Abstract
Objective
To assess longitudinal tremor outcomes with ventral intermediate nucleus deep brain stimulation (VIM DBS) in patients with dystonic tremor (DT) and to compare with DBS outcomes in essential tremor (ET).
Methods
We retrospectively investigated VIM DBS outcomes for 163 patients followed at our center diagnosed with either DT or ET. The Fahn-Tolosa-Marin tremor rating scale (TRS) was used to assess change in tremor and activities of daily living (ADL) at 6 months, 1 year, 2–3 years, 4–5 years, and ≥6 years after surgery.
Results
Twenty-six patients with DT and 97 patients with ET were analyzed. Compared to preoperative baseline, there were significant improvements in TRS motor up to 4–5 years (52.2%; p = 0.032) but this did not reach statistical significance at ≥6 years (46.0%, p = 0.063) in DT, which was comparable to the outcomes in ET. While the improvements in the upper extremity tremor, head tremor, and axial tremor were also comparable between DT and ET throughout the follow-up, the ADL improvements in DT were lost at 2–3 years follow-up.
Conclusion
Overall, tremor control with VIM DBS in DT and ET was comparable and remained sustained at long term likely related to intervention at the final common node in the pathologic tremor network. However, the long-term ADL improvements in DT were not sustained, possibly due to inadequate control of concomitant dystonia symptoms. These findings from a large cohort of DT indicate that VIM targeting is reasonable if the tremor is considerably more disabling than the dystonic features.
Classification of evidence
This study provides Class IV evidence that VIM DBS improves tremor in patients with DT or ET.
Dystonic tremor (DT), defined as tremor presenting in body regions with dystonia, has been reported as a frequent clinical manifestation in recent epidemiologic studies1,2; however, the pathophysiology and treatment have remained relatively underinvestigated. DT is frequently misdiagnosed as essential tremor (ET), although DT has some distinguishing characteristics such as asymmetry, irregularity in amplitude and frequency, and phenomenologic features of dystonia including overflow, sensory trick, and position specificity.3,4
There has been limited literature on effective treatments for DT with many options overlapping with ET.5 Similar to ET, deep brain stimulation (DBS) targeted to the ventral intermediate nucleus (VIM), an essential node in the tremor circuitry, is also considered for medication-refractory DT.6–11 However, the numbers of participants in these studies were small and little is known about the long-term outcomes.6–9 Cury et al.7 reported that DBS effects in DT were modest and transient in comparison to ET and Parkinson disease (PD); however, the DT cohort comprised only 6 patients and there was no direct comparison of outcomes across patient groups. At our center, we recommend the VIM target for patients with DT if the tremor was endorsed to be more prominent and bothersome compared to the dystonia symptoms, given our experience that implantation in the globus pallidus interna (GPi) did not ameliorate tremor to the same extent as dystonia. In the current study, we analyzed the outcomes in these patients with DT who underwent VIM DBS. We aimed to compare the outcomes of VIM DBS between patients with DT and patients with ET.
Methods
Standard protocol approvals, registrations, and patient consents
Upon receipt of approval from the local institutional review board (IRB201702279), we retrospectively investigated the patients with DT and patients with ET who underwent VIM DBS. Written informed consent was obtained for video disclosures.
Study design
The latest consensus criteria by the Movement Disorders Society define DT as tremor syndromes combining dystonia and tremor in a body part affected by dystonia and ET as an isolated tremor syndrome of bilateral upper limb action tremor with or without tremor in other body regions.3 The inclusion criteria for our study were (1) diagnosis of DT or ET according to the abovementioned criteria, (2) VIM DBS performed at the University of Florida between 2003 and 2017, (3) no history of stereotactic brain surgery, (4) no acquired etiologies or neurodegenerative diseases including PD, (5) preoperative clinical assessments with postoperative assessments at 6 months or longer, and (6) optimally placed DBS leads confirmed by the postoperative lead measurements. We excluded patients with tremor syndromes with additional features of parkinsonism, myoclonus, ataxia, or questionable dystonia.3 Because of the absence of a control group without DBS, the current study provides Class IV evidence for the clinical effectiveness of DBS in DT and ET populations.
Standard perioperative procedures
All patients received a thorough evaluation from multiple disciplines including neurosurgery, psychiatry, neuropsychology, and rehabilitation services to establish that the benefits of the surgery outweighed the risks. DBS leads (model 3387; Medtronic, Minneapolis, MN) were implanted under local anesthesia with additional guidance obtained with intraoperative microelectrode recordings and macrostimulation testing. The initial implantations and replacements were performed with the latest available pulse generators (Activa PC/SC, Soletra, or Kinetra; Medtronic). CT was performed 1 month after implantation and fused with the preoperative MRI using the in-house software. The electrode positions were graphically identified with a 3D deformable brain atlas overlay as described in our previous report.12 Patients were followed monthly to optimize stimulation settings and medication for the first 6 months after surgery. After the optimal setting was established, patients had biannual visits up to 3 years and annual visits afterward.
Outcome measures
We assessed the tremor outcomes with DBS using the Fahn-Tolosa-Marin tremor rating scale (TRS).13 The TRS consists of 3 parts: part A (resting, postural, and action tremor of the 9 body regions), part B (action tremor of the upper extremities [UE] during handwriting, drawing, and pouring tasks), and part C (activities of daily living [ADL]). Higher scores indicate worse conditions. We extracted TRS total (items 1–21), TRS motor (part A, items 1–9), TRS ADL (part C, items 15–21), axial tremor total (face, tongue, voice, head, and trunk; items 1–4 and 7), and head tremor (item 4). The lateralized scores of UE resting, postural, and action tremor were analyzed as combined (UE tremor total) and separately (item 5/6). The handwriting score (item 10) for the dominant extremity and the lateralized hand function score (drawing and pouring tasks; items 11–14) were also analyzed.
The postoperative scores were sorted into 5 follow-up intervals (6 months, 1 year, 2–3 years, 4–5 years, and ≥6 years after surgery). DBS outcomes were primarily assessed by comparing the “on”-stimulation condition with the baseline status within the DT and ET groups. The “off”-stimulation assessments were performed at least 30 minutes after the stimulation was turned off and TRS motor was compared between the “on”- and “off”-stimulation conditions at each follow-up. The Δ changes (i.e., subtraction) compared to baseline were calculated for all measures and were used for the comparisons between the DT and ET groups. We also separately compared patients treated with unilateral and bilateral VIM DBS to analyze the DBS effects on axial tremor within the DT and ET groups. Furthermore, we compared the percentages of good responders between the DT and ET groups with the threshold of a clinically relevant DBS response defined as >40% improvement in TRS motor at 6 months or 1 year compared to baseline. A responder analysis was restricted to the short-term follow-up because a subset of patients in both groups did not have follow-ups longer than 1 year.
Statistical analysis
Statistical analysis was performed using IBM (Armonk, NY) SPSS Statistics 25. We compared the demographics, baseline outcome measures, and adverse events (AEs) between the 2 groups using Mann-Whitney U tests, χ2 tests, or Fisher exact tests, as appropriate. The statistical significance was set to a threshold of p < 0.05. In the DT and ET groups, the outcome measures at each follow-up were compared with baseline status using Wilcoxon signed-rank tests. The “on”- and “off”-stimulation comparison of TRS motor at each follow-up was also performed using Wilcoxon signed-rank tests. We performed Mann-Whitney U tests for the between-group comparisons (DT vs ET; unilateral vs bilateral DBS) at each follow-up. The type I error rates for multiple comparisons were corrected with Holm-Bonferroni method, which adjusts p values for each hypothesis with a range of significance thresholds (0.01–0.05).
Data availability
Anonymized data can be made available at the request of qualified investigators for the purpose of replicating procedures and results.
Results
Participants
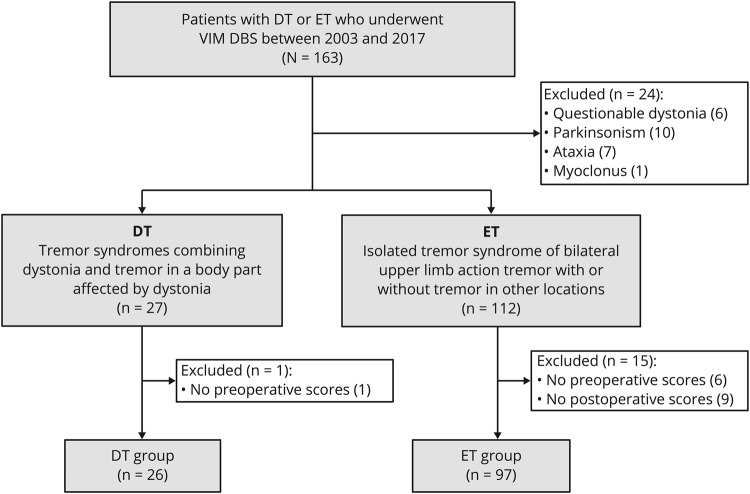
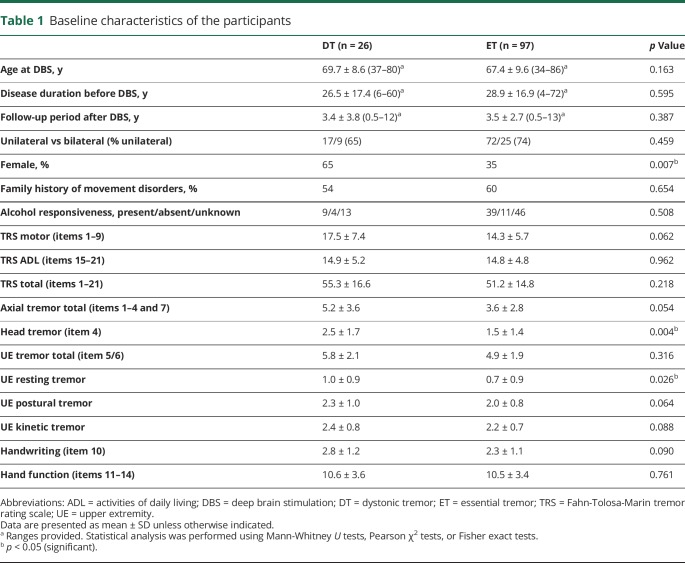
We identified 163 VIM DBS patients who met the diagnostic criteria for DT or ET (figure 1). Out of these, we excluded 24 patients for the following reasons: 6 patients were initially diagnosed with ET, but the clinicians noted questionable features of dystonia; 10 had additional parkinsonism; 7 presented with ataxia; and 1 had myoclonus. Furthermore, we excluded 16 patients (1 DT and 15 ET) due to the lack of preoperative or postoperative assessments in our database. The final cohort for analysis comprised 97 patients with ET and 26 patients with DT (21 patients with segmental dystonia and 5 with focal neck dystonia). In all the patients with DT, neck (n = 21) or arms (n = 14) were proven to be affected by both dystonia and tremor (i.e., DT). UE postural and action tremor was present in all the patients with DT and was the main therapeutic target. Some patients also had tremor in the face, voice, trunk, or legs. Except for female predominance (p = 0.007) and more severe head tremor (p = 0.004) and UE resting tremor (p = 0.026) in the DT group, there were no significant differences between the 2 groups in the demographic, clinical, or tremor characteristics (table 1).
Figure 1. Patient flowchart.
DT = dystonic tremor; ET = essential tremor; VIM DBS = ventral intermediate nucleus deep brain stimulation.
Table 1.
Baseline characteristics of the participants
Motor and ADL outcomes with VIM DBS in DT and ET
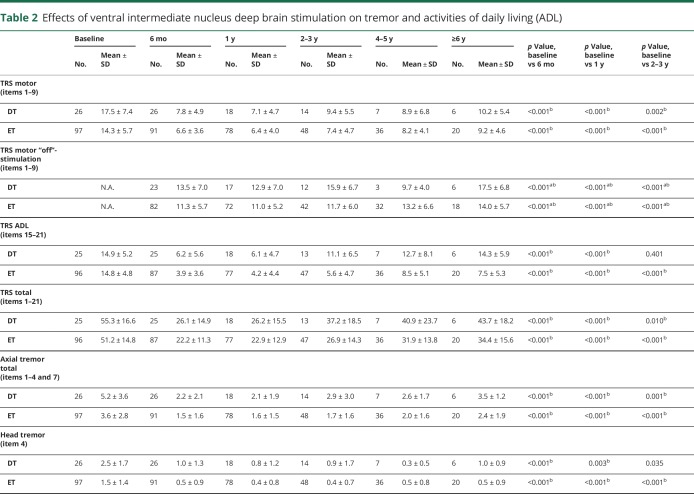
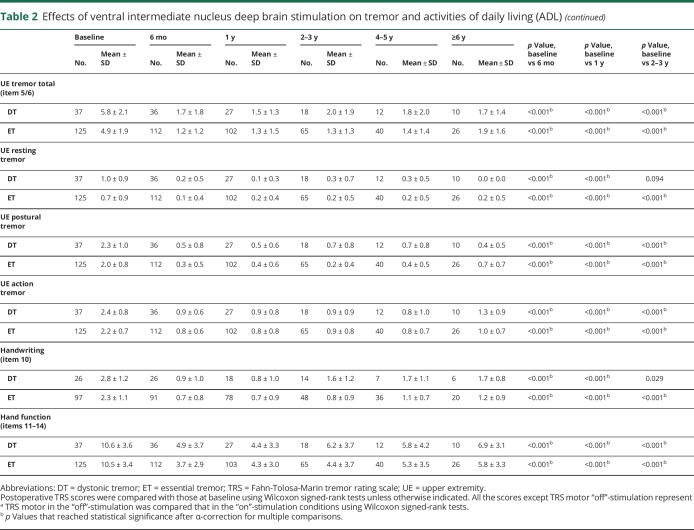
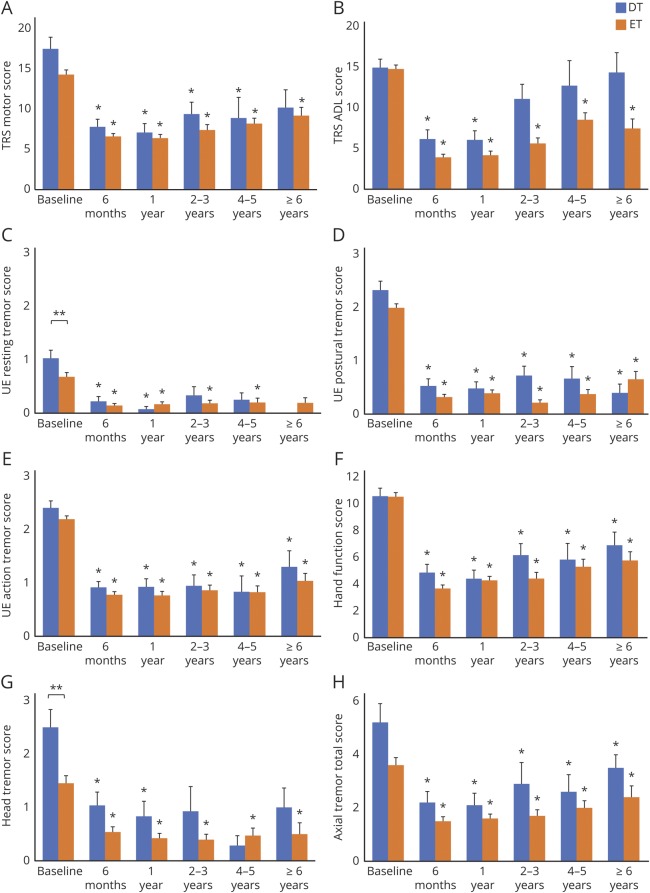
With regard to TRS motor, the DT group showed significant improvements at 6 months (55.7%, p < 0.001), 1 year (56.4%, p < 0.001), 2–3 years (44.0%, p = 0.002), and 4–5 years (52.2%, p = 0.016) but did not reach statistical significance at ≥6 years (46.0%, p = 0.063) in comparison with the baseline status (table 2 and figure 2). In the DT group, the “on”- and “off”-stimulation comparison showed significantly improved TRS motor in the “on”-stimulation conditions up to 2–3 years follow-up (∼46% improvement, all p < 0.001) but did not reach statistical significance in the “on”-stimulation condition at ≥6 years follow-up (41.9% improvement, p = 0.031). Likewise, the ET group showed significant improvements at 6 months (54.5%, p < 0.001), 1 year (53.2%, p < 0.001), 2–3 years (50.0%, p < 0.001), 4–5 years (45.0%, p < 0.001), and ≥6 years (32.5%, p = 0.002) and the “on”- and “off”-stimulation comparisons were significant at all follow-ups. There was no significant difference in the percentage of good responders for the DT vs ET groups (84.6% vs 78.4%, p = 0.481). Furthermore, there were no significant differences in improvement of TRS motor between the DT and ET groups at any follow-up (table e-1, doi.org/10.5061/dryad.380hc70).
Table 2.
Effects of ventral intermediate nucleus deep brain stimulation on tremor and activities of daily living (ADL)
Figure 2. Longitudinal changes in motor function and activities of daily living (ADL).
Scores for (A) Fahn-Tolosa-Marin Tremor Rating Scale (TRS) motor, (B) TRS ADL, (C) upper extremity (UE) resting tremor, (D) UE postural tremor, (E) UE action tremor, (F) hand function, (G) head tremor, and (H) axial tremor total are shown. Blue and orange bars represent mean scores for dystonic tremor (DT) and essential tremor (ET), respectively. Whiskers represent the standard error. *Significant differences between the baseline and postoperative scores. **Significant differences between the DT and ET groups.
The DT group showed significant improvements in TRS ADL at 6 months (55.7%, p < 0.001) and 1 year (56.4%, p < 0.001), whereas the results were not significant at 2–3 years and subsequent follow-ups. In contrast, the ET group experienced significant improvements at all follow-ups (6 months, 73.3%; 1 year, 71.4%; 2–3 years, 61.6%; 4–5 years, 45.9%; and ≥6 years, 53.7%; all p < 0.001). ADL improvements in the ET group tended to be greater than in the DT group but not statistically significant after Holm-Bonferroni correction at 6 months, 1 year, 2–3 years, and ≥6 years follow-up (all p < 0.05).
The effects of VIM DBS in the patients with DT are illustrated in the legends provided with the video 1.
Patient 1: Video segments before and after right ventral intermediate nucleus deep brain stimulation (VIM DBS) surgery. Before surgery, the patient had pronation–supination resting tremor and postural tremor in the left arm. He could not perform spiral drawing with the left hand. Eleven years after right VIM surgery, DBS effectively controlled the postural tremor, although the spiral drawing task continued to remain difficult and there were dystonic features in the left arm. Patient 2: Video segments comparing right VIM DBS turned on and off reveal improvements in the spiral drawing and the pouring tasks. Patient 3: Video segments recorded before surgery and while the left VIM DBS was turned on. Before surgery, a pronation–supination resting tremor was observed in the right arm and postural tremor was worse in the right hand with clear dystonic posturing. There was mild torticollis with dystonic head tremor. Left VIM DBS improved the resting tremor and the postural tremor in the right side, and there was mild suppression of head tremor.Download Supplementary Video 1 (18MB, wmv) via http://dx.doi.org/10.1212/008875_Video_1
Control of UE tremor with VIM DBS
UE tremor total, UE postural tremor, UE action tremor, and hand function significantly improved in the DT group at all follow-ups compared to the baseline status (table 2 and figure 2), while UE resting tremor showed statistically significant improvements only at 6 months (p < 0.001) and at 1 year follow-up (p < 0.001). Similarly, the ET group showed improvements in UE tremor total, UE postural tremor, UE action tremor, and hand function at all follow-ups, whereas significant improvements in UE resting tremor were observed up to 4–5 years follow-up (p = 0.010). There were no significant differences between DT and ET in any aspect of UE tremor assessments (tremor total, resting, postural, action, and hand function) at any follow-up (table e-1, doi.org/10.5061/dryad.380hc70).
Axial outcomes with VIM DBS
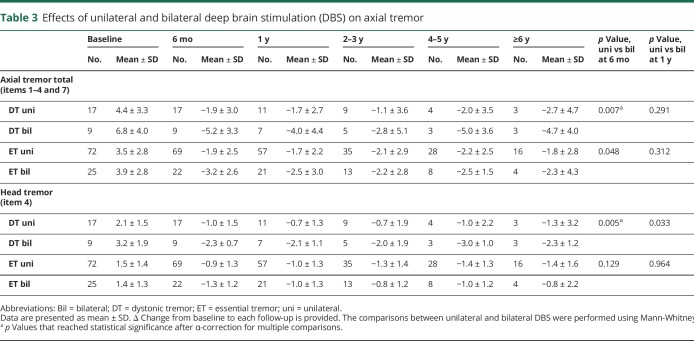
The DT group showed significant improvements in axial tremor total up to ≥6 years (p = 0.031); however, the statistically significant improvements in head tremor were observed only up to 1 year follow-up (table 2 and figure 2). The ET group demonstrated significant improvements in head tremor and axial tremor total up to ≥6 years. When comparing DT and ET, there were no significant differences at any follow-up (table e-1, doi.org/10.5061/dryad.380hc70). When comparing the subgroups treated with unilateral and bilateral DBS within the DT group (table 3), improvements in head tremor and axial tremor total were significantly greater in the patients with DT treated with bilateral DBS than those treated with unilateral DBS at 6 months (p = 0.007 and 0.005, respectively). At subsequent follow-ups, there was no statistical difference with the substantially reduced sample sizes. In the ET subgroups, there were no significant differences between patients treated with unilateral and bilateral DBS for the head tremor and axial tremor total throughout the follow-up.
Table 3.
Effects of unilateral and bilateral deep brain stimulation (DBS) on axial tremor
Anatomical location of the contacts and stimulation settings
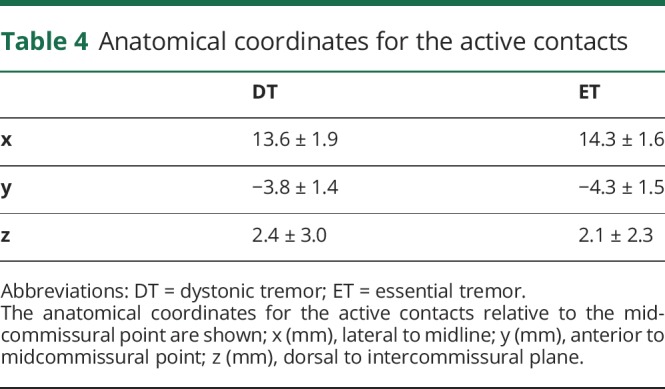
The anatomical coordinates for the active contacts relative to the midcommissural points are shown in table 4. The stimulation settings in the 2 groups at the last follow-up were as follows (mean ± SD, range): voltage (DT, 2.6 ± 0.9, 1.0–4.4; ET, 2.7 ± 0.7, 1.0–4.7), pulse width (DT, 102.3 ± 34.7, 60–210; ET, 92.0 ± 25.7, 60–210), and frequency (DT, 154.6 ± 23.7, 130–210; ET, 92.0 ± 25.7, 60–210).
Table 4.
Anatomical coordinates for the active contacts
Adverse events
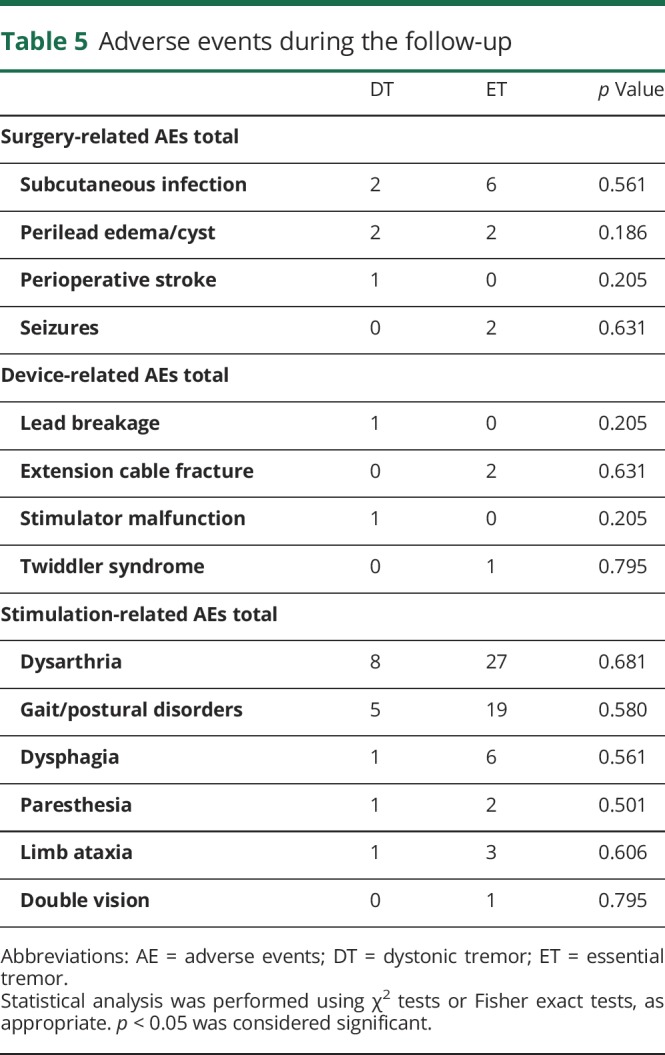
The surgery-related, device-related, and stimulation-related AEs for DT and ET are summarized in table 5. The most common AEs in both groups were dysarthria and gait and postural disorders. In most patients, the stimulation-related AEs were mild and were controlled with adjustments of stimulation settings. There were no significant differences in the frequency of AEs between DT and ET (all p > 0.05).
Table 5.
Adverse events during the follow-up
Discussion
This single-center study utilized a large dataset of patients who underwent VIM DBS to address whether VIM DBS effectively controlled the tremor in DT and whether the longitudinal outcomes were comparable to ET. In the patients with DT, VIM DBS effectively controlled UE postural and action tremor and axial tremor for 6 years or beyond, whereas the outcomes of TRS motor, UE resting tremor, and head tremor did not reach statistical significance at long term, presumably related to limited statistical power with smaller samples for analysis. When comparing DT and ET, the improvements in the TRS motor, UE tremor, head tremor, and axial tremor with VIM DBS were comparable throughout the follow-up; however, the ADL improvements were lost at 2–3 years follow-up only in the patients with DT.
There is scant literature on VIM DBS outcomes in DT, with mostly case reports and small case series.6–11 Hedera et al.6 reported that 6 patients with DT had a mean tremor improvement in the Washington Heights–Inwood Genetic Study of Essential Tremor scale of 85% at 6–9 months after VIM DBS, whereas the patients with DT in our cohort experienced improvements in TRS motor of 56% at 1 year and 46% at ≥6 years. The discrepancy between the outcomes reported by Hedera et al. and ours might be explained by differences in the tremor scales employed or the study cohorts. Our short- and long-term outcomes were similar to or slightly better than the findings of Cury et al.7 (41% at 1 year and 30% at >6 years), which were also assessed with TRS motor. Our long-term results at ≥6 years were not significant, which could be attributable to a small number of patients available for analysis and progressive worsening in TRS motor in the “on”-stimulation condition. As suggested in patients with ET,14–16 habituation or disease progression likely contributed to less impressive long-term outcomes for the patients with DT. Although a marginally nonsignificant difference between the “on”- and “off”-stimulation conditions at ≥6 years suggests the long-term efficacy of VIM DBS in DT, long-term studies with larger cohorts are warranted for further clarification.
A novel aspect of the present study is direct comparisons of DBS outcomes between DT and ET. The extent of tremor improvements in the patients with ET with VIM DBS was consistent with the previously published cohorts16,17 and the short- and long-term outcomes were comparable between the patients with DT and patients with ET except for ADL. There was no significant difference in the percentages of good responders in the 2 groups. The incidence of AEs was not significantly different between the patients with DT and patients with ET and was similar to that of previous VIM DBS studies.18,19 In both groups, the most common AEs were dysarthria and gait and postural disorders that are presumably related to the current spread to adjacent pathways such as the corticobulbar and corticospinal fibers, the afferent and efferent fibers of the red nucleus, or the cerebellar efferent fibers from the vermis.20–24
The patients with DT and patients with ET had similar outcomes of UE tremor total, postural tremor, action tremor, and hand function and the significant improvements in both groups were sustained for 6 years or beyond. However, the resting component of UE tremor did not demonstrate significant improvements at longer follow-ups in either group, which might be related to small sample size available for analysis. The baseline scores for resting tremor were relatively low, and the follow-up assessments were likely affected by a floor effect of the rating scale.
Whereas the patients with DT had significant improvements in axial tremor total for 6 years or beyond, the head tremor control was not significant in the long term. This might be attributable to the smaller numbers of patients for comparison because the extent of head tremor control at short term and long term was comparable. Bilateral DBS in the patients with DT seemed to control head and axial tremor better than unilateral DBS; however, we found statistical significance only at 6 months with the substantially reduced sample sizes at subsequent follow-ups. Whereas previous ET studies showed bilateral stimulation to be more effective,25–27 we found similar head tremor improvements when comparing unilateral with bilateral DBS in the ET group. Nonsuperiority of bilateral DBS might be explained by a relative insensitivity of head tremor scales and a floor effect. More sensitive tremor assessments may be preferable for future studies. In a recent large pooled analysis, bilateral VIM DBS for patients with ET was associated with additional control of axial tremor at the cost of an increased rate of AEs.27 Future studies with larger cohorts examining the long-term risk/benefit ratio of bilateral vs unilateral DBS may shed further insights.
The ADL improvements in the patients with DT were lost at 2–3 years, whereas the patients with ET demonstrated significant benefits for 6 years or beyond. The extent of improvements in the patients with ET (48% improvements) corroborates the previously published outcomes.17 To our knowledge, no DT studies have assessed ADL outcomes with DBS therapy. The smaller ADL improvements and diminished benefits at longer follow-ups in DT may be explained by the presence of concomitant dystonia symptoms although we did not assess dystonia symptoms with a standardized dystonia rating scale. Whereas there have been a few reported cases who experienced improvements in dystonia after VIM DBS,6,9,10 other studies have shown nonsignificant benefits.6,8 Hedera et al.6 reported that VIM DBS improved tremor by 85% without significant changes in dystonia, whereas GPi DBS improved tremor by 40% and dystonia by 64%. A few DT case reports showed tremor improvements after GPi DBS by up to 82% at follow-ups ranging from 10 months to 3.5 years, which was in parallel to dystonia improvements.28,29 Collectively, limited evidence suggests that VIM DBS may have better tremor outcomes compared to GPi DBS and that GPi DBS may offer superior control of other dystonia symptoms. Besides, there have been reports that demonstrated adequate control of both tremor and dystonia with combined VIM and GPi DBS.6,10 Future studies should compare VIM and GPi DBS for DT with long-term quality of life as the primary outcome. Another potential target may be the subthalamic nucleus (STN).30 Directional leads may provide better control of both dystonia and tremor by stimulating STN and the cerebellar pathway simultaneously with a minimal adverse effect.31
Patients with DT and patients with ET share many clinical characteristics. Patients with ET frequently have alcohol responsiveness and positive family history.3 Recent large studies have also found alcohol responsiveness (29.3%–56.5%) in patients with dystonia32–34 and the presence of positive family history was a strong predictor for alcohol responsiveness.33 Dystonia has been reported to manifest frequently in patients with familial ET.2 In some patients carrying the ANO3 mutation (DYT24), dystonia symptoms emerged after the manifestation of initial ET-like tremor.35 These findings collectively suggest that DT and ET may share common genetic backgrounds and pathophysiologic substrates for these 2 tremor disorders.
Similar to ET,36 there is growing evidence for the cerebellum and the cerebellar output pathway to participate in the pathogenesis of tremor in dystonia.37 For instance, an eyeblink conditioning study found cerebellar impairment in patients with dystonia who manifested tremor in contrast to those patients without a tremor.38 In another study, genetic silencing of the olivocerebellar projections in mice led to dystonic movement and tremor with aberrant cerebellar nuclear activities.39 Those symptoms were alleviated with DBS targeted to the cerebellar nuclei, suggesting that abnormal cerebellar activities can simultaneously cause both tremor and dystonia. In a recent fMRI study, both patients with DT and patients with ET showed common cerebellar impairment, although patients with DT demonstrated significantly more widespread abnormalities in functional connectivity.40 Our findings of a comparable tremor control with VIM DBS in DT and ET may support the hypothesis that dysfunction in the cerebello-thalamo-cortical pathway plays a key role in tremor occurrence in both DT and ET. However, the current spread into the adjacent structures, especially the Voa/Vop nuclei, might also contribute to control of tremor and dystonia.41–43 This possibility needs to be examined in future imaging studies.
Several limitations of this study must be considered. First, the data were analyzed retrospectively, all assessments were open-label, and the number of patients with DT with long-term follow-up was small, which limited the statistical power. Second, a standardized dystonia rating scale was not employed for a formal assessment of dystonia symptoms. Finally, all participants in our cohort had focal and segmental dystonia; thus caution should be taken for broader applicability of findings to other forms of dystonia.
Our findings suggest that VIM DBS is effective for DT with a potential for sustained long-term benefits when tremor is the predominant symptom in patients with focal or segmental dystonia. In DT, VIM DBS effectively controlled UE postural and action tremor and axial tremor for 6 years or beyond, whereas the improvements in TRS motor, UE resting tremor, and head tremor did not reach statistical significance at long term. Bilateral stimulation showed significantly better control of head and axial tremor compared to unilateral DBS only at short term. However, larger samples are needed for further clarification. Except for ADL, the tremor controlling effects of VIM DBS in DT and ET were comparable, likely related to the intervention at the final common node in the pathologic tremor network. Inadequate control of ADL in DT possibly due to suboptimal control of concomitant dystonia symptoms is an important limitation of VIM DBS. Thus our study findings indicate that the choice of a thalamic brain target for DBS in DT is reasonable when tremor is the predominant feature; however, GPi may be a better choice for patients with significant dystonia. Studies on formal head-to-head comparisons between VIM and GPi DBS will further assist clinicians by clarifying the target selection process.
Acknowledgment
The authors thank Tyler's Hope Foundation for a Dystonia Cure, the Uehara Memorial Foundation for the research fellowship program, and Eli Lilly Japan for the Lilly scientific fellowship program.
Glossary
- ADL
activities of daily living
- AE
adverse event
- DBS
deep brain stimulation
- DT
dystonic tremor
- ET
essential tremor
- GPi
globus pallidus interna
- PD
Parkinson disease
- STN
subthalamic nucleus
- TRS
Fahn-Tolosa-Marin tremor rating scale
- UE
upper extremities
- VIM
ventral intermediate nucleus
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study funding
No targeted funding reported.
Disclosure
T. Tsuboi, Z. Jabarkheel, P. Zeilman, and M. Barabas report no disclosures relevant to the manuscript. K. Foote reports grants from NIH and other funding from Donnellan/Einstein/Merz Chair during this study; grants and nonfinancial support from Medtronic; grants from St Jude, Functional Neuromodulation, and Boston Scientific; and grants and other funding from Neuropace; and has a patent (US 8295935 B2) issued for a DBS cranial lead fixation device. M. Okun serves as consultant for the National Parkinson's Foundation; has received research grants from the NIH, National Parkinson's Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and UF Foundation; has previously received honoraria, but in the past >60 months has received no support from industry; has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books); is an associate editor for New England Journal of Medicine Journal Watch Neurology; has participated in CME and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, Quantia, Henry Stewart, and the Vanderbilt University; Dr. Okun’s institution and not Dr. Okun receives grants from Medtronic, AbbVie, and ANS/St. Jude, and the PI has no financial interest in these grants; and has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. A. Wagle Shukla reports grants from the NIH and has received grant support from Benign Essential Blepharospasm Research Foundation, Dystonia Coalition, Dystonia Medical Research Foundation, and National Organization for Rare Disorders, and grant support from NIH (KL2 and K23 NS092957-01A1). Go to Neurology.org/N for full disclosures.
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey S, Sarma N. Tremor in dystonia. Parkinsonism Relat Disord 2016;29:3–9. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors: from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED. The evolving definition of essential tremor: what are we dealing with? Parkinsonism Relat Disord 2018;46(suppl 1):S87–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasano A, Bove F, Lang AE. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry 2014;85:759–769. [DOI] [PubMed] [Google Scholar]
- 6.Hedera P, Phibbs FT, Dolhun R, et al. Surgical targets for dystonic tremor: considerations between the globus pallidus and ventral intermediate thalamic nucleus. Parkinsonism Relat Disord 2013;19:684–686. [DOI] [PubMed] [Google Scholar]
- 7.Cury RG, Fraix V, Castrioto A, et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 2017;89:1416–1423. [DOI] [PubMed] [Google Scholar]
- 8.Vercueil L, Pollak P, Fraix V, et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol 2001;248:695–700. [DOI] [PubMed] [Google Scholar]
- 9.Woehrle JC, Blahak C, Kekelia K, et al. Chronic deep brain stimulation for segmental dystonia. Stereotact Funct Neurosurg 2009;87:379–384. [DOI] [PubMed] [Google Scholar]
- 10.Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson CE, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor? Stereotact Funct Neurosurg 2010;88:98–104. [DOI] [PubMed] [Google Scholar]
- 11.Patel A, Deeb W, Okun MS. Deep brain stimulation management of essential tremor with dystonic features. Tremor Other Hyperkinet Mov 2018;8:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morishita T, Hilliard JD, Okun MS, et al. Postoperative lead migration in deep brain stimulation surgery: incidence, risk factors, and clinical impact. PLoS One 2017;12:e0183711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahn S, Tolosa E, Marín C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, eds. Parkinson's Disease and Movement Disorders, 2nd ed. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- 14.Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CE, Okun MS. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain 2012;135:1455–1462. [DOI] [PubMed] [Google Scholar]
- 15.Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol 2011;258:434–439. [DOI] [PubMed] [Google Scholar]
- 16.Paschen S, Forstenpointner J, Becktepe J, et al. Long-term efficacy of deep brain stimulation for essential tremor: an observer-blinded study. Neurology 2019;92:e1378–e1386. [DOI] [PubMed] [Google Scholar]
- 17.Dallapiazza RF, Lee DJ, De Vloo P, et al. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry 2019;90:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord 2008;23:1146–1153. [DOI] [PubMed] [Google Scholar]
- 19.Wharen RE, Okun MS, Guthrie BL, et al. Thalamic DBS with a constant-current device in essential tremor: a controlled clinical trial. Parkinsonism Relat Disord 2017;40:18–26. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi T, Watanabe H, Tanaka Y, et al. Distinct phenotypes of speech and voice disorders in Parkinson's disease after subthalamic nucleus deep brain stimulation. J Neurol Neurosurg Psychiatry 2015;86:856–864. [DOI] [PubMed] [Google Scholar]
- 21.Fasano A, Herzog J, Raethjen J, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain 2010;133:3635–3648. [DOI] [PubMed] [Google Scholar]
- 22.Reich MM, Brumberg J, Pozzi NG, et al. Progressive gait ataxia following deep brain stimulation for essential tremor: adverse effect or lack of efficacy? Brain 2016;139:2948–2956. [DOI] [PubMed] [Google Scholar]
- 23.Mücke D, Hermes A, Roettger TB, et al. The effects of thalamic deep brain stimulation on speech dynamics in patients with essential tremor: an articulographic study. PLoS One 2018;13:e0191359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain 2014;137:109–121. [DOI] [PubMed] [Google Scholar]
- 25.Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, Wharen RE. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology 2000;54:2342–2344. [DOI] [PubMed] [Google Scholar]
- 26.Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE. Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry 2005;76:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell KT, Larson P, Starr PA, et al. Benefits and risks of unilateral and bilateral ventral intermediate nucleus deep brain stimulation for axial essential tremor symptoms. Parkinsonism Relat Disord 2018;5:331–338. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez-Zamora A, Kaszuba B, Gee L, et al. Clinical outcome and intraoperative neurophysiology for focal limb dystonic tremor without generalized dystonia treated with deep brain stimulation. Clin Neurol Neurosurg 2016;150:169–176. [DOI] [PubMed] [Google Scholar]
- 29.Torres CV, Moro E, Dostrovsky JO, Hutchison WD, Poon YY, Hodaie M. Unilateral pallidal deep brain stimulation in a patient with cervical dystonia and tremor. J Neurosurg 2010;113:1230–1233. [DOI] [PubMed] [Google Scholar]
- 30.Chou KL, Hurtig HI, Jaggi JL, Baltuch GH. Bilateral subthalamic nucleus deep brain stimulation in a patient with cervical dystonia and essential tremor. Mov Disord 2005;20:377–380. [DOI] [PubMed] [Google Scholar]
- 31.Falconer RA, Rogers SL, Shenai M. Using directional deep brain stimulation to co-activate the subthalamic nucleus and zona incerta for overlapping essential tremor/Parkinson's disease symptoms. Front Neurol 2018;9:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio-Agusti I, Pareés I, Kojovic M, et al. Tremulous cervical dystonia is likely to be familial: clinical characteristics of a large cohort. Parkinsonism Relat Disord 2013;19:634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junker J, Brandt V, Berman BD, et al. Predictors of alcohol responsiveness in dystonia. Neurology 2018;91:e2020–e2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol 2015;262:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamelou M, Charlesworth G, Cordivari C, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord 2014;29:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieuwhof F, Panyakaew P, van de Warrenburg BP, Gallea C, Helmich RC. The patchy tremor landscape: recent advances in pathophysiology. Curr Opin Neurol 2018;31:455–461. [DOI] [PubMed] [Google Scholar]
- 37.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 2014;260:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antelmi E, Di Stasio F, Rocchi L, et al. Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Parkinsonism Relat Disord 2016;31:23–27. [DOI] [PubMed] [Google Scholar]
- 39.White JJ, Sillitoe RV. Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat Commun 2017;8:14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSimone JC, Archer DB, Vaillancourt DE, Wagle Shukla A. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain 2019;142:1644–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagatti D, D'Ammando A, Franzini A, Messina G. Deep brain stimulation of the caudal zona Incerta and motor thalamus for postischemic dystonic tremor of the left upper limb: case report and review of the literature. World Neurosurg 2019;125:191–197. [DOI] [PubMed] [Google Scholar]
- 42.Pauls KA, Hammesfahr S, Moro E, et al. Deep brain stimulation in the ventrolateral thalamus/subthalamic area in dystonia with head tremor. Mov Disord 2014;29:953–959. [DOI] [PubMed] [Google Scholar]
- 43.Fukaya C, Katayama Y, Kano T, et al. Thalamic deep brain stimulation for writer's cramp. J Neurosurg 2007;107:977–982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient 1: Video segments before and after right ventral intermediate nucleus deep brain stimulation (VIM DBS) surgery. Before surgery, the patient had pronation–supination resting tremor and postural tremor in the left arm. He could not perform spiral drawing with the left hand. Eleven years after right VIM surgery, DBS effectively controlled the postural tremor, although the spiral drawing task continued to remain difficult and there were dystonic features in the left arm. Patient 2: Video segments comparing right VIM DBS turned on and off reveal improvements in the spiral drawing and the pouring tasks. Patient 3: Video segments recorded before surgery and while the left VIM DBS was turned on. Before surgery, a pronation–supination resting tremor was observed in the right arm and postural tremor was worse in the right hand with clear dystonic posturing. There was mild torticollis with dystonic head tremor. Left VIM DBS improved the resting tremor and the postural tremor in the right side, and there was mild suppression of head tremor.Download Supplementary Video 1 (18MB, wmv) via http://dx.doi.org/10.1212/008875_Video_1
Data Availability Statement
Anonymized data can be made available at the request of qualified investigators for the purpose of replicating procedures and results.