Abstract
Objective
To determine whether virally suppressed HIV neuropathogenesis, a chronic neuroinflammatory state, promotes abnormal brain amyloid deposition.
Methods
A total of 10 men with virally suppressed HIV-associated neurocognitive disorder (HAND), aged 46–68 years, underwent 11C-labeled Pittsburgh compound B PET. Data from the Australian Imaging, Biomarkers and Lifestyle (AIBL), including 39 cognitively normal individuals (aged 60–74 years), 7 individuals with mild cognitive impairment (MCI) (aged 64–71 years), and 11 individuals with Alzheimer disease (AD) (aged 55–74 years), were used as reference. Apart from more women, the AIBL cohort was demographically comparable with the HIV sample. Also, the AIBL PET data did not differ by sex. Cerebellum standardized uptake value ratio amyloid values within 22 regions of interest were estimated. In the HIV sample, apolipoprotein E (APOE) was available in 80%, CSF biomarkers in 60%, and 8–10 years of long-term health outcomes in 100%.
Results
HAND and the AIBL group with no cognitive deficits had similar amyloid deposition, which was lower than that in both the MCI and AD groups. At the individual level, one HAND case showed high amyloid deposition consistent with AD. This case also had a CSF-AD–like profile and an E4/E4 for APOE. Clinically, this case declined over 18 years with mild HAND symptoms first, followed by progressive memory decline 8–9 years after the study PET, then progression to severe dementia within 2–3 years, and lived a further 6 years. Another HAND case showed increased amyloid deposition restricted to the hippocampi. Two other HAND cases showed abnormally decreased amyloid in subcortical areas.
Conclusions
Relative to cognitively normal older controls, brain amyloid burden does not differ in virally suppressed HAND at the group level. However, individual analyses show that abnormally high and low amyloid burden occur.
The neuropathogenesis of HIV is commonly characterized by chronic neuroinflammation in people living with HIV infection (PLHIV) who are aging and virally suppressed on combination antiretroviral treatment (cART).1 Both age and HIV-associated chronic inflammation may be associated with a higher risk of developing neurodegenerative disease such as Alzheimer disease (AD),1 especially as PLHIV are entering age where dementia starts to rise in the general population.2 AD and other forms of dementia and predementia syndromes are often characterized by abnormal deposition of cortical amyloid.3,4 This neuropathologic process may be used as a way to identify aging PLHIV at risk of dementia and may represent one of the endpoints of HIV-related chronic neuroinflammation. One of the most efficient detection methods for brain amyloid deposition in the living brain is the β-amyloid radioligand 11C-labeled Pittsburgh compound B (11C-PiB) positron emission tomography (PET).5 To date, there is no clear evidence that middle-aged PLHIV are more at risk of developing AD than their HIV-negative (HIV−) counterparts. In particular, it remains unclear whether HIV leads to AD or to an AD-like neurodegeneration for the following reasons.
Evidence from the literature remains limited and inconsistent. Achim et al.6 studied 40–65-year-old HIV+ participants using the molecular imaging probe 2-(1-{6-[(2-[F-18]fluoroethyl)(methyl) amino]-2-naphthyl} ethylidene)malononitrile (FDDNP)-PET and found increased binding of β-amyloid and tau protein aggregates in the posterior cingulate gyrus and the parietotemporal areas in three cases with mild neurocognitive disorder (MND). Furthermore, immunohistochemistry of the neocortex, subcortical white matter, and basal ganglia in HIV+ patients on cART found that 34 of 35 of these cases had intraneuronal deposition of β-amyloid. Tau and α-synuclein were also abundant with the distribution following axonal tracts. APOE genotyping, the major genetic AD risk factor, was not performed, and there were no concomitant neurocognitive data. Another study by Ances et al.7 that included 10 cognitively unimpaired HIV+ individuals (mean age 52 years, age range 46–58 years, 50% men) did not show an overall increased amyloid deposition using 11C-PiB/PET compared with 20 HIV− controls (age range 41–53 years, 80% men). This was despite 4 of 10 participants having low CSF amyloid beta (Aβ) 42 levels. The same research group led by Ances et al.8 also found no significant evidence of pathologic amyloid deposition with 11C-PiB/PET when compared with HIV− community age-comparable controls (88% men) in 5 HIV-associated neurocognitive disorder (HAND) cases (82% men) and 11 cognitively unimpaired HIV+ patients (100% men) aged in their 40s. The HIV+ groups had a significantly lower amyloid deposition than 11 AD cases aged in their 70s (55% men). APOE genotyping was conducted in >90% of the HIV+ participants, and 45%–50% has at least one APOE 4 allele, but this was not associated with increased amyloid deposition. Analyses of CSF-AD markers were conducted in 77% of the HIV+ sample, with 5 HIV+ participants found to have low CSF-Aβ42 levels (<500 pg/mL). CSF-Aβ42 levels were not found to be associated with increased amyloid deposition. A larger study of 26 HIV+ adults aged 26–67 years old, reported as part of a review, found no difference in 11C-PiB PET amyloid deposition and CSF-Aβ42 levels between the HIV+ and the HIV− patients aged 32–62 years old.9 This study did not provide APOE genotyping, and levels of cognitive performance were not reported. Finally, a case study of an HIV-infected (HIV+) 71-year-old man with an undetectable plasma viral load found a combined diagnosis of HAND and probable AD.10 This was based on a multidisciplinary examination including neuropsychological testing, brain imaging, CSF-AD markers, fluorodeoxyglucose PET, and florbetaben PET.
Overall, 11C-PiB PET was the most commonly used method for detecting brain amyloid deposition. All studies conducted have focused on middle-aged PLHIV, mostly without HAND. All studies but one9 reported that most patients were on cART, although the degree of viral suppression was variable or not clearly reported. This represents a major caveat because detectable viral load has been found to be associated with increased Aβ-plaque in the hippocampus in a recent National NeuroAIDS Tissue Consortium (NNTC) analysis.11 APOE genotyping was conducted in only one study.8 While APOE4 status has been associated with HAND in some studies12,13 this has not always been replicated,14 although this result may be influenced by cohorts that included PLHIV of relatively young age, who were not virally suppressed and clinically stable.14 Nevertheless, APOE4 status is a major risk factor for AD and other dementias14 and, thus, remains relevant in aging PLHIV cohorts that could be at higher risk of coincidental AD. Also, APOE4 is a strong modulator of 11C-PiB PET data. In a cohort of 497 cognitively normal (NL) middle- and older aged participants with 11C-PiB PET,15 a greater proportion of APOE4 carriers developed amyloid-β pathology, at an earlier age, and with faster amyloid-β accumulation. Furthermore, a recent and large neuropathologic study from the NNTC showed that APOE4 and age were strong independent predictors of the deposition of 4G8-Aβ plaques in the frontal neocortex, evident in 29% of a selected sample.
11C-PiB PET has high affinity and selectivity for fibrillar Αβ in plaques and extracellular amyloid deposits.5 It may thus lead to lower binding in PLHIV and patients with HAND for whom plaques have been found to be more often diffuse and intracellular.16–18 However, these neuropathologic findings were derived from cohorts that included relatively young patients, active AIDS, as well as many without full viral control and sometimes significant alcohol and drug use comorbidity.17–19 In addition, the detection of abnormal amyloid deposition in the oldest PLHIV with HAND using 11C-PiB PET is important, as AD may be coincidental. On the other hand, the diffuse type of plaques may only reflect the early phase of the disease.
This study aimed to compare brain amyloid deposition quantified by 11C-PiB PET in 10 formally diagnosed HAND cases who were part of the Australian HIV and Aging Cohort. HAND cases were screened for hepatitis C infection, non-HIV neurologic conditions, alcohol and drug use to minimize non–HIV-related neuroinflammation, and other forms of brain pathology.20 These were compared with 39 NL cases, 7 cases with mild cognitive impairment (MCI), and 11 AD cases aged 55–74 years old, all from the Australian Imaging, Biomarkers and Lifestyle (AIBL) cohort.5,21 Because of the relatively small sample size and heterogeneous pattern of amyloid deposition in the HIV+ group, we also conducted a series of case analyses. Finally, we collected long-term health outcomes 8–10 years after the end of the PET data collection to determine dementia progression.
Methods
The University of New South Wales, St. Vincent's Hospital, and Austin Health Human Research Ethics Committees approved this research protocol (HREC/08/096). Informed written consent or the guardian consent when appropriate was obtained from all participants.
Participants
Ten HIV+ men formally diagnosed with HAND (table 1) were enrolled in a study to quantify brain amyloid deposition using the β-amyloid radioligand 11C-PiB as part of the Australian HIV and Brain Aging Cohort Study between 2009 and 2011. The inclusion/exclusion criteria have been described in detail previously.22 All were on cART with undetectable plasma and CSF viral load (<50 copies/mL). Seven of 10 underwent a lumbar puncture and blood tests to determine CSF-AD markers and HIV markers, respectively, and 8 of 10 had APOE genotyping. All HAND cases underwent neuropsychological testing, and a high-resolution T1-weighted MRI scan was obtained for each person; these methods have been reported in detail elsewhere.13,22,23 Five cases had mild HAND, including 4 cases with asymptomatic neurocognitive impairment (ANI) and 1 case with MND. The 5 other cases had HIV-associated dementia (HAD) along its clinical spectrum (Memorial Sloan Kettering [MSK] scale24), including 1 case with mild HAD (MSK1), 2 with moderate HAD (MSK2), and 2 with severe HAD (MSK3). Note that on the MSK scale, MND is classified as MSK1.
Table 1.
Demographic and clinical characteristics of the study samples
AIBL data
A request was made to access the summed digital imaging and communications in medicine PET AIBL data on August 28, 2018, at aibl.csiro.au/research/support/ and was approved on August 30, 2018. These data were collected by the AIBL study group following a published protocol.25 The AIBL study methodology has also been reported previously.5,21 AIBL subjects aged between 55 and 74 years with a PET scanning protocol closest to that of the HIV+ sample were retained (that is PET scan with a duration of 1,200 seconds: 57/103). Demographic and clinical characteristics of the retained AIBL cohort are presented in table 1. The AIBL sample has comparable demographics with the HIV sample except for more women, which is typical of an elderly cohort, because women have a higher survival rate and are more represented in AD cases21; HIV in Australia remains most prevalent in men who have sex with men.22 It is important that there was no significant differences between sexes in the AIBL PET data (t test p values >0.50). Although HIV infection was not formally diagnosed in those controls, the AIBL exclusion criteria were stringent and included a wide range of neurologic, psychiatric, and medical conditions in addition to laboratory test abnormalities.5,21 Evidence of immune compromise would therefore have been detected. Furthermore, the age and demographics of this cohort limits the risk of HIV infection because older age and late diagnosis are more common in non–Australian-born people.26 See examples of AIBL PET data in figure e-1 and e-3, links.lww.com/NXI/A248, in supplemental material.
PET data acquisition in the HIV+ sample
All PET scanning took place at the Austin Health PET Centre in Melbourne (VIC, Australia). Patients were flown from Sydney and back, with their carer as appropriate. They were requested to fast for at least 6 hours before scanning. PET scans for the β-amyloid radioligand 11C-PiB were acquired using a Philips Allegro PET camera with a resolution of 5.0 × 5.0 × 6.5 mm3 (x y z). A transmission scan was performed for attenuation correction. PET images were reconstructed using a 3D row action maximum likelihood algorithm with a voxel size of 2.0 × 2.0 × 2.0 mm3 (x y z). Summed images from the 40- to 70-minute time frame were used in each study. Each participant received 370-MBq 11C-PiB IV over 1 minute. A 30-minute acquisition (6 × 5-minute frames) in a 3D mode starting 40 minutes after injection of 11C-PiB was performed (total PET scan duration of 1,800 ms).
PET processing and analyses in HIV and AIBL data
All imaging data sets were processed using the PETPVE12 toolbox27 for SPM12 (v6906; Wellcome Trust Centre for Neuroimaging, London, UK; fil.ion.ucl.ac.uk/spm) in Matlab r2012b (Mathworks Inc., Sherborn, MA), which has improved functions to handle amyloid PET partial volume effects (PVEs) compared with previous versions. First, all T1-weighted images were segmented and skull stripped using default parameters. Second, all PET images were coregistered to their associated T1 images using the toolbox's default parameters. PVE correction was performed using the Müller-Gärtner method,28 and the intensity of the PVE-corrected PET images was normalized to the average intensity of all cerebellar regions (cerebellum standardized uptake value ratio [SUVRc]) from the Automated Anatomical Labeling (AAL) atlas.29 The inverse deformation parameters from the segmentation step were used to transform all AAL regions from the Montreal Neurological Institute to native space, separately for all participants. Finally, SUVRc values were extracted for each region of the AAL atlas in native space. To reduce the total number of regions of interest (ROIs), the 116 regions from the AAL atlas were arranged based on a previous atlas nomenclature29 and averaged out to result in 22 final ROIs. Twenty-six regions related to the cerebellum and the vermis were used as the cerebellum reference. The remaining 90 regions were collapsed into the 22 ROIs. The algorithm used to collapse these regions was developed by M.S.K., neuroanatomist (see supplementary file table e-5, links.lww.com/NXI/A248).
Statistical analyses
Amyloid uptake and deposition distributions in the HIV+ sample
Two HIV cases had abnormal 11C-PiB uptake (figure e-4, links.lww.com/NXI/A248) in which the PiB uptake was higher in the skull and some parts of the white matter compared with cortical regions. These were subsequently excluded based on the recommendation of the Austin PET team. As a result, 8 HIV cases were considered in the following analyses. The SUVRc data were not normally distributed for any of the study groups. Therefore, all analyses were based on nonparametric statistics.
Group analyses
Study groups (HIV, NL, MCI, and AD) were compared using the Kruskal-Wallis test followed by the Steel-Dwass method to compare all pairs. There were no covariates entered in the analyses. Although the sex ratio did not significantly differ in the AIBL data (p > 0.50, and showed a slightly wider variance when women were included, table 2), the analyses were repeated excluding only the female participants in the AIBL NL group (N = 19) compared with the HIV sample, to account for a residual effect of sex. Owing to the limited sample size, this was not performed in the MCI (male = 4) and AD (male = 6) groups. These statistical analyses were conducted using the statistical package JMP 13 (SAS Institute Inc).
Table 2.
Comparisons of amyloid deposition on the study groups
Case analyses
Case 2, on visual inspection, clearly exhibited high amyloid deposition across the entire brain. To quantify how significant the amyloid deposition was in this case, we ran case analysis statistics using the program Singlims_ES.exe30–32 against the AD, MCI, and NL groups (AIBL women included).
Case 1 had increased amyloid deposition in the hippocampus on visual inspection. Based on this, a single-case analysis was run for this ROI against the AD, MCI, and NL groups. Two other cases (cases 3 and 5) had a noticeable decrease in amyloid deposition across the basal ganglia. To quantify any significant differences, case statistics were run for these 2 cases for the cingulum, caudate, pallidum, putamen, and thalamus against the AD, MCI, and NL groups.
Long-term clinical outcomes in the HIV+ sample
We collected long-term clinical information from medical records located at St. Vincent's Hospital (Sydney, NSW, Australia) and by contacting the participant's primary physicians. This allowed determination as to which patients had progressive dementia and other relevant comorbid conditions 8–10 years after the PET data collection.
Data availability
The HIV sample data are available on the UNSW Australia repository: ros.unsw.edu.au/ (search by author “Cysique” and title of the current study). The AIBL data are available upon request to the AIBL group at aibl.csiro.au.
Results
Group analyses
The HIV group did not differ statistically from the entire AIBL NL group on any comparisons. The HIV group showed a statistically lower amyloid deposition than the entire MCI group in the caudate and pallidum nuclei. Furthermore, the HIV group obtained a statistically lower amyloid deposition than the entire AD group in all regions, except for the hippocampi, amygdala, temporal pole, and thalamus. On all ROIs, the AD groups exhibited higher amyloid deposition than the NL group. The MCI group obtained higher amyloid deposition than the NL group on all regions, except for the thalami, hippocampi, occipital, parietal, and superior temporal regions (table 2 and figure 1). The analyses including only male participants produced similar results (table 2). Although because of a slightly more restricted variance in the AIBL NL male subgroup, we found a significantly lower amyloid deposition in the HIV sample in the medial frontal and superior parietal cortex.
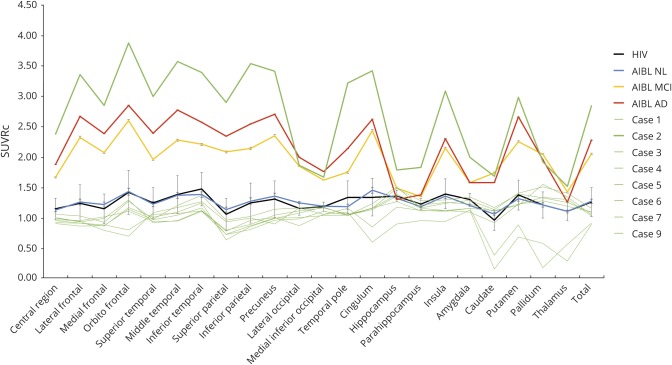
Figure 1. PiB SUVRc in the 22 ROIs in the study samples and individually in the HAND cases.
The HIV+ group data are represented by the black line, the AIBL NL group data are represented by the blue line, the AIBL MCI group is represented by the yellow line, and the AIBL AD group is represented by the red line. Mean and SE were provided for all 22 brain regions and total brain SUVRc. Individual HIV data were also represented by the green line, with the case who had amyloid deposition in line with AD in a bolded green line. AD = Alzheimer disease; AIBL = Australian Imaging, Biomarkers and Lifestyle; HAND = HIV-associated neurocognitive disorder; MCI = mild cognitive impairment; NL = cognitively normal; ROI = region of interest; SUVRc = standardized uptake value ratio with reference to the cerebellum.
Case analyses
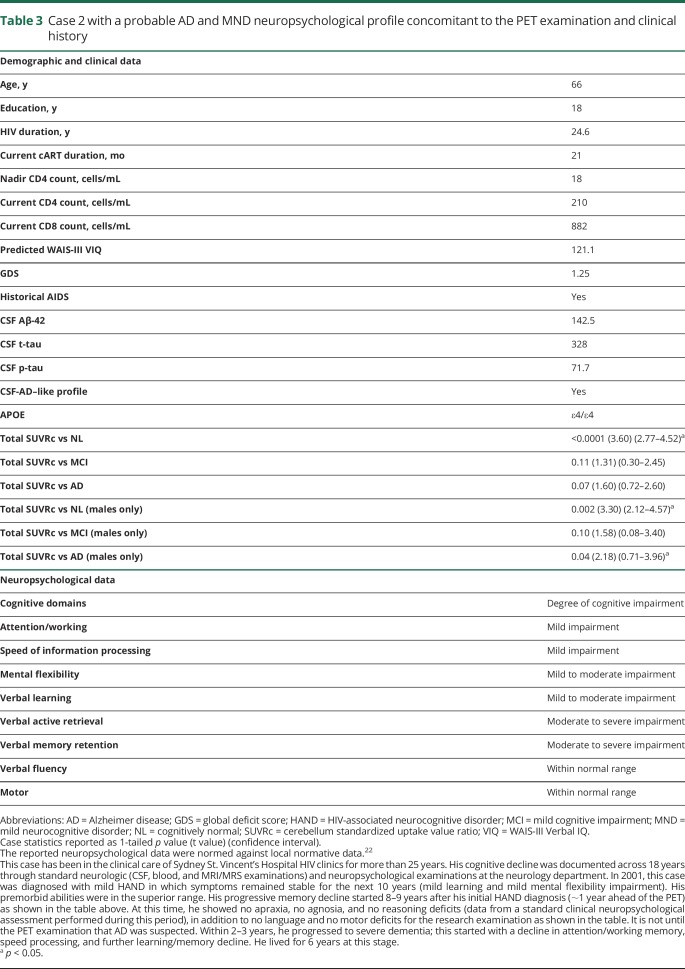
On individual inspection, case 2 (aged 66 years) had high amyloid deposition consistent with AD (whole-brain SUVRc = 2.83) (figures 1 and 2). On quantitative single-case analysis, case 2 showed a significantly increased deposition compared with the NL group, but no difference when compared with the MCI and AD cases. When including only men in the analyses, significant differences were again found between case 2 and the NL and AD groups, but not compared with the MCI group. These results are compiled in table 3, and individual 11C-PiB PET images are provided (figure 2) and show that case 2 had the highest amyloid deposition compared with all the other groups. As described in table 3, case 2 was APOE ε4 homozygous with a CSF profile, based on standard cutoff values, consistent with AD. A detailed clinical history and neuropsychological profile for case 2 is presented in table 2. It shows a mix of mild and moderate impairment in cognitive domains affected by HIV-related brain injury (attention/working memory and speed of information processing), but not for motor functions. The memory profile was consistent with probable AD with moderate to severe impairment.
Figure 2. Raw PiB PET data in the 8 HIV-associated neurocognitive disorder cases.
Raw PiB PET data are presented in the 8 HIV cases. Case 2 is the case with high amyloid deposition. Case 1 is the case with a trend for higher hippocampi binding compared with the Australian Imaging, Biomarkers and Lifestyle NL group. Cases 3 and 5 were the cases with the lowest amyloid deposition across all groups. Note that case example for the NL, MCI, and Alzheimer disease groups is presented in supplemental material in figures e-1, e-2, and e-3, respectively, links.lww.com/NXI/A248. MCI = mild cognitive impairment; NL = cognitively normal.
Table 3.
Case 2 with a probable AD and MND neuropsychological profile concomitant to the PET examination and clinical history
Case 1 (aged 62 years) had elevated amyloid deposition in both hippocampi (table e-1, links.lww.com/NXI/A248). Single-case analysis showed that there was no significant increase in deposition compared with the MCI and AD groups, but there was a trend compared with the NL group (p = 0.06; table 2 and figure 2). This individual was also APOE ε4 homozygous; however, CSF biomarkers were not consistent with an AD profile (CSF Aβ-42 = 168.21 pg/mL; t-tau = 132.18 pg/mL; and p-tau = 36.23 pg/mL). Neuropsychological deficits included mildly impaired speed of information processing and working memory and moderately impaired motor functions with preserved verbal fluency and memory.
Case analysis statistics, as well as demographic, neuropsychological, HIV, CSF, and APOE data for case 3 (aged 52 years) and case 5 (aged 54 years) are presented in table e-2, links.lww.com/NXI/A248, and e-3, links.lww.com/NXI/A248, respectively, and in figure 2. There was a significant decrease in amyloid deposition for both cases in all areas tested (range p < 0.04–p < 0.0001), except for the cingulum and putamen when compared with the NL group (p = 0.15–0.07). Furthermore, case 5 was identified as having an AD-like CSF profile. Both were cases with severe HAD.
Long-term follow-up
The 2 patients who originally had clinically severe HAD (MSK3) and 1 who had clinically moderate HAD (MSK2) and had normal amyloid died because of other comorbidities (see table e-4, links.lww.com/NXI/A248). Case 2 who had clinically moderate HAD + AD (MSK2) and significantly elevated amyloid also died after progression to severe dementia. Case 1 who had ANI, with elevated amyloid in the hippocampi, developed a progressive supranuclear palsy (PSP)-like illness, which has remained static. Case 1 also showed microinfarcts and hyperintensities of the white matter consistent with vascular injury on MRI and evidence of cognitive decline reaching an MND (MSK1) diagnosis within 3 years after study. Other cases, which had normal amyloid, included 1 ANI case (case 9), who did not progress clinically according to their physicians and are currently living independently. Case 6, who had MND (MSK1) at entry, has been cognitively stable despite a series of cancers, which have been successfully treated. Case 7, who had clinically mild HAD (MSK1) at entry, has been mostly stable except for progressing peripheral neuropathy. Of the 10 cases, 2 cases (cases 8 and 10) showed abnormal PiB PET uptake (figure e-4, links.lww.com/NXI/A248). Case 8 had ANI at entry that progressed clinically displaying worsening mood changes and behavioral problems with subsequent cognitive decline consistent with MND (MSK1). Case 10 also progressed clinically by reporting increasing cognitive difficulties in line with MND (MSK1). These 2 cases have not reached the HAD stage and still live independently.
Discussion
Consistent with previous studies,7–9 there was no statistical difference in amyloid deposition between the HIV group and NL cases (combining men and women) from the AIBL. However, the NL cases, as part of the AIBL study, were older than those in previous referenced studies. When repeating the analyses with only NL male participants from the AIBL, we found abnormally low amyloid deposition in the HIV group in the medial frontal and superior parietal cortex. This indicates that HIV may be associated with abnormally low amyloid deposition when compared with an older NL cohort. Our study has a small HIV sample size, yet it was a data-rich sample composed of well-characterized HIV+ cases aged 55.5 (46–68) years old, with formally diagnosed HAND, long-term HIV disease clinical stability, viral suppression, and low neurologic and psychiatric confounds. Critically, the sample had long-term follow-up of the participants over years (table e-4, links.lww.com/NXI/A248). This is unique compared with previous HIV PET studies. The small HIV sample size was amenable to a case analysis strategy, which showed abnormal increases of amyloid deposition in 2 HAND cases.
Case 2 had an overall deposition of amyloid consistent with AD supported on single-case analysis. The typical AD-like CSF profile and progressive cognitive impairment with a mixed picture of typical AD and HAND deficits supported a probable dominant AD diagnosis. It is important that case 2 was APOE E4 homozygous and was the second oldest participant in the sample (66 years of age). This is consistent with potential coexistence of HAND and AD in an advanced HIV+ patient, as previously described.10 From an AD prevalence perspective, it is logical that some HIV+ patients will develop AD based on genetic and other predisposing factors. It is, however, not clear whether the start of neurodegeneration may be earlier because of HIV. It is also uncertain whether amyloid deposition may have a faster deposition rate,33 although this is certainly possible given that this case had the highest amount of amyloid deposition across all our comparisons. Also, we noted that the cognitive profile was unusual compared with HAND (sparing of motor functions and long-term stability of psychomotor speed) and AD profiles (restricted memory deficits with no agnosia, apraxia, or language deficits except toward the end stage).34 From a clinical perspective, it has been argued that HAND and AD can be distinguished to some degree.2 However, an extensive test battery with the inclusion of agnosia, apraxia, and language would be needed in addition to the usual cognitive domains assessed in HAND to better address this question. It has been noted that 11C-PiB PET, which has high affinity and selectivity for fibrillar Αβ in plaques and extracellular amyloid deposits,5 may lead to lower binding in PLHIV and patients with HAND for whom plaques have been found to be more often diffuse and intracellular.16–18 In this study, case 2 shows that such an interpretation may not be correct in aging PLHIV with a possible coincidental AD pathology and profile.
A role for HIV in the dysregulation of brain amyloid is biologically plausible. Hypotheses include recent research into the antimicrobial activity of amyloid35,36 in which increased amyloid may reflect a long-term response to the presence of HIV infection in the brain (potentially in brain cellular reservoirs). Supporting this, Bourgade et al.37,38 suggest that β-amyloid can inhibit herpes simplex virus 1 and is associated with overproduction of amyloid more generally in infection-related neurologic diseases.
Although case 1 showed no statistical overall increase in amyloid deposition, a trend toward abnormal binding in the hippocampus was observed. The CSF biomarker profile was also not consistent with an AD-like profile. Recent literature on AD suggests that, even subthreshold increases in amyloid levels can result in memory deficits, and in some cases it is the rate of amyloid accumulation, rather than the overall amyloid level, that more accurately predicts worsening memory.39,40 However, case 1 had a PSP-like illness, the pathologic underpinning of which in the context of HIV is unknown at present. Nonetheless, it is interesting that he was homozygous for APOE E4. There is evidence that progression into a PSP-like illness is associated with a homozygous APOE E4 genotype.41
In this study, 2 of the 3 cases (cases 3 and 5) with severe HAD (pre-cART) who survived on cART showed low amyloid deposition. These cases were the most vulnerable and died within 8–10 years of the study's long-term outcomes collection. All were younger than 65 years with long HIV duration (>25 years). Cases 3 and 5 exhibited major subcortical atrophy, consistent with severe HIV-related brain injury.42–45 This level of subcortical atrophy potentially biased the measurement of amyloid in the basal ganglia. Nevertheless, there is at least 1 plausible mechanism for the abnormally low level of amyloid. Microglia and astrocytes primed by HIV may play important roles in engulfing and purging Aβ plaques from the brain.46 In severe HAD with a disrupted blood-brain barrier, amyloid clearance may be enhanced in some individuals. Also, in treated PLHIV, amyloid clearance may occur through HIV-1 Tat priming and activation of microglia.47 Case 5 had a CSF biomarker profile that supported AD (T-tau >350 Ab42/P-tau <6.5), but the PET scan did not confirm increased amyloid deposition suggesting caution in the clinical use of CSF for AD diagnosis in patients with HIV, especially those with HAND. Finally, we cannot exclude that in these younger cases, the plaques may have been diffuse rather than fibrillar, and this may have contributed to a lower 11C-PiB PET signal.16–18
Although our study does not provide statistical evidence of an overall increase in amyloid in PLHIV with HAND, it highlights the importance of interindividual variations in patients with chronic HIV and HAND and the need to focus the research on favorable and unfavorable phenotypic and/or biotypic clusters.48 In this small sample, we were able to detect cases with high, focused (albeit trending) and very low brain amyloid. Such complex phenotypes if already apparent in a pilot study suggest a need to focus on long-term individual phenotypic analyses. Future longitudinal studies monitoring amyloid deposition as PLHIV age are crucial in understanding the relationship between abnormal amyloid levels and HAND as well as other comorbidities. It is noteworthy that all the HIV+ patients in this study also had a large number of medical comorbidities (See table e-4). How exactly such comorbidities played a role in their amyloid pattern and then clinical progression or lack thereof remains to be fully elucidated. Although we used a PET protocol that retained the individual neuroanatomy, it would be important that larger studies use a multimodal imaging strategy because abnormal brain aging in HIV can be detected by using volumetric MRI.49
Our study had some limitations. The controls used in this study were obtained from AIBL and included a mix of male and female participants differing from the HIV+ sample who were all men. The sex difference had a minimal effect on the cases with AD + HAD, when specifically investigated. We also had missing data for the APOE genotyping and CSF profiles and recognize this, just as for previous studies have, as a limitation. Although 50% of the HAND cases had HAD as per Frascati criteria at study entry, it is important to note that the MSK clinical staging was well represented as well as those with mild HAND as per Frascati criteria. Finally, our pilot sample size and case series analyses yield indicative rather than definitive findings. It remains unclear why 2 HAND cases had no cortical amyloid uptake. Despite a thorough search, we could not find reports of similar issues in the HIV or non-HIV literature. There was no PET scan technical issue reported by the Austin Health PET Centre examiners.
In conclusion, relative to NL older controls, brain amyloid burden does not differ in virally suppressed HAND at the group level. However, individual analyses show that abnormally high and low amyloid burden occur. These results indicate that focus on individual phenotypes in longitudinal analyses in the HIV-aging cohort is needed.
Acknowledgment
The authors thank the participants for their time on the study and dedicate this work to the patients whom they cared for and who have since died. They thank the physicians of the HIV+ patients for responding in a timely and detailed fashion to their queries. They thank Dr. Jones, Prof. Villemagne, and Prof. Rowe from the Centre for PET, Austin Health, Melbourne, Australia, for their support in interpreting the data acquisition validity.
Glossary
- AAL
Automated Anatomical Labeling
- Aβ
amyloid beta
- AD
Alzheimer disease
- AIBL
Australian Imaging, Biomarkers and Lifestyle
- ANI
asymptomatic neurocognitive impairment
- APOE
apolipoprotein E
- 11C-PiB
11C-labeled Pittsburgh compound B
- cART
combination antiretroviral therapy
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorder
- MCI
mild cognitive impairment
- MND
mild neurocognitive disorder
- MSK
Memorial Sloan Kettering
- NNTC
National NeuroAIDS Tissue Consortium
- PLHIV
people living with HIV infection
- PSP
progressive supranuclear palsy
- PVE
partial volume effect
- ROI
region of interest
- SUVR
standardized uptake value ratio
Appendix. Authors

Study funding
This work was supported by the NHMRC project grants (APP568746; PI Cysique, APP1105808; PI Brew, and APP1045400; PI Cysique), Peter Duncan Neuroscience Research Unit at St. Vincent's Hospital Centre for Applied Medical Research (Director: Prof. Brew), and a UNSW Australia Faculty of Medicine development.
Disclosure
B.J. Brew reports personal fees from AbbVie, personal fees from ViiV, grants and personal fees from Biogen Idec, personal fees from Merck Sharp and Dohme, grants from the National Health and Medical Research Council, and grants from the National Institutes of Health, outside the submitted work. C.D. Rae reports personal fees from Philips Healthcare, personal fees from Springer, and personal fees from Elsevier, outside the submitted work. Other authors report no declaration of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.Mackiewicz MM, Overk C, Achim CL, Masliah E. Pathogenesis of age-related HIV neurodegeneration. J Neurovirol 2019;21:019–00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer's disease: an emerging issue in geriatric neuroHIV. Curr HIV/AIDS Rep 2017;14:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmonds EC, Bangen KJ, Delano-Wood L, et al. Patterns of cortical and subcortical amyloid burden across stages of preclinical Alzheimer's disease. J Int Neuropsychol Soc 2016;22:978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarro L, Senjem ML, Lundt ES, et al. Amyloid-β deposition and regional grey matter atrophy rates in dementia with Lewy bodies. Brain 2016;139:2740–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 6.Achim CL, Barrio JR, Kepe V, et al. Abnormal protein aggregation in the brains of long-term surviving HIV patients in the HAART era. 8th International Symposium on NeuroVirology: Journal of Neurovirology 2007:12.
- 7.Ances BM, Christensen JJ, Teshome M, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology 2010;75:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ances BM, Benzinger TL, Christensen JJ, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol 2012;69:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega M, Ances BM. Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol 2014;9:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst) 2016;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Sinsheimer JS, Levine AJ. Associations of regional amyloid-beta plaque and phospho-tau pathology with biological factors and neuropsychological functioning among HIV-infected adults. J Neurovirol 2019;29:019–00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Jiang C, Cunningham E, et al. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology 2014;82:2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cysique LA, Hewitt T, Croitoru-Lamoury J, et al. APOE epsilon4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals—a cross-sectional observational study. BMC Neurol 2015;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geffin R, McCarthy M. Aging and apolipoprotein E in HIV infection. J Neurovirol 2018;24:529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra S, Blazey TM, Holtzman DM, et al. Longitudinal brain imaging in preclinical Alzheimer disease: impact of APOE epsilon4 genotype. Brain 2018;141:1828–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rempel HC, Pulliam L. HIV-1 tat inhibits neprilysin and elevates amyloid beta. AIDS 2005;19:127–135. [DOI] [PubMed] [Google Scholar]
- 17.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005;19:407–411. [DOI] [PubMed] [Google Scholar]
- 18.Umlauf A, Soontornniyomkij B, Sundermann EE, et al. Risk of developing cerebral beta-amyloid plaques with posttranslational modification among HIV-infected adults. AIDS 2019;33:2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E; Neurobehavioral Research Center. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 2009;4:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cysique LA, Jugé L, Gates T, et al. Covertly active and progressing neurochemical abnormalities in suppressed HIV infection. Neurol Neuroimmunol Neuroinflamm 2018;5:e430 10.1212/NXI.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis KA, Bush AI, Darby D, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) Study of Aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr 2009;21:672–687. [DOI] [PubMed] [Google Scholar]
- 22.Cysique LA, Heaton RK, Kamminga J, et al. HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J Neurovirol 2014;20:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cysique LA, Moffat K, Moore DM, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One 2013;8:e61738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079–1083. [DOI] [PubMed] [Google Scholar]
- 25.Rowe CC, Ng S, Ackermann U, et al. Imaging β-amyloid burden in aging and dementia. Neurology 2007;68:1718. [DOI] [PubMed] [Google Scholar]
- 26.The Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia Annual Surveillance Report 2018: Sydney: UNSW Australia, 2018. [Google Scholar]
- 27.Gonzalez-Escamilla G, Lange C, Teipel S, Buchert R, Grothe MJ; Alzheimer's Disease Neuroimaging Initiative. PETPVE12: an SPM toolbox for partial volume effects correction in brain PET–Application to amyloid imaging with AV45-PET. Neuroimage 2017;147:669–677. [DOI] [PubMed] [Google Scholar]
- 28.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12:571–583. [DOI] [PubMed] [Google Scholar]
- 29.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 30.Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia 2002;40:1196–1208. [DOI] [PubMed] [Google Scholar]
- 31.Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards. Cogn Neuropsychol 2010;27:245–260. [DOI] [PubMed] [Google Scholar]
- 32.Crawford JR, Howell DC. Comparing an individual's test score against norms derived from small samples. Clin Neuropsychologist 1998;12:482–486. [Google Scholar]
- 33.Fulop T, Witkowski JM, Larbi A, Khalil A, Herbein G, Frost EH. Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer's disease? J Neurovirol 2019;25:637–647. [DOI] [PubMed] [Google Scholar]
- 34.Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer's disease. J Neurovirol 2019;25:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brothers HM, Gosztyla ML, Robinson SR. The physiological roles of amyloid-ß peptide hint at New ways to treat Alzheimer's disease. Front Aging Neurosci 2018;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimer's Dement 2018;14:1602–1614. [DOI] [PubMed] [Google Scholar]
- 37.Bourgade K, Garneau H, Giroux G, et al. beta-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology 2015;16:85–98. [DOI] [PubMed] [Google Scholar]
- 38.Bourgade K, Le Page A, Bocti C, et al. Protective effect of amyloid-beta peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis 2016;50:1227–1241. [DOI] [PubMed] [Google Scholar]
- 39.Leal SL, Lockhart SN, Maass A, Bell RK, Jagust WJ. Subthreshold amyloid predicts tau deposition in aging. J Neurosci 2018;38:4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landau SM, Horng A, Jagust WJ; Alzheimer's Disease Neuroimaging Initiative. Memory decline accompanies subthreshold amyloid accumulation. Neurology 2018;90:e1452–e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuboi Y, Josephs KA, Cookson N, Dickson DW. APOE E4 is a determinant for Alzheimer type pathology in progressive supranuclear palsy. Neurology 2003;60:240–245. [DOI] [PubMed] [Google Scholar]
- 42.Tesic T, Boban J, Bjelan M, Todorovic A, Kozic D, Brkic S. Basal ganglia shrinkage without remarkable hippocampal atrophy in chronic aviremic HIV-positive patients. J Neurovirol 2018;24:478–487. [DOI] [PubMed] [Google Scholar]
- 43.Sanford R, Fernandez Cruz AL, Scott SC, et al. Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART era. J Acquir Immune Defic Syndr 2017;74:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aylward EH, Henderer JD, McArthur JC, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 1993;43:2099–2104. [DOI] [PubMed] [Google Scholar]
- 45.Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011;5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li JW, Zong Y, Cao XP, Tan L, Tan L. Microglial priming in Alzheimer's disease. Ann Transl Med 2018;6:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. HIV-1 Tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J Neurosci 2017;37:3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul R. Neurocognitive phenotyping of HIV in the era of antiretroviral therapy. Curr HIV/AIDS Rep 2019;16:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang L, Shukla DK. Chapter 18 - imaging studies of the HIV-infected brain. In: Brew BJ, editor. Handbook of Clinical Neurology. Amsterdam, The Netherlands: Elsevier; 2018:229–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The HIV sample data are available on the UNSW Australia repository: ros.unsw.edu.au/ (search by author “Cysique” and title of the current study). The AIBL data are available upon request to the AIBL group at aibl.csiro.au.