Abstract
It is well established that the inadequate intake of key nutrients can lead to nutrient deficiency-related phenomena. However, even when the intake of nutrients is sufficient, the inadequate digestion and/or absorption of macronutrients, micronutrients or other therapeutic compounds from the diet (i.e., phytonutrients) can result in similar clinical consequences. These consequences include classic GI-related symptoms related to malabsorption, as well as a broad range of clinical and subclinical signs and symptoms (though many nutrient insufficiencies are difficult to diagnose). Along with food matrix issues, the integrative and functional medicine community has long considered inadequate levels of stomach acid, pancreatic enzymes and/or bile acid secretion to greatly contribute to an individual’s risk for maldigestion or malabsorption.
It is well established that the inadequate intake of key nutrients can lead to nutrient deficiency-related phenomena. However, even when the intake of nutrients is sufficient, the inadequate digestion and/or absorption of macronutrients, micronutrients or other therapeutic compounds from the diet (i.e., phytonutrients) can result in similar clinical consequences. These consequences include classic GI-related symptoms related to malabsorption, as well as a broad range of clinical and subclinical signs and symptoms (though many nutrient insufficiencies are difficult to diagnose). Along with food matrix issues, the integrative and functional medicine community has long considered inadequate levels of stomach acid, pancreatic enzymes and/or bile acid secretion to greatly contribute to an individual’s risk for maldigestion or malabsorption. Indeed, routine mealtime “replacement” of one or more of these agents is commonly recommended by such practitioners to improve digestion and absorption. In this paper, we outline the evidence for one of these common recommendations- the supplementation of betaine HCl to support inadequate stomach acid production (hypochlorhydria)- while exploring what is known about the prevalence of this condition.
Inadequate Stomach Acid Production (Hypochlorhydria/Achlorhydria)
A variety of different methods can be used to measure gastric acid production and stomach pH (e.g., gastric intubation, catheter electrodes, radio-telemetric capsules and pH-sensitive tablets); therefore, a variety of different cut-off points have been used to define hypochlorhydria and achlorhydria in the literature. Generally, a fasting gastric pH less than 3.0 is considered “normal,” while values above 3.0 are deemed to be gradually more hypochlorhydric. True achlorhydria results in a gastric pH above 7, which is characterized by very limited acid production even when stimulated by gastrin or histamine (e.g., chronic atrophic gastritis).1 Subjects taking proton-pump inhibitors will generally have a fasting gastric pH between 5-7.
Inadequate levels of stomach acid (regardless of the root cause) can result in many nutritional and digestive issues. For instance, a reduction in gastric acid secretion prevents adequate denaturing of folded proteins resulting in poor protein digestion and increased food allergenicity.2 Activation of pepsin (from pepsinogen) is greatest at a pH of 2 or less and its protease activity is optimal at a pH of 1.8 to 2.3.3 A low-acid environment is linked to reduced absorption of key micronutrients such as calcium, iron, folic acid, vitamin B6 and vitamin B12.4,5 Also, since gastric acid helps to eliminate harmful ingested microorganisms and hinders bacterial overgrowth in the stomach and small bowel; low stomach acid can increase the risk for small intestinal bacterial overgrowth (SIBO) and specific microbial overgrowth from organisms like Clostridium difficile.6,7,8 While most of these consequences of low stomach acid are undisputed, there is much less agreement on the prevalence of this condition in the general population, how to test for such a condition and, especially, whether there is an appropriate therapy for low stomach acid.
Prevalence of Low Stomach Acid
A large variance in the reported prevalence of hypochlorhydria is noted in the literature. While aging is regularly associated with decreased gastric acid production, fasting hypochlorhydria is reported to be less common (~10% or less) in elderly American subjects, while it is reported to be more common (>60%) in elderly Japanese subjects, and as high as 80% in a small cohort of Norwegian subjects in their eighth and ninth decades of life (average age: 84 years, range: 80-91).8-11 These studies illustrate the lack of consensus in the literature for the prevalence of fasting hypochlorhydria and achlorhydria in the aging population as many factors are likely to affect fasting gastric pH (e.g., gender, testing method and cutoff values, number of parietal cells to produce HCl, coincident disease states such as H. pylori infection and overall health, etc.).12
Nevertheless, while fasting gastric pH is likely an important marker for achlorhydria, especially when this condition is related to chronic atrophic gastritis, the gradual “functional” decline in gastric acid secretion during and after consuming a meal (a biomarker rarely reported in the literature) may be a much more important measure of acid-related digestive issues. Interestingly, studies performed by researchers at the University of Michigan nearly two decades ago give us some clues to investigate this phenomenon. They reported on the fasting, mealtime and postprandial stomach pH levels in healthy young and elderly subjects.13,14 Gastric pH levels were measured using a tethered radio-telemetric capsule (Heidelberg) in 15-second intervals. After 12 hours of fasting, gastric pH was measured for one hour before a “standard meal”i and continuously for another four hours once subjects commenced eating the meal. In the fasted state, the average gastric pH was similar in both the younger (mean age 25 years) and the older (mean age 71 years) subjects, with a slight statistical trend toward lower pH (more acid) in the elderly subjects (See Figure 1). However, it should be noted that while none of the younger subjects had a fasting pH > 5.0 (the study’s definition of achlorhydria), 11% of the elderly subjects had a fasting gastric pH >5.0, similar to the prevalence noted above in US elderly subjects. Using this fasting data alone, one might conclude that ~90% of elderly subjects have similar gastric acid production compared to younger subjects. However, while both groups saw an expected rise in stomach pH upon consumption of the meal, the time required to re-acidify the gastric contents was much slower in the older subjects. For instance, while the average pH after consuming the meal was similar in young and old subjects (5.0 and 4.9, respectively), the time it took to re-acidify the stomach to a pH of 3.0 was 42 minutes in the younger subjects and 89 minutes in the older subjects, averaging nearly an hour longer to reach a pH of 2.0 (16.4% of the elderly subjects did not return to pH of 2.0 within four hours).
Figure 1.
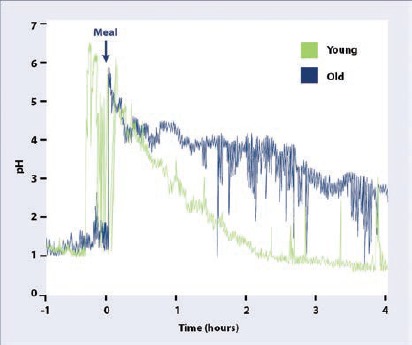
Stomach pH during meal in younger and older subjects.
These data suggest that a diminution of gastric acid secretion may gradually worsen with aging, which cannot be readily detected in the fasted state (i.e., independent of atrophic gastritis/achlorhydria). This extended mealtime/postprandial hypochlorhydria may contribute to poor protein digestion, reduced micronutrient absorption, increased risk of dysbiosis, SIBO, or other symptoms associated with functional dyspepsia. Therefore, based upon this progressive “functional” hypochlorhydria in older subjects, it is not unreasonable for integrative and functional medicine clinicians to consider oral supplementation of “gastric acid” in the form of betaine HCl (often with pepsin) to help reduce meal-time stomach pH; but what is the evidence for this approach?
Supplementing “Acid” to Improve Digestion: What is the Evidence?
The debate about the utility of supplementing acid is related to the debate about the relationship between endogenous stomach acid production and gastrointestinal outcomes. While conventional medical literature routinely suggests that the amount of stomach acid production is more than adequate for the purposes of digestion in healthy subjects, meal-time “functional hypochlorhydria” may be much more common in older subjects. In addition, the rampant use of drugs to suppress acid production increases the frequency of mealtime hypochlorhydria in many subjects. Therefore, it is common within the functional and integrative medicine community to recommend supplementing agents that directly or indirectly increase stomach acid during a meal.
Agents Suggested to Indirectly Increase Stomach Acid
Bitter-tasting plants or plant extracts (bitters), have been commonly used in many herbal medicinal traditions to promote digestion and/or to relieve digestive complaints, and mechanisms have been studied in in vitro models for the potential role of bitters in acid secretion; however, there is little in the way of systemic research in humans to suggest specific preparations and dosages.15-18 Further, in vitro research has identified several compounds in brewed coffee that stimulate gastric acid secretion, some of which are altered by the roasting process, with one study suggesting this gastric acid stimulation may be signaled via bitter taste receptors.19-22 Interestingly, cola beverages (e.g., Coca-Cola, pH 2.5) have been used to increase gastric acidity in clinical trials aiming to lower stomach pH to increase the absorption of some pH-dependent pharmaceutical drugs (e.g., ketoconazole, itraconazole, etc.); however, the use of cola beverages to promote low stomach pH for drug absorption (or nutrient absorption) is not a healthy option.23-25 Instead researchers and clinicians have turned to the supplementation of betaine HCl for its direct ability to reduce stomach pH as it can be delivered in a tablets or capsules while avoiding contact with the oral cavity and esophagus.25-28
Supplementing Betaine HCl
Betaine HCl is the hydrochloride salt of betaine, a different but important supplemental compound. It is important to distinguish between betaine HCl and betaine (or trimethylglycine (TMG)) as these agents have very different chemistry and clinical indications. The non-acidic betaine is used primarily as a methyl donor, especially to treat homocystinuria (for which it is approved as a prescription drug). In contrast, betaine HCl readily releases H+ in an aqueous environment (approximately 0.65 mmol/100 mg). Confusion between these two compounds is common, even noted recently in a medication error report when a pharmacy dispensed anhydrous betaine (Cystadane) instead of the physician-prescribed betaine HCl (which was intended to help the patient absorb another prescription drug while taking a PPI drug; see below).29 Because betaine HCl readily donates H+ in an aqueous environment, it is important that betaine HCl supplements are in the form of capsules or tablets when ingested. Betaine HCl is most often measured in milligrams; however, some recommendations still use “grains” to measure this compound. One grain of betaine HCl is equal to 65 mg.
Within the integrative medicine community, the most common recommendation for the use of betaine HCl supplements is usually implemented using an empirical test for low stomach acid whereby increasing doses of betaine HCl are given during sequential meals until such time as an uncomfortable sensation is noticed by the patient (see sidebar for a typical protocol). Along with improvements in symptoms of dyspepsia (or laboratory analysis of improved protein digestion), the lack of side-effects acts is an empirical confirmation that low gastric acid production was contributing to poor digestion and/or dyspeptic symptoms. At this time, we are unaware that this popular protocol has been rigorously tested in a research setting, though thousands of clinicians follow such recommendations with positive anecdotal outcomes.
Supplementation with Betaine HCl and Stomach pH
While betaine HCl supplementation is widely recommended, there is limited published data evaluating the effects of this agent on stomach pH and, subsequently, digestive outcomes. However, recent published data that specifically investigates how betaine HCl supplementation alters stomach pH may be helpful to the clinician recommending this therapy. These researchers investigated the ability of betaine HCl to re-acidify the gastric environment in subjects taking proton pump inhibitors, with a specific goal to improve the solubility and efficacy of specific pH-sensitive drugs.25,26 Using six healthy volunteers with normal fasting gastric pH (pH < 2), hypochlorhydria (defined by the study as a pH > 4) was induced by giving 20 mg of rabeprazole sodium (PPI) twice daily with food for four days prior to the study day. On the fifth day, radio-telemetric Heidelberg capsules were positioned in the stomach and each subject was given an additional 20 mg of rabeprazole. When the subjects’ gastric pH remained above 4.0 for a minimum of 15 minutes, they were given 1,500 mg of betaine HCl (two capsules, 750 mg each) with 250 ml of water and monitored for changes in gastric pH for several hours in the fasted state. Gastric pH in all subjects fell rapidly from an average pH 5.2 in the half hour prior to the ingestion of betaine HCl to an average pH of 0.6 thirty minutes after supplementation. While the gastric acidification was rapid, averaging 6.25 minutes to reach pH < 3, the total duration of re-acidification lasted just longer than one hour (average time to rebound to pH > 3 was 73 minutes, rebound to pH>4 was 77 minutes, though there was a wide inter-individual range [±30 minutes] for rebound). They later showed that, indeed, betaine HCl co-administered with a pH-sensitive drug (dasatinib) greatly enhanced solubility and absorption during PPI-induced hypochlorhydria. It is important to note that these dramatic results occurred in the fasted state, whereas subsequent research by this group suggests that food can alter the re-acidification dynamics.27
Using a similar study design as their previous work, Faber et al. evaluated whether betaine HCl supplementation could affect the absorption of a different pH-dependent drug (atazanavir) in healthy subjects (N = 8) in whom hypochlorhydria was induced using a PPI drug (i.e., rabeprazole, 20 mg twice daily).27 This study differed from their previous work with dasatinib in that this drug was taken with a standardized light meal containing 336 kcal, 5.1 g fat and 9.3 g protein. The meal was administered at T0 followed by betaine HCl (1500 mg) ten minutes later (T10) and five minutes later the drug was given (T15). In this study, 1500 mg of betaine HCl was not shown to significantly improve the absorption of atazanavir in subjects with PPI-induced achlorhydria given a meal, though interestingly, betaine HCl supplementation did decrease the gastric pH. However, compared to the previous study performed in the fasted state, it took nearly three-times longer to reduce the pH below 1.0 in the fed state. It is possible that consuming the betaine HCl just prior to the meal (rather than 10 minutes after starting the meal) could have diminished the time for re-acidification. Nonetheless, these studies (along with the mealtime pH studies in the young and old mentioned previously) allow us to make some clinical observations.
First, these data clearly show the potency of betaine HCl to quickly acidify gastric pH in achlorhydric subjects using a dose of 1500 mg (~23 grains), as well as the safe use of betaine HCl in subjects treated with proton pump inhibitors. The rebound time to higher gastric pH reported in these subjects is, however, confounded by the fact that these subjects were fasting in addition to taking acid-suppressing therapy. Even when given 1500 mg of betaine HCl, most subjects had returned to their PPI-induced gastric hypochlorhydria in less than 75 minutes on an empty stomach. Since 1500 mg of betaine HCl was less potent when a small (336 kcal) meal was consumed 10 minutes prior, this suggests that higher doses of betaine HCl dosed just before the meal may be needed to compensate for the average meal (600-1000 kcal). These same researchers have conducted a similar study using 1500, 3000 and 4500 mg of betaine HCl in their attempt to overcome the mealtime suppression of drug absorption noted when using only 1500 mg of betaine HCl; however, the results of this trial have not yet been made available.30
While limited in scope, these results generally agree with the empirical supplementation of meal-time betaine HCl and suggest that the dose(s) of betaine HCl may need to be taken minutes prior to the meal or divided and taken throughout the meal. Furthermore, these data strongly suggest that supplemental mealtime betaine HCl may be safe and appropriate (and necessary) in subjects on PPI therapy, in order to decrease common PPI side-effects (e.g., protein/micronutrient/drug malabsorption, food-borne microbial survival, or slow gastric emptying).
Basic Protocol for using Betaine HCl.
(For empiric testing of mealtime hypochlorhydria and for supplementing gastric acid)
This protocol involves giving patients increasing doses of betaine HCl at mealtimes until such time as noticeable discomfort is reported. Patients who have exceeded the necessary dose will experience tingling, heartburn, diarrhea, or any type of discomfort including a feeling of unease, digestive discomfort, neck ache, backache, headache, or any new odd symptom. Upon experiencing tingling, burning, or any uncomfortable symptom, patients can neutralize the acid with 1 tsp baking soda in water or milk.
Patients with suspected mealtime hypochlorhydria should begin by taking one (1) capsule containing 350–750 mg (~5-12 grains) of betaine HCl with a protein-containing meal† (Capsules containing betaine HCl with added pepsin can also be used and may be superior for overall benefit).
If no discomfort or burning sensation is noted, the patient can begin taking two (2) capsules with each protein-containing meal.
If a burning sensation or any discomfort is noted after taking this (or any) dose, the patient can neutralize the acid with 1 tsp baking soda in water or milk and reduce the dose of betaine HCl to a previously tolerated dose at subsequent meals or discontinue the protocol.
If there are no noticeable reactions to the betaine HCl after two days, patients should increase the number of capsules with each meal to three (3).a
Continue increasing the number of capsules every two days (maximum 3,000 mg of betaine HCl) with each meal if necessary, until a dose results in tingling, burning, or any other type of discomfort. At such point, the patient should decrease the dose by one (1) capsule per meal. If the discomfort continues, they should be instructed to discontinue the betaine HCl supplementation and consult with their healthcare professional.
Once a dose is established, continue this dose at subsequent meals.
With smaller meals, less betaine HCl is needed, so a reduced dose may be adequate.
Individuals with very moderate HCl deficiency generally show rapid improvement in symptoms and have early signs of intolerance to the acid. This typically indicates a return to normal acid secretion.
Precautions: Administration of HCl/pepsin is contraindicated in peptic ulcer disease. HCl can irritate sensitive tissue and can be corrosive to teeth; therefore, capsules should NOT be emptied into food or dissolved in beverages.
aIt is important that this be done with a meal of sufficient size (500 calories or more) containing adequate protein. Betaine HCl should not be given on an empty stomach unless it is followed immediately by consuming a meal.
Does the Betaine (TMG) portion of Betaine HCl Benefit Digestion
Because of the confusion between these two compounds, some have suggested that betaine itself is the active ingredient and the HCl is not needed (or acidic). While we have clearly dismissed the notion that betaine HCl is not acidic, human clinical trials exploring the effect of betaine (independent of its HCl counterpart) on GI outcomes are currently lacking. However, a 2018 animal study in rats found that high salt stress decreased the activities of amylase, lipase, trypsin and chymotrypsin (P < .05) and that anhydrous betaine (TMG) supplementation was able to increase the activities of these enzymes under these conditions.31 High salt stress also disturbed the morphology of the intestinal villi by reducing gut villus heights in the duodenum, jejunum and ileum; whereas supplementation with betaine resulted in higher villus heights than the control group consuming normal chow (P<.05). Supplementation of the high salt diet with betaine was also able to offset the diminished gut microbial diversity induced by the high salt diet. Since these effects were seen only in rats (and only under high salt stress), it is unknown if any of these benefits would be realized in humans (with or without hypochlorhydria).
Footnotes
Author Disclosure Statement
Thomas G. Guilliams, PhD, is a medical advisor for Ortho Molecular Products.
i. The “standard meal” used in this early 1990s report was a 6 oz. hamburger, 2 slices of bread, 2 oz. of hash browns, 1 oz. of tomato, some lettuce, mayo and ketchup and 8 oz. of milk (1000 calories).
References
- 1.Feldman M, Barnett C. Fasting gastric pH and its relationship to true hypochlorhydria in humans. Digestive diseases and sciences. 1991;36(7):866-869. [DOI] [PubMed] [Google Scholar]
- 2.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. The Journal of allergy and clinical immunology. 2008;121(6):1301-1308; quiz 1309-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirschowitz BI. Pepsinogen. Postgraduate medical journal. 1984;60(709):743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassarjian Z, Russell RM. Hypochlorhydria: a factor in nutrition. Annual review of nutrition. 1989;9:271-285. [DOI] [PubMed] [Google Scholar]
- 5.Russell RM, Krasinski SD, Samloff IM, Jacob RA, Hartz SC, Brovender SR. Folic acid malabsorption in atrophic gastritis. Possible compensation by bacterial folate synthesis. Gastroenterology. 1986;91(6):1476-1482. [DOI] [PubMed] [Google Scholar]
- 6.Schubert ML. Functional anatomy and physiology of gastric secretion. Current opinion in gastroenterology. 2015;31(6):479-485. [DOI] [PubMed] [Google Scholar]
- 7.McDonald EG, Milligan J, Frenette C, Lee TC. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA internal medicine. 2015;175(5):784-791. [DOI] [PubMed] [Google Scholar]
- 8.Husebye E, Skar V, Hoverstad T, Melby K. Fasting hypochlorhydria with gram positive gastric flora is highly prevalent in healthy old people. Gut. 1992;33(10):1331-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhutto A, Morley JE. The clinical significance of gastrointestinal changes with aging. Current opinion in clinical nutrition and metabolic care. 2008;11(5):651-660. [DOI] [PubMed] [Google Scholar]
- 10.Morihara M, Aoyagi N, Kaniwa N, Kojima S, Ogata H. Assessment of gastric acidity of Japanese subjects over the last 15 years. Biological & pharmaceutical bulletin. 2001;24(3):313-315. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz A, Brady DA, Schaal SE, Samloff IM, Dedon J, Ruhl CE. Gastric acidity in older adults. Jama. 1997;278(8):659-662. [PubMed] [Google Scholar]
- 12.Iijima K, Ohara S, Koike T, Sekine H, Shimosegawa T. Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scandinavian journal of gastroenterology. 2004;39(8):709-716. [DOI] [PubMed] [Google Scholar]
- 13.Dressman JB, Berardi RR, Dermentzoglou LC, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharmaceutical research. 1990;7(7):756-761. [DOI] [PubMed] [Google Scholar]
- 14.Russell TL, Berardi RR, Barnett JL, et al. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharmaceutical research. 1993;10(2):187-196. [DOI] [PubMed] [Google Scholar]
- 15.McMullen MK, Whitehouse JM, Towell A. Bitters: Time for a New Paradigm. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:670504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker J, Hell J, Liszt KI, et al. Identification of beer bitter acids regulating mechanisms of gastric acid secretion. Journal of agricultural and food chemistry. 2012;60(6):1405-1412. [DOI] [PubMed] [Google Scholar]
- 17.Stoeger V, Liszt KI, Lieder B, et al. Identification of Bitter-Taste Intensity and Molecular Weight as Amino Acid Determinants for the Stimulating Mechanisms of Gastric Acid Secretion in Human Parietal Cells in Culture. Journal of agricultural and food chemistry. 2018;66(26):6762-6771. [DOI] [PubMed] [Google Scholar]
- 18.Liszt KI, Hans J, Ley JP, Kock E, Somoza V. Characterization of Bitter Compounds via Modulation of Proton Secretion in Human Gastric Parietal Cells in Culture. Journal of agricultural and food chemistry. 2018;66(10):2295-2300. [DOI] [PubMed] [Google Scholar]
- 19.Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJ. Coffee and gastrointestinal function: facts and fiction. A review. Scandinavian journal of gastroenterology Supplement. 1999;230:35-39. [DOI] [PubMed] [Google Scholar]
- 20.Rubach M, Lang R, Hofmann T, Somoza V. Time-dependent component-specific regulation of gastric acid secretion-related proteins by roasted coffee constituents. Annals of the New York Academy of Sciences. 2008;1126:310-314. [DOI] [PubMed] [Google Scholar]
- 21.Rubach M, Lang R, Bytof G, et al. A dark brown roast coffee blend is less effective at stimulating gastric acid secretion in healthy volunteers compared to a medium roast market blend. Molecular nutrition & food research. 2014;58(6):1370-1373. [DOI] [PubMed] [Google Scholar]
- 22.Liszt KI, Ley JP, Lieder B, et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(30):E6260-e6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. European journal of clinical pharmacology. 1997;52(3):235-237. [DOI] [PubMed] [Google Scholar]
- 24.Chin TW, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrobial agents and chemotherapy. 1995;39(8):1671-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yago MR, Frymoyer A, Benet LZ, et al. The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. The AAPS journal. 2014;16(6):1358-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yago MR, Frymoyer AR, Smelick GS, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Molecular pharmaceutics. 2013;10(11):4032-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber KP, Wu HF, Yago MR, et al. Meal Effects Confound Attempts to Counteract Rabeprazole-Induced Hypochlorhydria Decreases in Atazanavir Absorption. Pharmaceutical research. 2017;34(3):619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang J, Dalziel G, Dean B, Ware JA, Salphati L. Pharmacokinetics and absorption of the anticancer agents dasatinib and GDC-0941 under various gastric conditions in dogs--reversing the effect of elevated gastric pH with betaine HCl. Molecular pharmaceutics. 2013;10(11):4024-4031. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MR, Smetzer JL. ISMP Medication Error Report Analysis: Betaine Anhydrous Versus Betaine Hydrochloride Look-Alike Generic Names Don’t Give Zurampic Without Allopurinol Lantus Overdose Tied to Confusing Vial Label More on Lipid Rescue. Hospital pharmacy. 2017;52(3):169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benet LZ, Frassetto L.A. Effect of Betaine and Food on Gastric pH. Available from: https://clinicaltrials.gov/ct2/show/NCT02758015?id=NCT02758015&rank=1&load=cart. NLM identifier: NCT02758015. Accessed December 7, 2018.
- 31.Wang H, Li S, Fang S, Yang X, Feng J. Betaine Improves Intestinal Functions by Enhancing Digestive Enzymes, Ameliorating Intestinal Morphology, and Enriching Intestinal Microbiota in High-salt stressed Rats. Nutrients. 2018;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]


