenolic, total flavonoids, and free radical scavenging antioxidant activity (DPPH). The HPLC-MS analysis showed that curry leaf was high in hydroxycinnamates. However, only four flavonoids were detected and the estimated total flavonoid content in curry leaf was low (0.60 mg g−1 FW). These results indicated that phenolic compounds in curry leaf were interfering with the aluminum chloride assay. In agreement with the HPLC-MS results, saponification of the methanol extracts showed that curry leaf was the highest in p-coumaric acid, which has 1.4 Trolox equivalent antioxidant capacity (TEAC).26 Our results were in agreement with previous reports, which showed high phenolic contents as well as high antioxidant capacities in curry leaves.27 In another study, curry leaf was also found to be rich in gallic acid and total phenolic compounds, and showed high DPPH value.28
The HPLC-MS analysis also showed that curry leaf was high in carbazole alkaloid contents. The alkaloid, mahanimbine, and the essential oil of curry leaf showed strong antibacterial activities against several antibiotic-resistant pathogenic bacteria and were effective against several cancer cell lines.29 In a separate study, the methanol extract of curry leaves showed strong antimicrobial activities against several pathogens including Staphylococcus, Streptococcus, and E. coli.30 The antibacterial activities of the curry leaf extract were comparable with gentamycin and amikacin.30 Carbazole compounds from curry leaf also showed high oil stability index and a strong radical scavenging activity against DPPH.31 The DPPH radical scavenging activities of most carbazoles were stronger than that of α-tocopherol.31 The previous results together indicated that carbazole alkaloids could have a strong antimicrobial activity against CLas. However, future research is needed to confirm this suggestion.
Our results showed that Dancy tangerine leaves were second highest in total phenolic compounds and the third in antioxidant activity. In agreement with the colorimetric assays, HPLC-MS also showed that Dancy tangerine was rich in hydroxycinnamates, flavanones and flavones, and polymethoxylated flavones. The saponification results showed that Dancy tangerine was rich in ferulic and sinapic acid. High levels of hydrolyzable hydroxycinnamic acids were earlier found in Dancy tangerine peel.32 Levels of polymethoxylated flavones in Dancy tangerines were several times higher than in most other citrus cultivars.32 Our earlier research showed that several hydroxycinnamates and polymethoxylated flavones were induced in CLas-infected sweet orange trees.33 These results suggested that hydroxycinnamates and polymethoxylated flavones could be involved in citrus defense against CLas. Induction of hydroxycinnamates was also observed in different plants upon bacterial and viral infections.33-35 It is believed that hydroxycinnamates could act as direct antimicrobial agents and signal molecules, and can be deposited into the cell wall to reinforce it against pathogen attack.36 These observations further indicate that Dancy tangerine could be tolerant to the HLB pathogen through its high phenolic content.
Bearss lime was the second in total flavonoids, DPPH, and it contained moderate amounts of total phenolic. In agreement with the colorimetric result, the HPLC-MS results showed that Bearss lime was rich in flavonoids (22 compounds). In addition, the saponification results showed that Bearss lime was the highest in sinapic acid and the fourth in total hydroxycinnmates. These results indicated that it could show some tolerance to the HLB disease.
Previous studies showed that C. citrange was relatively tolerant to the CLas pathogen.6 Our current results showed that C. citrange was the third highest variety in total phenolic and the fifth in total flavonoids, and the seventh in DPPH. In agreement with these results, the HPLC-MS analysis showed that C. citrange was rich in hydroxycinnamates (15 compounds) and it contained moderate amount of flavonoids (20 compounds). Saponification of the methanol extract also showed that C. citrange was rich in ferulic acid. Our previous study showed that C. citrange was the highest among 13 different varieties in total volatiles and sesquiterpenes and second in total monoterpenes.8 With respect to single volatiles, C. citrange was the highest cultivar in t-caryophyllene, γ-elemene, d-limonene, β-elemene, and germacrene D.8 In addition, C. citrange was the second highest variety in quinic acid.9
Flavonoids, which are also considered as a group of phenolic compounds, have a good antioxidant activity.26,37 Recently, Zuo et al. (2019) showed that flavones such as luteolin and apigenin and flavonols (quercetin) had potent inhibitory effects on YbeY of CLas bacterium.38 YbeY is considered part of the 206 genes that make up the minimal bacterial genome set, which is necessary for the maturation of RNAs. In addition, the YbeY portion of the bacterium’s genetic code was found to play an important role in bacterial symbiosis and pathogenicity.38 Tested flavones and flavonols showed a significant reduction in CLas titer in HLB-infected citrus trees.38 On the other hand, the flavanones (hesperetin and naringenin) were found to have little to no inhibitory effect.38 The previous results suggested that HLB resistant plants could be generated via genetic or metabolic modification of the biosynthetic pathways of flavones and flavonols.38 Our previous study showed that several flavonoids compounds such as 6,8-di-C-glucosylapigenin, 2”-O-xylosylvitexin, hesperidin, sinensetin, and polymethoxylated flavones were induced in CLas-infected sweet orange trees.33 Taken together, these previous results suggest that flavonoids could play a role in citrus defense against CLas.
Conclusion
Our results showed that tolerant varieties contained higher levels of phenolic compounds and flavonoids than sensitive varieties. Curry leaf, which is immune toCLas, contained high level of hyrdoxycinnamates and carbazole alkaloids. Our results suggest that high levels of flavonoids and phenolic compounds might enhance citrus tolerance to HLB. Further studies into the roles of these compounds in imparting tolerance in citrus to the CLas bacterium may provide useful information for the control of citrus HLB disease.
Funding Statement
This work was supported by the Citrus Research and Development Foundation [19-015].
Acknowledgments
We thank Yasser Nehela for the technical assistance and Lorraine Jones for maintaining the trees in greenhouses. This work was kindly funded by Citrus Research and Development Foundation, grant number 19-015.
References
- 1.Tiwari S, Killiny N, Stelinski LL.. Dynamic insecticide susceptibility changes in Florida populations of Diaphorina citri (Hemiptera: psyllidae). J Econ Entomol. 2013;106:1–9. doi: 10.1603/EC12281. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein RA, Lorca GL, Teplitski M. Challenges for Managing Candidatus Liberibacter spp. (Huanglongbing disease pathogen): current control measures and future directions. Phytopathology. 2018;108:424–435. doi: 10.1094/PHYTO-07-17-0260-RVW. [DOI] [PubMed] [Google Scholar]
- 3.Halbert SE, Manjunath KL. Asian citrus psyllids (Sternorrhyncha: psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomol. 2004;87:330–353. doi: 10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2. [DOI] [Google Scholar]
- 4.Tsai JH, Liu YH. Biology of Diaphorina citri (Homoptera: psyllidae) on four host plants. J Econ Entomol. 2009;93:1721–1725. doi: 10.1603/0022-0493-93.6.1721. [DOI] [PubMed] [Google Scholar]
- 5.Richardson ML, Hall DG, Westbrook R, Stover EW, Duan YP. Resistance of Poncirus and Citrus x Poncirusgermplasm to the Asian Citrus Psyllid. J Citrus Pathol. 2014;1:266. [Google Scholar]
- 6.Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO. Examination of the responses of different genotypes of citrus to Huanglongbing (citrus greening) under different conditions. Phytopathology. 2009;99:1346–1354. doi: 10.1094/PHYTO-99-12-1346. [DOI] [PubMed] [Google Scholar]
- 7.Shokrollah. Differential reaction of citrus species in Malaysia to Huanglongbing (HLB) disease using grafting method. Am J Agric Biol Sci. 2009;4:32–38. doi: 10.3844/ajabssp.2009.32.38. [DOI] [Google Scholar]
- 8.Hijaz F, Nehela Y, Killiny N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal Behav. 2016;11:e1138193. doi: 10.1080/15592324.2016.1138193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killiny N, Hijaz F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal Behav. 2016;11:e1171449. doi: 10.1080/15592324.2016.1171449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killiny N, Valim MF, Jones SE, Omar AA, Hijaz F, Gmitter FG, Grosser JW. Metabolically speaking: possible reasons behind the tolerance of ‘Sugar Belle’ mandarin hybrid to Huanglongbing. Plant Physiol Biochem. 2017;116:36–47. doi: 10.1016/j.plaphy.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Killiny N, Jones SE, Nehela Y, Hijaz F, Dutt M, Gmitter FG, Grosser JW. All roads lead to Rome: towards understanding different avenues of tolerance to Huanglongbing in citrus cultivars. Plant Physiol Biochem PPB. 2018;129:1–10. doi: 10.1016/j.plaphy.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21(10):pii: E1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. doi: 10.1146/annurev.py.30.090192.002101. [DOI] [Google Scholar]
- 14.Michalek S, Mayr U, Treutter D, Lux-Endrich A, Gutmann M, Feucht W, Geibel M. Role of flavan-3-OLS in resistance of apple trees to venturia inaequalis. Acta Hortic. 1998;484:535–539. doi: 10.17660/ActaHortic.1998.484.91. [DOI] [Google Scholar]
- 15.Arici SE, Kafkas E, Kaymak S, Koc NK. Phenolic compounds of apple cultivars resistant or susceptible to Venturia inaequalis. Pharm Biol. 2014;52:904–908. doi: 10.3109/13880209.2013.872674. [DOI] [PubMed] [Google Scholar]
- 16.Treutter D. Significance of flavonoids in plant resistance: A review. Environ Chem Lett. 2006;4:147–157. doi: 10.1007/s10311-006-0068-8. [DOI] [Google Scholar]
- 17.Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A. Plant Flavonoids-Biosynthesis, Transport and involvement in stress responses. Int J Mol Sci. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez-Rangel JC, Benavides J, Heredia JB, Cisneros-Zevallos L, Jacobo-Velázquez DA. The Folin-Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal Methods. 2013;5:5990–5999. doi: 10.1039/c3ay41125g. [DOI] [Google Scholar]
- 19.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 20.Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005;89:569–575. doi: 10.1016/j.foodchem.2004.03.013. [DOI] [Google Scholar]
- 21.Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohana H. Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry. 2003;63:97–104. doi: 10.1016/S0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 22.Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 23.Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem Toxicol. 2013;55:290–296. doi: 10.1016/j.fct.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 25.Moon JK, Shibamoto T. Antioxidant assays for plant and food components. J Agric Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 26.Baderschneider B, Winterhalter P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J Agric Food Chem. 2001;49:2788–2798. doi: 10.1021/jf010396d. [DOI] [PubMed] [Google Scholar]
- 27.Singh AP, Wilson T, Luthria D, Freeman MR, Scott RM, Bilenker D, Shah S, Somasundaram S, Vorsa N. LC-MS-MS characterisation of curry leaf flavonols and antioxidant activity. Food Chem. 2011;127:80–85. doi: 10.1016/j.foodchem.2010.12.091. [DOI] [Google Scholar]
- 28.Ghasemzadeh A, Jaafar HZE, Rahmat A, Devarajan T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.). Evidence-based Complement Altern Med. 2014;2014:1–8. doi: 10.1155/2014/873803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagappan T, Ramasamy P, Wahid MEA, Segaran TC, Vairappan CS. Biological activity of carbazole alkaloids and essential oil of murraya koenigii against antibiotic resistant microbes and cancer cell lines. Molecules. 2011;16:9651–9664. doi: 10.3390/molecules16119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Harbi H, Irfan UM, Ali S. The antibacterial effect of curry leaves (Murraya Koenigii). EJPMR. 2016;3:382–387. [Google Scholar]
- 31.Yukari T, Hiroe K, Lajis NH, Nakatani N. Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii Leaves. J Agric Food Chem. 2003. doi: 10.1021/JF034700+. [DOI] [PubMed] [Google Scholar]
- 32.Manthey JA, Grohmann K. Phenols in citrus peel by products. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem. 2001;49:3268–3273. doi: 10.1021/jf010011r. [DOI] [PubMed] [Google Scholar]
- 33.Hijaz FM, Manthey JA, Folimonova SY, Davis CL, Jones SE, Reyes-De-Corcuera JI. An HPLC-MS Characterization of the changes in sweet orange leaf metabolite profile following infection by the bacterial pathogenCandidatus Liberibacter asiaticus. PLoS One. 2013;8(11):e79485. doi: 10.1371/journal.pone.0079485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Gresa MP, Torres C, Campos L, Lisón P, Rodrigo I, Bellés JM, Conejero V. Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ Exp Bot. 2011;74:216–228. doi: 10.1016/j.envexpbot.2011.06.003. [DOI] [Google Scholar]
- 35.Bellés JM, López-Gresa MP, Fayos J, Pallás V, Rodrigo I, Conejero V. Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant Sci. 2008;174:524–533. doi: 10.1016/j.plantsci.2008.02.008. [DOI] [Google Scholar]
- 36.Von Roepenack-Lahaye E, Newman MA, Schornack S, Hammond-Kosack KE, Lahaye T, Jones JDG, Daniels MJ, Dow JM. p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J Biol Chem. 2003;278:43373–43383. doi: 10.1074/jbc.M305084200. [DOI] [PubMed] [Google Scholar]
- 37.Chen GL, Fan MX, Wu JL, Li N, Guo MQ. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019;277:706–712. doi: 10.1016/j.foodchem.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 38.Zuo R, Oliveira A, Bullita E, Torino MI, Padgett‐Pagliai KA, Gardner CL, Harrison NA, da Silva D, Merli ML, Gonzalez CF, et al. Identification of flavonoids as regulators of YbeY activity in Liberibacter asiaticus. Environ Microbiol. 2019;21(12):4822–4835. doi: 10.1111/1462-2920.14831. [DOI] [PubMed] [Google Scholar]


