Abstract
Objective
To characterize risk factors for spontaneous intracerebral hemorrhage (sICH) occurrence and severity among West Africans.
Methods
The Stroke Investigative Research and Educational Network (SIREN) study is a multicenter case-control study involving 15 sites in Ghana and Nigeria. Patients were adults ≥18 years old with CT-confirmed sICH with age-, sex-, and ethnicity-matched stroke-free community controls. Standard instruments were used to assess vascular, lifestyle, and psychosocial factors. Factors associated with sICH and its severity were assessed using conditional logistic regression to estimate odds ratios (ORs) and population-attributable risks (PARs) with 95% confidence intervals (CIs) for factors.
Results
Of 2,944 adjudicated stroke cases, 854 were intracerebral hemorrhage (ICH). Mean age of patients with ICH was 54.7 ± 13.9 years, with a male preponderance (63.1%), and 77.3% were nonlobar. Etiologic subtypes of sICH included hypertension (80.9%), structural vascular anomalies (4.0%), cerebral amyloid angiopathy (0.7%), systemic illnesses (0.5%), medication-related (0.4%), and undetermined (13.7%). Eight factors independently associated with sICH occurrence by decreasing order of PAR with their adjusted OR (95% CI) were hypertension, 66.63 (20.78–213.72); dyslipidemia, 2.95 (1.84–4.74); meat consumption, 1.55 (1.01–2.38); family history of CVD, 2.22 (1.41–3.50); nonconsumption of green vegetables, 3.61 (2.07–6.31); diabetes mellitus, 2.11 (1.29–3.46); stress, 1.68 (1.03–2.77); and current tobacco use, 14.27 (2.09–97.47). Factors associated with severe sICH using an NIH Stroke Scale score >15 with adjusted OR (95% CI) were nonconsumption of leafy green vegetables, 2.03 (1.43–2.88); systolic blood pressure for each mm Hg rise, 1.01 (1.00–1.01); presence of midline shift, 1.54 (1.11–2.13); lobar ICH, 1.72 (1.16–2.55); and supratentorial bleeds, 2.17 (1.06–4.46).
Conclusions
Population-level control of the dominant factors will substantially mitigate the burden of sICH in West Africa.
Spontaneous intracerebral hemorrhage (sICH) caused by a rupture of arteries in the brain is a devastating type of stroke associated with high mortality and morbidity. Intracerebral hemorrhage (ICH) accounts for 10%–20% of all strokes in developed countries with higher rates reported in low and middle-income countries (LMIC) in Africa and Asia.1 While the burden of ICH is receding in high-income countries due largely to improved control of vascular risk factors, in particular hypertension, LMIC in sub-Saharan Africa (SSA) continue to experience a high and rising burden of ICH.2 Limited data emanating from SSA suggest that ICH comprises 25%–35% of all strokes, with 30-day mortality rates ranging between 30% and 70%.3 A better understanding of the epidemiology and biology of ICH among Africans is critical given that it occurs frequently in the young,4–6 it is associated with higher mortality/morbidity, and contributions of genetic predisposition remain to be elucidated in this population. We present a detailed characterization of ICH among Africans regarding its clinical and radiologic features, subtypes, risk factors for occurrence, and severity from the ongoing Stroke Investigative Research and Educational Networks (SIREN) study. SIREN is the largest study to date to comprehensively evaluate the contributions of traditional vascular and genetic risk factors for stroke among indigenous Africans.
Methods
Study design
The SIREN study is a multicenter case-control study involving 15 medical centers in Ghana and Nigeria. A detailed protocol has been published previously.7 We recruited consenting patients with stroke who were ≥18 years old with first clinical stroke within 8 days of current symptom onset or last seen without deficit. In unconscious/aphasic patients, we sought consent from the next of kin. The clinical diagnosis of sICH was confirmed by neuroimaging with CT scan within 10 days of symptom onset. Patients with stroke were recruited from hospitals but not from communities to ensure rapid and accurate phenotyping. The study covered costs of neuroimaging, ECG, echocardiography, carotid Doppler, HbA1c, and lipid profile for all eligible participants who could not afford these to minimize investigation bias.
Control recruitment
We recruited stroke-free adults, mostly from the communities in the catchment areas of the SIREN hospitals, to serve as controls. The study team at each site had a Community Engagement Core that went into urban, periurban, and rural communities within the catchment area of the SIREN hospitals where stroke cases were recruited to enroll control participants. Potential control participants were screened using the 8-item Questionnaire for Verifying Stroke-Free Status (QVSFS). In a validation study performed in 3 key languages spoken in West Africa namely Hausa, Yoruba, and Akan, the QVSFS had 98% negative predictive value.8 Controls were matched to cases by age (±5 years), sex, and ethnicity, to reduce the potential for confounding from these variables.
Standard protocol approvals, registrations, and patient consents
Ethical approval was obtained from all study sites and informed consent was obtained from all participants.7
ICH phenotyping
The diagnosis of ICH was confirmed in all cases using a cranial CT scan. The anatomic location of ICH was noted and classified as lobar (frontal, parietal, temporal, occipital) or nonlobar (basal ganglia, thalamus, cerebellum, brainstem) based on the epicenter of the bleed. The presence of intraventricular bleeding and the dimensions of ICH were recorded, and the volume of hematoma determined using the ABC/2 formula with approximation of hematoma to an ellipsoid.9 sICH was further classified etiologically into structural lesions such as aneurysms/arteriovenous malformations, medication-related, amyloid angiopathy, systemic/other disease, hypertension, and undetermined causes (SMASH-U).10 Ascertainment of etiology followed the proposed algorithm by Meretoja et al.,10 and involved (1) obtaining a cranial CT scan with angiography or magnetic resonance angiography where indicated (CT angiography was done in only 3% of the study population based on clinical suspicion) to characterize structural lesions11; (2) assessing for systemic causes such as liver cirrhosis and disseminated intravascular coagulopathy using history, physical examination, spontaneously elevated international normalized ratio (INR) or liver enzymes 3× upper limit of normal, and thrombocyte count <50 × 109; (3) using warfarin with INR >2.0, novel oral anticoagulants or heparin, or systemic thrombolysis for nonischemic stroke; (4) determining lobar, cortical, or cortico-subcortical hemorrhage among participants ≥55 years of age; and (5) determining deep or infratentorial ICH with pre-ICH hypertension or ECG evidence of left ventricular hypertrophy.
Data collection
Basic demographic, socioeconomic, lifestyle (including cigarette smoking and alcohol use), physical activity, dietary, psychosocial stress, and depression information was obtained by self-report using validated instruments. Blood samples for HbA1c and early morning samples after overnight fast in patients (postacute phase when fasting is feasible) and controls for measurement of fasting lipid panel (total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides [TG]) and fasting glucose were obtained following a uniform standard operating procedure across all study sites. We assessed stroke severity using either the NIH Stroke Scale (NIHSS) (categorized as severe if score >15) or the Stroke Levity Scale12 (categorized as severe if score is between 0 and 5, and not severe if score >5).
Definition of risk factors
Hypertension: an average of 3 blood pressure (BP) measurements was obtained at baseline and daily for 7 days or until death.13 Hypertension was defined using a cutoff of ≥140/90 mm Hg at 72 hours after stroke, a history of hypertension, or use of antihypertensive drugs before stroke or >72 hours after stroke. Many patients were unaware of premorbid hypertensive status or aphasic or unconscious and unable to provide information on history of hypertension. Hence diagnosis of hypertension relied on BP measurements taken after ICH and to account for elevations in poststroke BP in relation to premorbid BP, we made adjustments to systolic BP (SBP) by applying a correction factor of 0.8755 to SBPs measured in accordance with recommendations of the Oxford Vascular Study (OXVASC) in sensitivity analyses.14 In controls, hypertension was defined as self-reported history of hypertension or use of antihypertensive drugs or average BP at first clinical encounter ≥140/90 mm Hg.13
Diabetes mellitus (DM) was defined using a history of DM, use of medications for DM, an elevated HBA1c ≥6.5%, or fasting blood glucose (FBG) level ≥7.0 mmol/L for controls or FBG taken after 7 days for patients with stroke due to stress-induced transient rise in plasma glucose.15
Dyslipidemia was defined as a fasting TC ≥5.2 mmol/L, LDL-C ≥3.4 mmol/L, HDL-C ≤1.03 mmol/L, or TG ≥1.7 mmol/L in accordance with National Cholesterol Education Program guidelines16 or use of statin prior to stroke onset. The LDL/HDL ratio was categorized into the lowest 2 tertiles (≤1.97 and 1.98–2.95) as normal and compared with the highest tertile (≥2.96) based on distribution of the LDL/HDL ratio in our study.
Cardiac disease was defined on the basis of history, clinical examination, and ECG or echocardiographic evidence of atrial fibrillation, heart failure, ischemic heart disease, cardiomyopathy, rheumatic heart disease, or valvular heart diseases.
Obesity: participants with waist-to-hip ratio (WHR) of 0.90 for men and 0.85 for women or a body mass index ≥30 kg/m2 were classified as obese using WHO cutoffs.17
Individuals were classified as physically active if they were regularly involved in moderate exercise (walking or cycling) or strenuous exercise (jogging, football, vigorous swimming) for 4 hours or more per week.13
Dietary history: the regularity of consumption of food items such as leafy green vegetables, addition of salt at table, meat, fish, nuts, sugar, and other local staple food items were classified as daily, weekly, at least once monthly, and none in a month. Dose-response associations were assessed for leafy green vegetable consumption.
Alcohol use was categorized into current users (users of any form of alcoholic drinks) or never/former drinkers. We dichotomized alcohol intake as low (for men, 1–3 drinks per day; for women, 1–2 drinks per day) or high if consumption exceeded these sex-specific cutoffs.13
Smoking status was defined as current smoker (individuals who smoked any tobacco in the last 12 months) or former smoker (stopped for >12 months) or never smoked.
Psychosocial factors such as psychosocial stress and depression were assessed using validated instruments from the INTERSTROKE study.13 Combined measures of psychosocial stress at home or work such as anxiety, sleeping difficulties, or irritability and life events experienced in the 2 weeks preceding stroke onset were used to define presence of stress. The presence of depression was screened for using a combined checklist of depression symptoms experienced in the 4 weeks before stroke.
Family history of cardiovascular diseases (CVDs) was defined on the basis of self-reported history of any of hypertension, dyslipidemia, DM, stroke, cardiac disease, or obesity in the participant’s father, mother, sibling, or second-degree relative.
Data availability
M.O. had full access to the study data and had the final responsibility for the decision to submit this manuscript for publication. Datasets will be made available upon reasonable request to the principal investigator (M.O.).
Statistical analysis
Bivariate associations between risk factors and ICH (including anatomic subtypes: lobar and nonlobar) were assessed using the McNemar test for paired categorical outcomes. We used conditional logistic regression models to determine the adjusted associations among ICH, its subtypes (lobar vs nonlobar), and risk factors. Adjustments were made for potential confounders not used in the matching except baseline age, which was included due to the nonexact age matching.
ICH stroke severity was dichotomized as severe (using NIHSS score >15 or Stroke Levity Score <5) vs nonsevere and factors associated with severe stroke were assessed using multivariate logistic regression modeling. Here, we adjusted for demographic and vascular risk factors and selected neuroimaging features including location of bleed, tentorial location, and midline shift. In general, covariates were selected for inclusion in adjusted models after literature review, our clinical understanding of ICH risk, and empirical evidence on the basis of significant associations found in our initial bivariable analyses. The final adjusted models were assessed for collinearity using goodness of fit via residual analysis and variance inflation factor approaches. The odds ratio (OR) and 95% confidence intervals (CIs) in our models were estimated using conditional likelihood. The adjusted population-attributable risks (PARs) of ICH (and its subtypes) for each risk factor included in the best-fitted adjusted models were determined. We calculated the 95% CI for the PARs using the AF R-package18 and estimated the variance using the delta method. Composite PARs for the risk factors for ICH and its subtypes were calculated using the ATTRIBRISK R package with its 95% CI computed via the bootstrap method.19 Using this method, the addition of the PARs for individual risk factors included as covariates in the model usually exceeds 100%, but the composite or overall PAR of these risk factors is less than 100%. All statistical tests of hypotheses were 2-sided with a p value <0.05 considered significant. Statistical analyses and graphics were produced with SAS 9.4 and R statistical program (version 3.4.2).
Results
Clinical features and subtypes of ICH
Between August 2014 and June 2018, approximately 7,000 patients with clinically suspected stroke reported to the 15 study sites in Ghana and Nigeria, of whom 4,050 (58%) had head CT scans performed to assess eligibility for our study. A significant majority of participants were excluded after CT scan was done because they had had stroke symptoms lasting more than 10 days, making it difficult for phenotyping into ischemic or hemorrhagic types. We enrolled 854 patients with ICH out of 2,944 adjudicated stroke cases from Ghana and Nigeria for the present analysis. There was a preponderance of men (63.1%), with an overall mean age of 54.7 years (95% CI 53.8–55.7). Nearly 64% of participants presented with a focal motor neurologic deficit, followed by impaired speech (57.4%), headaches (57.3%), loss of consciousness (56.2%), and vomiting (35.5%) as common symptoms as shown in table 1.
Table 1.
Presenting symptomatology of intracerebral hemorrhage among West Africans

Nonlobar ICH constituted 77.0%, with the remainder being lobar. In decreasing order, SMASH-U subtypes of ICH were hypertension (80.9%), structural anomalies of blood vessels (4.0%), cerebral amyloid angiopathy (0.7%), systemic illnesses (0.5%), medication-related (0.4%), and undetermined in the remainder.
Neuroimaging features of ICH
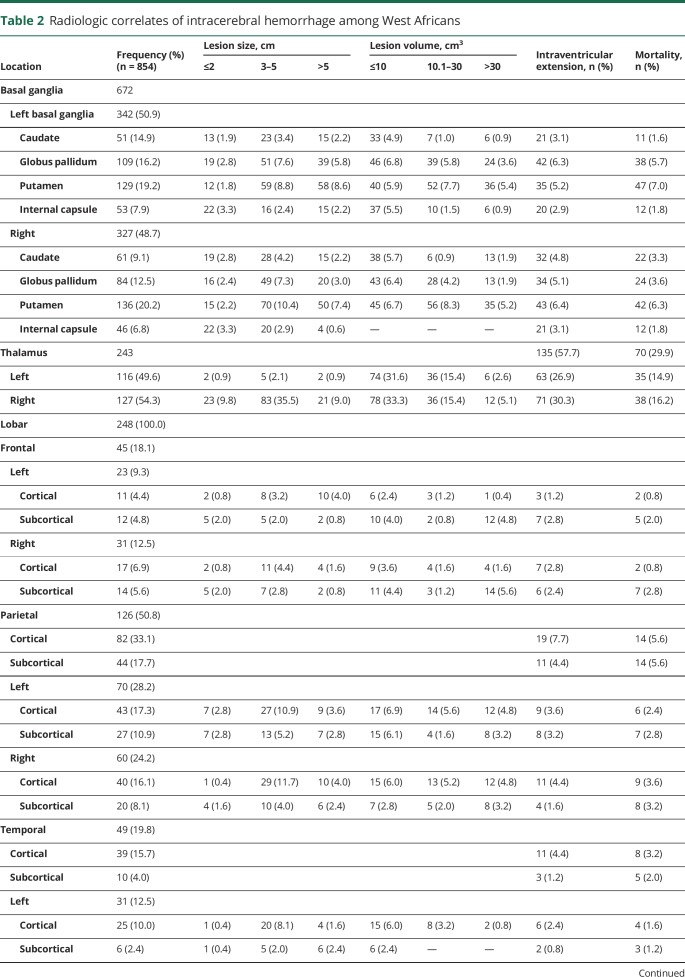
Out of 854 ICH cases, nonlobar bleeds occurred in decreasing order of anatomic location in the basal ganglia in 672 (78.7%), thalamus in 234 (27.4%), brainstem in 91 (10.7%), and cerebellum in 42 (4.9%), with some overlaps. The 248 lobar ICHs that occurred involved the parietal lobe in 126 (50.8%), temporal lobe in 49 (19.8), frontal lobe in 45 (18.1%), and occipital lobe in 28 (11.2%). Intraventricular extension of bleeds was commonly observed with thalamic bleeds in 57.7%, cerebellar bleeds in 16.5%, and pontine hemorrhage in 10.9%, with corresponding mortality rates of 29.9%, 15.4%, and 13.2%, respectively (table 2).
Table 2.
Radiologic correlates of intracerebral hemorrhage among West Africans
Characteristics of participants with ICH compared with controls
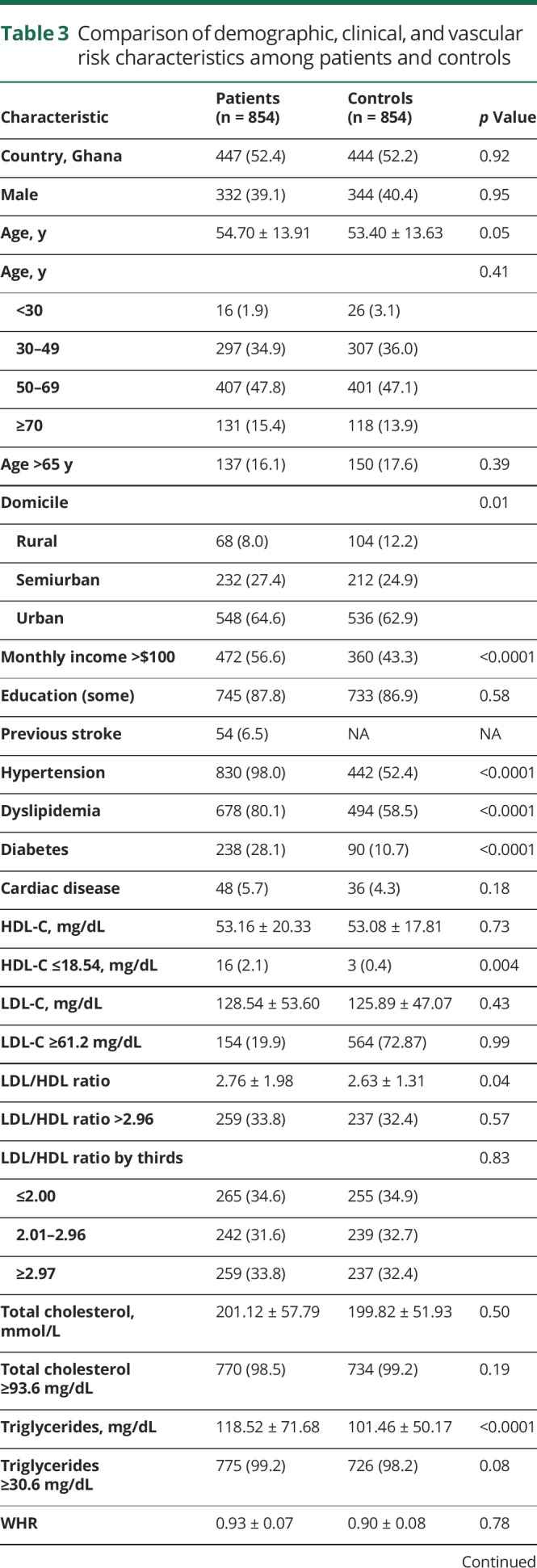
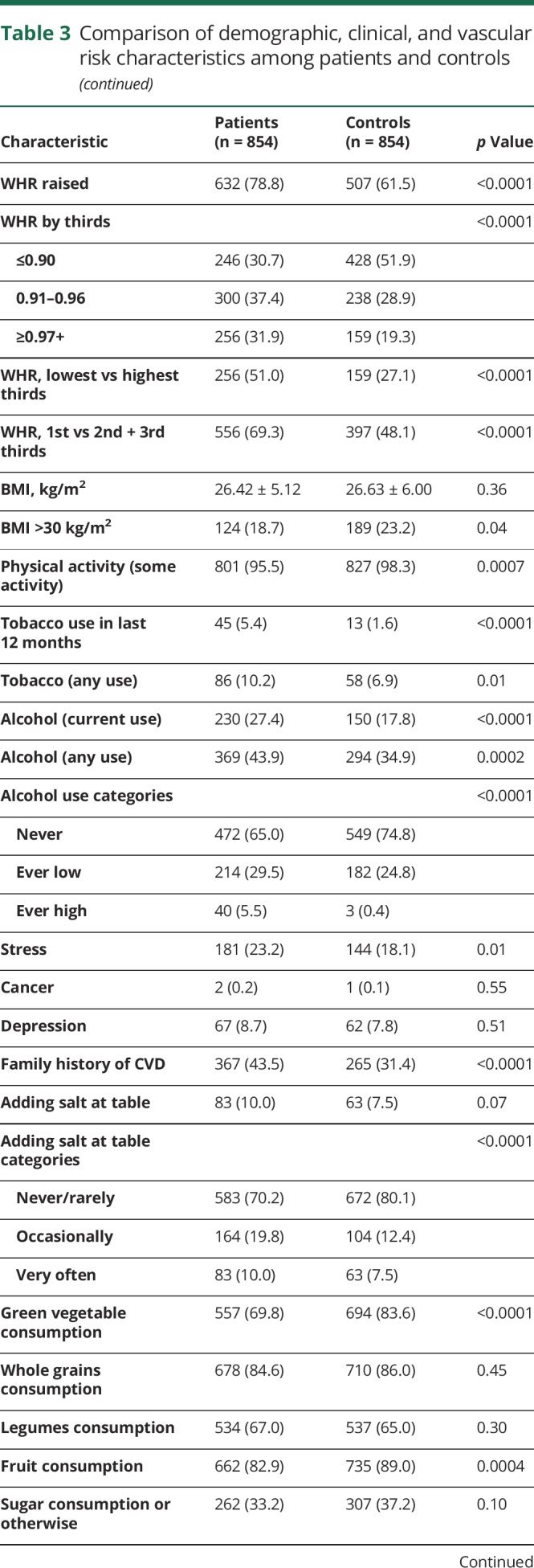
Comparing patients with ICH with controls, 8.0% vs 12.2% resided in rural locations (p = 0.01), and 56.6% vs 43.3% earned >$100 USD per month (p < 0.0001). Regarding cardiometabolic risk factors among cases compared with controls, respectively, 98.0% vs 52.4% had hypertension (p < 0.0001), 80.1% vs 58.5% had dyslipidemia (p < 0.0001), 28.1% vs 10.7% had DM (p < 0.0001), 78.8% vs 61.5% had increased WHR (p < 0.0001), 95.5% vs 98.3% had regular physical activity (p < 0.0001), 10.2% vs 6.9% had any use of tobacco (p < 0.0001), and 27.3% vs 17.8% currently used alcohol (p < 0.0001), as shown in table 3.
Table 3.
Comparison of demographic, clinical, and vascular risk characteristics among patients and controls

Risk factors for ICH and its subtypes
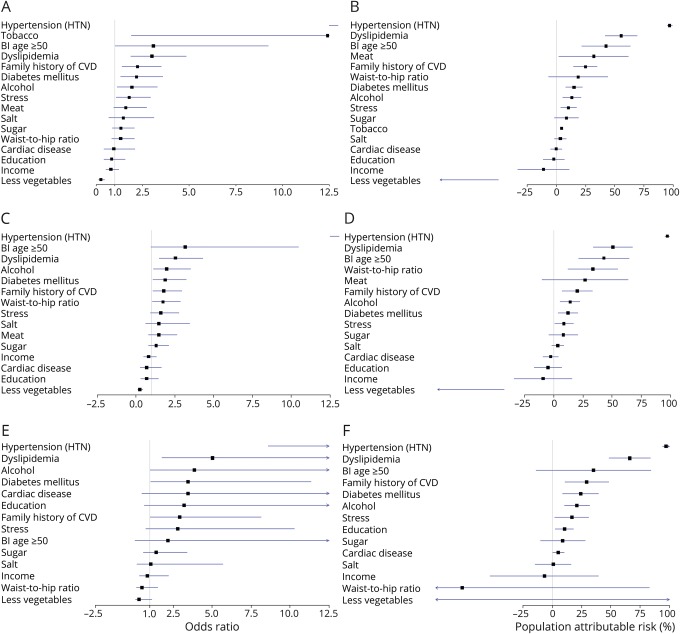
Eight factors independently associated with sICH occurrence by decreasing order of PAR with their adjusted OR (95% CI) were hypertension, 66.63 (20.78–213.72); dyslipidemia, 2.95 (1.84–4.74); meat consumption, 1.55 (1.01–2.38); family history of CVD, 2.22 (1.41–3.50); nonconsumption of leafy green vegetables, 3.61 (2.07–6.31); DM, 2.11 (1.29–3.46); stress, 1.68 (1.03–2.77); and current tobacco use, 14.27 (2.09–97.47) (table 4 and figure). These factors accounted for 90.7% (95% CI of 81.6–99.8) of PARs for ICH.
Table 4.
Risk factors of intracerebral hemorrhage (ICH) and its subtypes among West Africans from conditional multivariate logistic regression models showing adjusted odds ratio (aOR) and population attributable risk (PAR) (95% confidence interval [CI])
Figure. Forest plots show risk factors for intracerebral hemorrhage (ICH) among West Africans.
(A) Odds ratios (ORs) and 95% confidence intervals (CIs) of risk factors for ICH. (B) Population attributable risks (PARs) and their 95% CIs for ICH. (C, D) ORs and PARs for nonlobar ICH. (E, F) ORs and PARs for lobar ICH. Bl = baseline; CVD = cardiovascular disease.
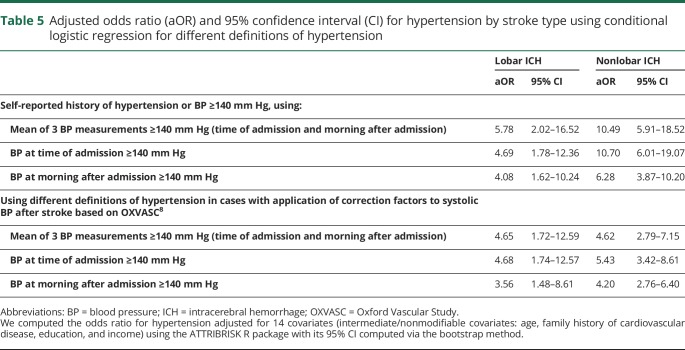
Both nonlobar and lobar ICH shared 3 common risk factors: hypertension, dyslipidemia, and nonconsumption of leafy green vegetables. In addition, current tobacco use and family history of CVD were independently associated with nonlobar ICH while DM was independently associated with lobar ICH. The effect sizes of hypertension as a risk factor for nonlobar compared with lobar ICH were adjusted ORs of 4.62 (2.79–7.15) and 4.65 (1.72–12.59), respectively, after application of a correction factor to the SBP at time of admission based on the OXVASC study14 (table 5).
Table 5.
Adjusted odds ratio (aOR) and 95% confidence interval (CI) for hypertension by stroke type using conditional logistic regression for different definitions of hypertension
Protective association between leafy green vegetable consumption and ICH risk
We observed a dose-response association between regularity of leafy green vegetable consumption and ICH risk. Daily consumption of vegetables had an adjusted OR (aOR) of 0.27 (0.20–0.36), and weekly consumption had an aOR of 0.67 (0.51–0.86), compared with monthly or no consumption. For lobar ICH, daily or weekly consumption had aORs of 0.47 (0.26–0.83) and 0.78 (0.45–1.34), respectively, while among those with nonlobar ICH, the adjusted odds were 0.22 (0.16–0.31) and 0.65 (0.48–0.87), respectively, compared with monthly or no consumption.
Factors associated with ICH severity
Factors independently associated with severe ICH as defined by an initial NIHSS cutoff of >15 were presenting SBP, nonconsumption of leafy green vegetables, lobar hemorrhages, supratentorial bleeds, and presence of a midline shift. These factors were similarly associated with stroke severity assessed using the Stroke Levity Scale (table 6). A total of 268 (268) out of 747 (35.9%) patients with adjudicated follow-up data died within 30 days of ictus.
Table 6.
Factors associated with intracerebral hemorrhage severity from multivariate logistic regression models

Discussion
Our study shows that ICH affected a relatively younger and productive segment of the population with an average age of 55 years. We have previously shown among Nigerians and Ghanaians that the average age at ICH onset was 52 years compared with 62 years among those with ischemic stroke.5 The Global Burden of Disease estimates, with representation from Africa, suggest that about 50% of ICH cases occurred among an age group younger than 65 years.20 In the present study, we found that nearly 75% of patients with ICH were younger than 65 years.
Risk factors of ICH
The 8 dominant factors associated with ICH among indigenous Ghanaians and Nigerians were hypertension, dyslipidemia, regular meat consumption, family history of CVD, nonconsumption of leafy green vegetables, DM, psychosocial stress, and current tobacco use. There is a general agreement on the spectrum of some of the factors identified in our study and those of the Global Burden of Disease21 and the INTERSTROKE studies,6 which included some representation of participants from Africa. These 8 factors compositely accounted for 90.7% of the PAR of ICH.
The overwhelming contribution of hypertension to occurrence of ICH in many LMIC regions worldwide has been amply reflected in the present study, with 98% of patients with ICH presenting with hypertension, with an adjusted PAR of 97% for ICH occurrence. The high aOR for hypertension observed in our main analysis may be largely attributable to the inclusion of antihypertensive use after ICH in our definition of hypertension. Ascertainment of premorbid hypertensive status of patients with ICH was challenged by lack of awareness, aphasia, or profound perturbations in level of consciousness and lack of electronic medical records at study centers. Sensitivity analyses revealed ORs between 3.6 and 10.7 using different definitions for hypertension without inclusion of antihypertensive use after ICH occurrence. Hypertension control in Ghana22 and Nigeria, as in many LMIC, is severely challenged by a myriad of adversely perpetuating systemic-, provider-, and personal-level factors, leading to an explosion of CVDs, of which ICH is a prototype.23 Indeed, among the control population, over 52% had hypertension.
Our study further highlights the importance of smoking and excessive alcohol intake, which have been posited as major contributors to the disproportionate rise in ICH burden on the LMIC.24,25 We have also identified an inverse dose-response association between vegetable consumption and ICH occurrence, which persisted in both lobar and nonlobar anatomic subtypes of ICH. This is probably the first such report, although it is similar to meta-analytic data that had a heavy tilt towards ischemic stroke.26 Regular vegetable consumption exerts a plenitude of beneficial effects on cardiovascular health, including favorable modulation of BP, dyslipidemia, obesity, inflammation, and oxidative stress.27
Risk factors for ICH subtypes
Three-quarters of patients with ICH in this study had nonlobar ICH, with the remainder having lobar ICH. Lobar and nonlobar ICH shared 3 modifiable risk factors: hypertension, dyslipidemia, and nonconsumption of leafy green vegetables. Together with associations with current tobacco use found in nonlobar ICH, these risk factors may mechanistically cause small vessel vasculopathy leading to ICH occurring predominantly in the basal ganglia and thalamus. Lobar hemorrhage commonly attributed to β-amyloid accumulation in the large cortical and leptomeningeal vessels was independently also associated with DM in the present study. However, the contribution of cerebral amyloid angiopathy to the occurrence of lobar ICH in our study was low, probably due to our younger population. Of interest, we found nonsignificant associations between family history of CVD and lobar ICH occurrence, aOR of 2.05 (0.66–6.38), but significant for nonlobar ICH 2.10 (1.24–3.57). First-degree history of ICH and APOE ε2 or ε4 genotypes have been associated with ICH occurrence in US cohorts.28 We could not obtain family history of ICH specifically from study participants, most of whom were not aware of this information.29
The high crude mortality rate of 36% is consistent with the generally poor outcomes associated with ICH in LMIC.1,2,20,30 The factors identified with severe ICH included known radiologic predictors such as midline shift, lobar, and supratentorial bleeds as well as high presenting SBP and nonconsumption of leafy green vegetables. The association between SBP and stroke severity is explained by the relationship between BP and hematoma volume expansion.31 However, our finding that nonconsumption of leafy green vegetables is associated with severe ICH is intriguing and has hitherto not been reported. It is possible that regular consumption of leafy green vegetables may interact favorably with other known risk factors of ICH to mitigate its severity. We have not conducted formal mediation and interaction analyses due to limited sample size.
Limitations and strengths
Case-control studies are limited in establishing causality but seek to establish associations and quantify effect sizes of risk factors such as done in the present study. Many participants with ICH were either unconscious or aphasic, hence proxy evaluation (by spouses or first-degree relatives) of medical history of vascular risk factors such as premorbid hypertension or DM, lifestyle, and behavioral history was undertaken, with associations observed in the same direction as those assessed directly (not shown). CT angiography or magnetic resonance angiography was performed only in cases of suspected vascular malformations or lobar bleeds of uncertain etiology according to international guidelines.11 A larger sample size will permit further characterization and establishment of dose-response relationships and interactions between the factors associated with sICH and its subtypes with greater precision.
We consider the deployment of active community engagement activities throughout the study duration as a major strength towards minimizing presentation bias for patients and controls, thus enhancing generalizability of our study findings. We acknowledge the possibility of patients not being referred to the hospital—for example, those with very mild stroke symptoms—in spite of our community engagement activities. We recruited 84% of study control participants from communities from which ICH cases emanated, which enhanced our efforts to fulfil the ideal recommendation of community-based controls. We performed 1:1 matching of cases to controls and used conditional logistic regression analysis to attain unbiased ORs.
Implications of our findings and future directions
Given the enormous and rising burden of stroke and poststroke mortality and morbidity in Africa,32–40 aggressive public health interventions aimed at improving the control of hypertension41,42 and other vascular risk factors at the population level are urgently needed to stem the rising tide of CVDs. A precise resolution of the contribution of genetic predispositions to ICH occurrence and gene-environment interactions is a priority to elucidate the reasons for the inordinately high burden of ICH among individuals of African ancestry. In support of this, a comparative tri-population study among study participants in SIREN and Reasons for Geographic and Racial Differences in Stroke (REGARDS) identified a gradient of increased ICH occurrence among indigenous Africans (27%), African Americans (8%), and European Americans (5.8%).43 This increased risk of ICH among Africans has also been observed in the South London Ethnicity and Stroke Study.44
The significant burden of ICH among West Africans is driven predominantly by hypertension and other modifiable risk factors. Modification of these factors, in particular hypertension at the population level, will significantly mitigate the burden of ICH.
Glossary
- aOR
adjusted odds ratio
- BP
blood pressure
- CI
confidence interval
- CVD
cardiovascular disease
- DM
diabetes mellitus
- FBG
fasting blood glucose
- HDL-C
high-density lipoprotein cholesterol
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- LDL-C
low-density lipoprotein cholesterol
- LMIC
low and middle-income countries
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- OXVASC
Oxford Vascular Study
- PAR
population-attributable risk
- QVSFS
Questionnaire for Verifying Stroke-Free Status
- SBP
systolic blood pressure
- sICH
spontaneous intracerebral hemorrhage
- SIREN
Stroke Investigative Research and Educational Networks
- SMASH-U
structural lesions such as aneurysms/arteriovenous malformations, medication-related, amyloid angiopathy, systemic/other disease, hypertension, and undetermined causes
- SSA
sub-Saharan Africa
- TC
total cholesterol
- TG
triglycerides
- WHR
waist-to-hip ratio
Appendix 1. Authors




Appendix 2. Coinvestigators



Footnotes
Editorial, page 417
Study funding
SIREN was funded by NIH grant U54 HG007479 under the H3Africa initiative and R01NS107900. Hugh Markus was supported by an NIHR Senior Investigator award. Mayowa Owolabi and Hugh Markus were supported by an Academy of Medical Sciences Global Challenges Research Fund Networking Grant (GCRFNGR2\10190).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Feigin VL, Lawes CM, Bennet DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischemic and hemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owolabi MO, Akarolo-Anthony S, Akinyemi R, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr 2015;26(2 suppl 1):S27–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarfo FS, Ovbiagele B, Gebregziabher M, et al. Stroke among young West Africans: evidence from the SIREN (Stroke Investigative Research and Educational Network) large multisite case-control study. Stroke 2018;49:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owolabi MO, Sarfo F, Akinyemi R, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health 2018;6:e436–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donnel MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 7.Akpalu A, Sarfo FS, Ovbiagele B, et al. Phenotyping stroke in sub-Saharan Africa: Stroke Investigative Research and Education Network (SIREN) phenomics protocol. Neuroepidemiology 2015;45:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarfo F, Gebregziabher M, Ovbiagele B, et al. Multilingual validation of the Questionnaire for Verifying Stroke-Free Status in West Africa. Stroke 2016;47:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 10.Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012;43:2592–2597. [DOI] [PubMed] [Google Scholar]
- 11.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 12.Owolabi MO, Platz T. Proposing the Stroke Levity Scale: a valid, reliable, simple, and time-saving measure of stroke severity. Eur J Neurol 2008;15:627–633. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell M, Xavier D, Diener C, et al. Rationale and design of INTERSTROKE: a global case-control study of risk factors for stroke. Neuroepidemiology 2010;35:36–44. [DOI] [PubMed] [Google Scholar]
- 14.Fischer U, Conney MT, Bull LM, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral hemorrhage versus major ischemic stroke: a population-based study. Lancet Neurol 2014;13:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (Adult Treatment Panel III). Circulation 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO Expert Consultation. 2008. Available at: who.int/nutrition/publications/obesity/WHO_report_waistcircumference_and_waisthip_ratio/en/. Accessed May 27, 2017. [Google Scholar]
- 18.Llorca J, Delgado- Rodriguez M. A comparison of several procedures to estimate the confidence interval for attributable risk in case-control studies. Stat Med 2000;19:1089–1099. [DOI] [PubMed] [Google Scholar]
- 19.Dahlqwist E, Sjölander A. AF: model based estimation of confounder-adjusted attributable fractions. Available at: cran.r-project.org/web/packages/AF/index.html. Accessed May 27, 2017.
- 20.Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 Study. Glob Heart 2014;9:101–106. [DOI] [PubMed] [Google Scholar]
- 21.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 22.Sarfo FS, Mobula LM, Burnham G, et al. Factors associated with uncontrolled blood pressure among Ghanaians: evidence from a multicenter hospital-based study. PLoS One 2018;13:e0193494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality and risk factors. Neurol Clin 2008;26:871–895. [DOI] [PubMed] [Google Scholar]
- 24.Jha P, Jacob B, Gajalakshmi V, et al. , for the RGI-CGHR Investigators. A nationally representative case-control study of smoking and death in India. N Engl J Med 2008;358:1137–1147. [DOI] [PubMed] [Google Scholar]
- 25.Zaridze D, Brennan P, Boreham J, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet 2009;373:2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke 2014;45:1613–1619. [DOI] [PubMed] [Google Scholar]
- 27.Woodside JV, Young IS, McKinley MC. Fruit and vegetable intake and risk of cardiovascular disease. Proc Nutr Soc 2013;72:399–406. [DOI] [PubMed] [Google Scholar]
- 28.Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012;79:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinyemi RO, Sarfo FS, Akinyemi J, et al. Knowledge, attitudes and practices of West Africans on genetic studies of stroke: evidence from the SIREN study. Int J Stroke 2019;14:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarfo FS, Awuah DO, Nkyi C, Akassi J, Opare-Sem OK, Ovbiagele B. Recent patterns and predictors of neurological mortality among hospitalized patients in central Ghana. J Neurol Sci 2016;363:217–224. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Luna D, Rodriguez-Villatoro N, Juega JM, et al. Prehospital systolic blood pressure is related to intracerebral volume on admission. Stroke 2018;49:204–206. [DOI] [PubMed] [Google Scholar]
- 32.Sarfo FS, Mobula LM, Sarfo-Kantanka O, et al. Estimated glomerular filtration rate predicts incident stroke among Ghanaians with diabetes and hypertension. J Neurol Sci 2018;396:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker RW, Jusabani A, Aris E, et al. Post-stroke case fatality within an incident population in rural Tanzania. J Neurol Neurosurg Psychiatry 2011;82:1001–1005. [DOI] [PubMed] [Google Scholar]
- 34.Danesi MA, Okubadejo NU, Ojini FI, Ojo OO. Incidence and 30-day case fatality rate of first-ever stroke in urban Nigeria: the prospective community based Epidemiology of Stroke in Lagos (EPISIL) phase II results. J Neurol Sci 2013;331:43–47. [DOI] [PubMed] [Google Scholar]
- 35.Gomes J, Damasceno A, Carrilho C, et al. Determinants of early case-fatality among stroke patients in Maputo, Mozambique and impact of in-hospital complications. Int J Stroke 2013;8(suppl 100):69–75. [DOI] [PubMed] [Google Scholar]
- 36.Sarfo FS, Akassi J, Kyem G, et al. Long-term outcomes of stroke in a Ghanaian outpatient clinic. J Stroke Cerebrovasc Dis 2018;27:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarfo FS, Jenkins C, Singh A, et al. Post-stroke depression in Ghana: characteristics and correlates. J Neurol Sci 2017;379:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfo FS, Akassi J, Adamu S, Obese V, Ovbiagele B. Burden and predictors of vascular cognitive impairment among long-term Ghanaian stroke survivors. J Stroke Cerebrovas Dis 2017;26:2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarfo FS, Nichols M, Qanungo S, et al. Stroke-related stigma among West Africans: patterns and predictors. J Neurol Sci 2017;375:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarfo FS, Berchie P, Singh A, et al. Prevalence, trajectory and predictors of poststroke fatigue among Ghanaians. J Stroke Cerebrovasc Dis 2019;28:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarfo F, Treiber F, Gebregziabher M, et al. PINGS (Phone-based Intervention under Nurse Guidance after Stroke): interim results of a pilot randomized controlled trial. Stroke 2018;49:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarfo FS, Treiber F, Gebregziabher M, et al. Phone-based intervention for blood pressure control among Ghanaian stroke survivors: a pilot randomized controlled trial. Int J Stroke 2018;14:630–638. [DOI] [PubMed] [Google Scholar]
- 43.Owolabi M, Sarfo F, Howard VJ, et al. Stroke in indigenous Africans, African Americans, and European Americans: interplay of racial and geographic factors. Stroke 2017;48:1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giosue G, Rutten-Jacobs LCA, Kalra L, Rudd AG, Wolfe CDA, Markus HS. Differences in the distribution of stroke subtypes in a UK black stroke population-final results from the South London Ethnicity and Stroke Study. BMC Med 2016;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
M.O. had full access to the study data and had the final responsibility for the decision to submit this manuscript for publication. Datasets will be made available upon reasonable request to the principal investigator (M.O.).