Abstract
The T lymphocyte, especially its capacity for antigen-directed cytotoxicity, has become a central focus for engaging the immune system in the fight against cancer. Basic science discoveries elucidating the molecular and cellular biology of the T cell have led to new strategies in this fight, including checkpoint blockade, adoptive cellular therapy and cancer vaccinology. This area of immunological research has been highly active for the past 50 years and is now enjoying unprecedented bench-to-bedside clinical success. Here, we provide a comprehensive historical and biological perspective regarding the advent and clinical implementation of cancer immunotherapeutics, with an emphasis on the fundamental importance of T lymphocyte regulation. We highlight clinical trials that demonstrate therapeutic efficacy and toxicities associated with each class of drug. Finally, we summarize emerging therapies and emphasize the yet to be elucidated questions and future promise within the field of cancer immunotherapy.
Subject terms: Immunology, Drug discovery, Cancer immunotherapy
T cells play a central role in immune responses to cancer. In this guide to cancer immunotherapy, the authors provide a comprehensive historical and biological perspective on cancer immunotherapy, with a focus on current and emerging therapeutic approaches that harness T cells to fight cancer.
Introduction
The idea to deploy the immune system as a tool to treat neoplastic disease originated in the nineteenth century1. Wilhelm Busch and Friedrich Fehleisen were the first to describe an epidemiological association between immune status and cancer. They noticed spontaneous regression of tumours following the development of erysipelas, a superficial skin infection most commonly caused by Streptococcus pyogenes1. Later, William Coley, often called the ‘Father of Cancer Immunotherapy’, retrospectively demonstrated that erysipelas was associated with a better outcome in patients with sarcoma2. With hopes of prospectively verifying his epidemiological evidence, Coley treated patients with cancer with extracts of heat-inactivated S. pyogenes and Serratia marcescens to boost immunity3. This extract, termed ‘Coley’s toxins’, possessed potent immunostimulatory properties and achieved favourable responses in various cancers2. However, lack of scientific rigour and reproducibility, in concert with the discovery of radiotherapy and chemotherapeutic agents, prevented treatment with ‘Coley’s toxins’ from becoming standard practice1.
The concept of cancer immunotherapy resurfaced in the twentieth century and made significant headway with the advent of new technology. In 1909, Paul Ehrlich hypothesized that the human body constantly generates neoplastic cells that are eradicated by the immune system3. Lewis Thomas and Sir Frank Macfarlane Burnet independently conceived the ‘cancer immunosurveillance’ hypothesis, stating that tumour-associated neoantigens are recognized and targeted by the immune system to prevent carcinogenesis in a manner similar to graft rejection1. Productive immune responses following tumoural adoptive transfer in mice4 and clinical reports of spontaneous regression of melanoma in patients with concomitant autoimmune disease5 provided additional evidence supporting this hypothesis, although a unifying mechanism was elusive. The advent of knockout mouse models provided the necessary technology to experimentally demonstrate a link between immunodeficiency and cancer6. Additional molecular and biochemical advances led to the identification of tumour-specific immune responses7. This provided unequivocal evidence that the immune system, in particular T cells (see Box 1 and Fig. 1), was capable of waging war on cancer tissue7. Cancer immunotherapy has now revolutionized the field of oncology by prolonging survival of patients with rapidly fatal cancers. The number of patients eligible for immune-based cancer treatments continues to skyrocket as these therapies position themselves as the first line for many cancer indications. Novel treatment combinations and newly identified druggable targets will only expand the role of immunotherapy in the treatment of cancer in the decades to come.
Fig. 1. Peripheral T cell fates after antigenic activation.
Resting T cells become activated after stimulation by cognate antigen in the context of an antigen-presenting cell and co-stimulatory signals. Activated T cells produce and consume proliferative/survival cytokines, for example, IL-2, IL-4 and IL-7, and begin to expand in number. If CD4+CD25+ regulatory T (Treg) cells are present, they can deprive the cycling T cells of proliferative/survival cytokines, especially IL-2, causing them to undergo apoptosis. Once cells are proliferating rapidly, they have different fates depending on their environment. If they receive acute strong antigenic stimulation, especially if it is encountered repeatedly, the cells will undergo restimulation-induced cell death. By contrast, if they receive chronic weak antigenic stimulation, the cells will survive but become reprogrammed into a specific unresponsive transcriptional state known as ‘T cell exhaustion’. Finally, as the antigen and cytokine stimulation diminishes as the immune response wanes, usually once the pathogen has been cleared, cytokine withdrawal can occur passively to contract the expanded population of antigen-specific T cells. A small fraction of cells will be reprogrammed to enter a ‘memory’ phenotype, and this differentiation step is facilitated by IL-7 and IL-15. Memory T cells will continue to persist in the immune system and form the basis of anamnestic responses. In these regulatory processes, T cell death usually takes the form of apoptosis.
In this Review, we emphasize the role of T cells in modern cancer immunotherapies and discuss three different categories of immunotherapeutic approaches to treat cancer: immune checkpoint blockade, an approach that is designed to ‘unleash’ powerful T cell responses; adoptive cellular therapies, which are based on the infusion of tumour-fighting immune cells into the body; and cancer vaccines, which can be designed to have either prophylactic or therapeutic activity. Finally, we introduce some of the emerging targets and approaches in cancer immunotherapy.
Box 1 T cell function, development, activation and fate.
The 1960s represented a period of enlightenment within the field of immunology because two major subtypes of lymphocytes, B lymphocytes and T lymphocytes, were characterized264,265. This was recognized by the 2019 Lasker Award for Basic Science, awarded for the pioneering work by Jacques A. F. P. Miller and Max Dale Cooper that defined the key roles of T cells and B cells in adaptive immunity. B cells recognize circulating antigen in its native form and respond by secreting protective antibodies266. By contrast, T cells recognize peptide antigens, derived from proteins degraded intracellularly, that are loaded onto cell surface MHC molecules, a process called antigen presentation. Two broad classes of T cells that have distinct effector mechanisms are delineated by the expression of either the CD4 or CD8 co-receptor: CD4+ T cells detect antigen in the context of MHC class II molecules and orchestrate the adaptive arm of the immune system by producing cytokines with chemotactic, pro-inflammatory and immunoprotective properties267. At least one CD4+ T cell subclass, CD4+CD25+ regulatory T cells, dampens the immune response following challenge268. CD8+ T cells detect antigen in the context of MHC class I molecules and carry out direct cytotoxic reactions that kill infected or neoplastic cells269.
A unique clone-specific cell surface protein complex, the T cell receptor (TCR), specifically recognizes antigens and participates in the developmental selection of T cells that can recognize pathogens but are self-tolerant270. The TCR complex comprises highly polymorphic single α- and β-glycoprotein chains (a small T cell population harbours γ- and δ-chains instead) that contain variable and constant regions, akin to immunoglobulins, and a group of non-polymorphic signalling chains, called CD3 γ, δ, ε and ζ. A vast repertoire of T cell clonotypes with unique specificities is generated through rearrangement of α- and β-chain gene segments within the genome of each T cell271. Following clonotype production, positive and negative thymic selection functions to entrain a ‘tolerant’ immune system, one that efficiently responds to pathogens or cancer cells but generally ignores or ‘tolerates’ self-tissues as non-immunogenic269,270.
Antigen stimulation of the TCR is necessary for T cell activation and proliferation, but an additional signal, termed co-stimulation, is required for phosphorylation events crucial for early signal transduction272. The non-polymorphic surface protein CD28 and its family members are the most potent co-stimulatory receptors on T cells, as elegantly demonstrated by the synergism of anti-CD28 stimulatory antibodies and TCR engagement on T cell activation and proliferation273,274. Additional evidence was provided by studies demonstrating the efficient inhibition of T cell activation and proliferation by inhibitory anti-CD28 antibodies275–278. The ligands for CD28, B7-1 and B7-2, are expressed on antigen-presenting cells and are upregulated when these cells encounter microorganisms that activate Toll-like receptors or other pathogen sensors279,280. Inhibitory molecules, including cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death 1 (PD1), are induced during immune responses and represent a ‘checkpoint’ to dampen T cell hyperactivation281 (see Fig. 2). The polymorphic TCR signals through a complex of three sets of dimeric CD3 chains, ε–δ, γ–δ and ζ– ζ282. The intracellular portions of the CD3 chains contain immunoreceptor tyrosine-based activation motifs that are phosphorylated by lymphocyte-specific protein kinase (LCK), a SRC family kinase283. At rest, the surface signalling protein CD45 exhibits phosphatase activity that blocks LCK function284. Following activation, CD45 removes an inhibitory phosphate on LCK, permitting phosphorylation of ζ chain-associated protein kinase 70 (ZAP70), a SYK kinase family member that binds to immunoreceptor tyrosine-based activation motifs in the CD3 ζ-chain and recruits the linker for activation of T cells (LAT) and phospholipase Cγ1 (PLCγ)285. With ample co-stimulation, downstream signalling affects calcium release, the activation of the GTPase RAS and transcriptional reprogramming essential for activated T cell function286.
Following activation, circulating naive T cells have three major fates in the periphery (Fig. 1). First, the effector T cell population can contract through apoptosis as the immune response resolves (cytokine withdrawal) or following repeated high-dose stimulation (restimulation-induced cell death)287–289. T cells can also exhibit an exhausted phenotype induced by repeated low-dose and low-affinity stimulation, as seen in chronic infections and neoplastic processes88. Lastly, a subset of these effector cells are involved in long-term immunological memory. Memory T cells are primed to react more vigorously to the same antigen during a subsequent encounter, making them critical mediators of immune recall responses to pathogens and tumours290. Leveraging the power of technological advances in molecular biology, recent single-cell RNA sequencing and epigenomic studies have provided additional molecular insight into T cell fates and the corresponding features of immunotherapy-responsive T cells. These studies collectively implicate that complex transcriptomic, epigenomic and clonotypic changes of tumour-infiltrating T cells determine the success of immunotherapy291–294.
Immune checkpoint therapy
Several evolutionarily conserved negative regulators of T cell activation act as ‘checkpoint molecules’ to fine-tune the immune response and regulate hyperactivation. Cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death 1 (PD1) are the most potent examples of T cell immune checkpoint molecules. They exert their biological effect at distinct body sites and times during the T cell lifespan8. Therefore, they complement each other functionally and ensure that T cell responses preserve self-tolerance while effectively protecting the body from pathogens and neoplasia. CTLA4 and PD1 have been successfully targeted by several pioneering research groups as treatments for a wide variety of recalcitrant cancers, research that ultimately earned James P. Allison and Tasuku Honjo the 2018 Nobel Prize in Physiology or Medicine.
CTLA4 biological function
After the discovery of T cell co-stimulation mediated by the surface protein CD28 (Box 1), the search for additional immune regulators led to the identification of CTLA4, a receptor with structural and biochemical similarities to CD28, as a new immunoglobulin superfamily member9,10. The CTLA4 and CD28 genes are found in the same region of chromosome 2 (2q33.2) and are selectively expressed in the haematopoietic compartment11. However, in contrast to the high levels of basal CD28 expression on conventional T cells, CTLA4 is expressed at a low basal level and is strongly induced following antigen activation. Interestingly, CD4+CD25+ regulatory T (Treg) cells, which have an immunosuppressive function, express CTLA4 constitutively. Structurally, both CTLA4 and CD28 form membrane-bound homodimers comprising an extracellular immunoglobulin-like domain, a transmembrane region and a cytoplasmic tail capable of recruiting signalling proteins and controlling surface expression10,12,13. The trafficking of CTLA4-containing vesicles to the cell surface after activation is controlled by a physical interaction with the lipopolysaccharide-responsive and beige-like anchor protein (LRBA)13. The sequence similarity between CTLA4 and CD28 is highest within their extracellular binding domain and they therefore bind to the same ligands, called B7-1 (also known as CD80) and B7-2 (also known as CD86), which are expressed by antigen-presenting cells (APCs; Box 1). However, CTLA4 has greater affinity and avidity than CD28 for B7 ligands, representing a key difference in their biology14–16.
With further characterization, it became clear that CD28 and CTLA4 had opposite immunoregulatory functions. For example, soluble CTLA4 was shown to inhibit the proliferation of T cells co-cultured with B7-expressing APCs because it interfered with the CD28–B7 interaction14. T cell receptor (TCR) signalling studies unequivocally demonstrated that CTLA4 inhibits T cell activation and proliferation12,17,18. The negative tolerogenic role of CTLA4 was also evident in vivo, because Ctla4-knockout mice developed a characteristic T cell-mediated lymphoproliferative autoimmune disease19. The absence of Ctla4 was sufficient to cause this phenotype, as treatment with an engineered soluble version of a CTLA4:Fc fusion protein (CTLA4Ig) and genetic crosses to B7-deficient mice ameliorated disease20,21. The autoimmune lymphoproliferative disorder caused by Ctla4 loss depends on the activity of CD28 because mutation of an LCK-binding carboxy-terminal proline motif in the intracellular tail of CD28 abrogates disease in mouse models22. Moreover, human patients with CTLA4 haploinsufficiency exhibit similar severe multiorgan lymphocytic infiltration and autoimmunity (CHAI disease) that can be treated with abatacept, an FDA-approved CTLA4Ig23,24.
CTLA4 restrains T cell activation through multiple mechanisms: by directly antagonizing CD28, by competing for co-stimulatory ligands, by preventing immune conjugate formation and by recruiting inhibitory effectors25 (Fig. 2). To directly oppose CD28 activity, intracellular vesicles release CTLA4 at the immunological synapse where it associates with the TCR26. In the context of the immunological synapse, CTLA4 can also reorganize the cytoskeleton and disturb T cell–APC immune conjugate formation27. CTLA4 also mediates the internalization of its ligands, thereby preventing their binding to CD28, which, in turn, reduces IL-2 secretion and T cell proliferation17,28,29. Lastly, phosphatases, including SH2 domain-containing tyrosine phosphatase 2 (SHP2) and protein phosphatase 2A (PP2A), are recruited and interact with the cytoplasmic tail of CTLA4, thereby contributing to its negative effect on T cell activation. SHP2 is an inhibitor of phosphorylation of the CD3 ζ-subunit of the TCR and also inhibits phosphorylation of the adaptor protein linker of activated T cells (LAT)30,31. PP2A is hypothesized to inhibit extracellular signal-regulated kinase (ERK), a kinase that acts as a signalling protein downstream of the TCR32. However, there is significant debate about which of the molecules that associate with the cytoplasmic tail of CTLA4 are most important for inhibiting T cell activity. Nevertheless, these inhibitory signals reduce the activation of transcription factors, such as activator protein 1 (AP-1), nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT), which reprogrammes T cells towards an anergic fate29,33.
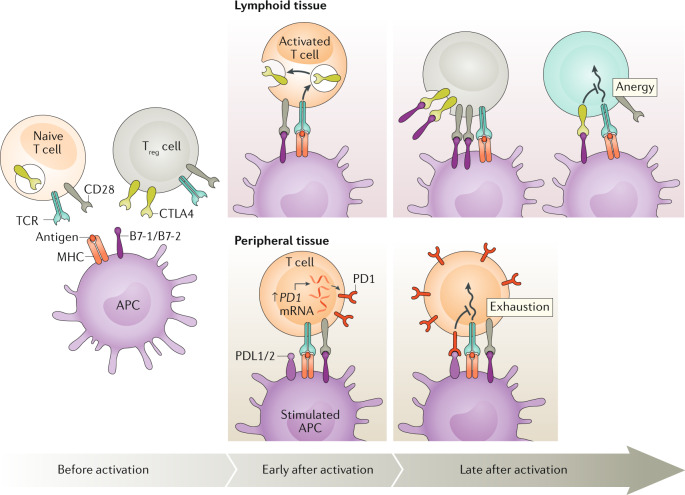
Fig. 2. Mechanisms of T cell activation and regulation.
Before activation, antigen-presenting cells (APCs) load antigen onto MHC molecules to prepare for contact with a T cell that displays a cognate T cell receptor (TCR) while also providing necessary co-stimulatory ligands B7-1 and B7-2. The inhibitory molecule cytotoxic T lymphocyte antigen 4 (CTLA4) is contained within intracellular vesicles in naive T cells, whereas it is constitutively expressed on the cell surface of CD4+CD25+ regulatory T (Treg) cells. Both classes of T cells express the co-stimulatory receptor CD28. Early after activation, generally in the lymphoid tissue, T cells are activated when their TCRs bind to their cognate antigen presented by APCs in conjunction with CD28 binding to B7-1/B7-2. Also, the activated T cells begin the process of displaying CTLA4 on the cell surface. T cells within peripheral tissues upregulate PD1 at the mRNA level early after activation. Late after activation, in lymphoid tissue, CTLA4 expressed by activated T cells binds to the B7-1 and B7-2 molecules on APCs, thereby preventing their binding to CD28 and promoting anergy by decreasing the T cell activation state. At the same time, constitutive expression of CTLA4 on Treg cells leads to trans-endocytosis of B7 ligands and interferes with the CD28 co-stimulatory ability of APCs. Late after activation in peripheral tissues, PD1 is further upregulated transcriptionally, leading to greater surface expression of programmed cell death 1 (PD1), which binds to its ligands PDL1 and PDL2, thereby promoting T cell exhaustion at sites of infection or when confronted with neoplasms. Image courtesy of the National Institute of Allergy and Infectious Diseases.
Beyond its function in activated conventional T cells, CTLA4 expression on Treg cells is essential for the direct and indirect immunosuppressive activity of these cells34,35. In vitro studies showed that CTLA4 was necessary for anti-inflammatory cytokine release by Treg cells, which reduces polyclonal activation and proliferation of conventional T cells nearby36,37. This result was confirmed in vivo by adoptive transfer of CTLA4-bearing Treg cells to prevent autoimmunity induced by CTLA4-deficient T cells that had been transferred to T cell- and B cell-deficient mice (Rag–/– mice)38,39. This treatment effect was nullified by antibody-mediated neutralization of CTLA4 (refs38,40,41). Thus, Treg cell-expressed CTLA4 can compensate for lack of CTLA4 expression by conventional T cells42,43. Beyond direct immunosuppression, Treg cells also prime dendritic cells to induce anergy of conventional T cells in a CTLA4-dependent fashion by binding to B7 ligands on APCs, followed by internalizing and degrading them, a process termed trans-endocytosis28,44.
CTLA4 blockade in cancer
The recognition of CTLA4 as a negative regulator of T cell activation gave rise to the idea that blocking its actions could unleash a therapeutic response of T cells against cancer45 (Fig. 3). James Allison and colleagues first tested this idea and demonstrated that neutralizing anti-CTLA4 antibodies enhanced antitumoural immunity in mice against transplanted and established colon carcinoma and fibrosarcoma46. In addition, during rechallenge, animals treated with anti-CTLA4 were able to rapidly eliminate tumour cells through immune mechanisms, providing evidence that blocking of CTLA4 induces long-lasting immunological memory46,47. Although CTLA4-targeted monotherapy was shown to confer benefit in animal models of brain48, ovarian49, bladder50, colon46, prostate47 and soft tissue46 cancers, less immunogenic cancers, including SM1 mammary carcinoma51 and B16 melanoma52, did not respond as favourably. Furthermore, heterogeneity between cancer models yielded discordant tissue-specific results45,53. In addition, a greater tumour burden correlated with reduced tumour responses to anti-CTLA4 treatment because larger tumours foster a more robust anti-inflammatory tumour microenvironment45,49.
Fig. 3. Effects of CTLA4-blocking antibodies.
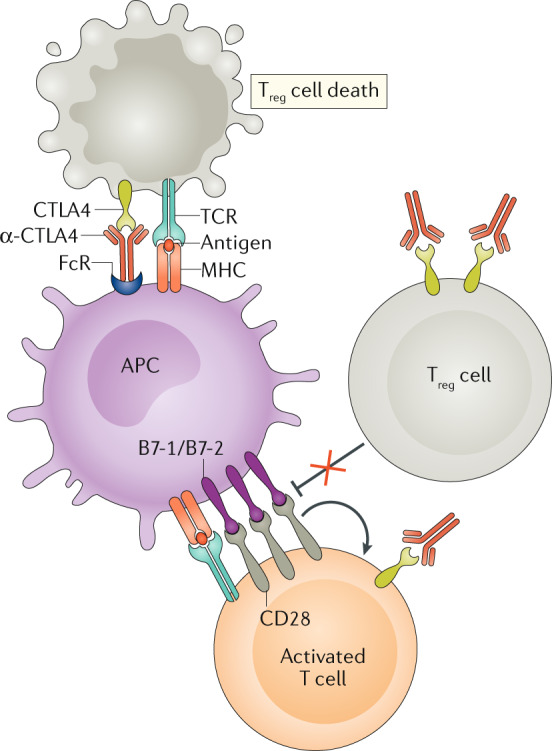
Cytotoxic T lymphocyte antigen 4 (CTLA4)-blocking antibodies (α-CTLA4), especially when bound to an Fc receptor (FcR) on an antigen-presenting cell (APC), can promote antibody-dependent cellular cytotoxicity (ADCC). CD4+CD25+ regulatory T (Treg) cells express higher amounts of CTLA4 than conventional T cells and are therefore more prone to α-CTLA4-induced ADCC than conventional T cells. In addition, α-CTLA4 can bind to CTLA4 on the surface of the Treg cell and prevent it from counter-regulating the CD28-mediated co-stimulatory pathways that are playing a role in T cell activation. At the same time, α-CTLA4 can also promote T cell responses by blocking CTLA4 on the surface of conventional T cells as they undergo activation. TCR, T cell receptor. Adapted from ©2019 Fritz, J. M. & Lenardo, M. J. Originally published in J. Exp. Med. 10.1084/jem.20182395 (ref.135).
Despite the mixed success in preclinical studies, mAbs targeting CTLA4 proved effective in clinical trials of melanoma45. Ipilimumab, a human IgG1κ anti-CTLA4 mAb, gained FDA approval in 2011 for non-resectable stage III/IV melanoma following evidence that it elicited potent tumour necrosis54 and conferred a 3.6-month short-term survival benefit55. Long-term survival data demonstrated that 22% of patients with advanced melanoma treated with ipilimumab benefited from an additional 3 years or more of life56. Additional long-term studies have demonstrated the durability of this survival benefit, indicating the persistence of antitumoural immunity following CTLA4 blockade56,57. Unfortunately, trial results in renal cell carcinoma58, non-small-cell lung cancer59, small-cell lung cancer60 and prostate cancer61 have yielded less impressive effects than those seen in patients with melanoma. Tremelimumab, an IgG2 isotype form of a CTLA4-blocking antibody, has yet to receive FDA approval as it did not increase survival in advanced melanoma62. It is hypothesized that effectiveness varies between ipilimumab and tremelimumab owing to differences in binding kinetics and the capacity to mediate antibody-dependent cell-mediated cytotoxicity63,64.
The mechanisms of CTLA4-mediated tumour regression are pleiotropic but unified by the action of one cell type, the T lymphocyte (Fig. 3). T cell responses are necessary for the therapeutic effects of CTLA4-targeted agents because T cell depletion in animal models abolishes tumoricidal activity65. Inhibition of CTLA4 enhances T cell clonal responses to tumour-associated neoantigens and a high neoantigen burden portends a favourable response to anti-CTLA4 therapy66,67. Apart from boosting effector T cell responses, anti-CTLA4 therapy depletes local intratumoural Treg cells through antibody-dependent cell-mediated cytotoxicity in mouse models and shifts the balance of the tumour microenvironment away from immunosuppression68,69. This phenomenon requires further study in human cancer as current data are inconclusive70,71. The relative role of effector T cells and Treg cells in conferring a clinical benefit has been contested, although specific blocking of CTLA4 in both cell populations can lead to synergistic increases in tumour regression69. Overall, current data suggest that the most critical factor in predicting outcome is the ratio of effector T cells to Treg cells infiltrating the tumour45,49.
PD1/PDL1 biological function
PD1 was first identified in 1992 as a putative mediator of apoptosis, although later evidence suggested a role in restraining immune system hyperactivation, analogous to CTLA4 (ref.72). As a type 1 transmembrane glycoprotein within the immunoglobulin superfamily, PD1 exhibits a 20% and 15% amino acid identity to CTLA4 and CD28, respectively73. Human PD1 is expressed on T cells after TCR stimulation and binds the B7 homologues PDL1 (also known as B7-H1) and PDL2 (also known as B7-DC), which are present constitutively on APCs and can be induced in non-haematopoietic tissues by pro-inflammatory cytokines74–76. In this review, we refer to PD1 and its ligands as the ‘PD1 axis’. The predominant role of the PD1 axis in the negative regulation of T cell activation became clear in 1999 when loss of the mouse PD1 orthologue, Pdcd1, was found to cause autoimmunity in vivo. C57BL/6 mice lacking functional PD1 protein developed splenomegaly77. Ageing of these animals led to mild T cell-mediated lupus-like glomerulonephritis and arthritis that was exacerbated by concurrent lpr mutations in the Fas gene78. Characterization of additional mouse strains showed that Pdcd1–/– mice of the BALB/c strain exhibited cardiac inflammation leading to dilated cardiomyopathy79. By comparison, non-obese diabeticPdcd1–/– mice had accelerated type 1 diabetes mellitus compared with their Pdcd1-sufficient counterparts80. The heterogeneous and late-onset autoimmune phenotypes of Pdcd1–/– mice were distinct from Ctla4–/– animals, demonstrating that the PD1 axis regulates T cell biology differently to CTLA4. Spatially, CTLA4 exerts its regulatory effect predominantly within lymphoid organs, whereas PD1 tends towards tempering T cell activation locally within peripheral tissues8. Temporally, PD1 acts later in the course of T cell activation and fate determination. Overall, the PD1 axis plays a unique role in maintaining T cell tolerance to self.
PD1 restrains immune responses primarily through inhibitory intracellular signalling in effector T cells and Treg cells81. The immunoreceptor tyrosine-based switch motif and the immunoreceptor tyrosine-based inhibitory motif of PD1 are phosphorylated and recruit the phosphatases SHP1 and SHP2, which dephosphorylate, and thereby inactivate, downstream effectors (that is, the CD3 ζ-subunit and ZAP70) that are important for early T cell activation76 and CD28 signalling82. Both CTLA4 and PD1 inhibit protein kinase B (PKB; also known as AKT) signalling to reduce glucose uptake and utilization, the former through PP2A and the latter by reducing phosphoinositide 3-kinase (PI3K) activity83. In contrast to CTLA4, the PD1 axis is essential for controlling the continued activation and proliferation of differentiated effectors; when PD1 engages its ligands, it can induce a state of T cell dysfunction called T cell exhaustion84–86. However, what determines whether PD1 mediates exhaustion or apoptosis in certain contexts is still an active area of research. One model suggests that the interaction between PI3K signalling and the mitochondrial B cell lymphoma-extra large (BCL-XL) protein is a critical control point at which PD1-mediated P13K inhibition reduces BCL-XL and promotes apoptosis25,83. Beyond regulating conventional T cells, PDL1 on APCs can control Treg cell differentiation and suppressive activity87. Unfortunately, tumour cells can exploit this mechanism by upregulating PD1 ligands to induce T cell exhaustion and generate a tumour microenvironment that facilitates tumour growth and invasion88.
PD1/PDL1 blockade in cancer
Once the PD1 axis was implicated in the negative regulation of T cells, preclinical work examined whether inhibitors of this pathway could be used for cancer treatment and biomarker discovery. First, overexpression of PDL1 or PDL2 in cancer cell lines was found to constrain the CD8+ T cell cytotoxic antitumour response, whereas tumours were rejected in mice without functional PD1 (refs89,90). Second, blockade of PD1 suppressed the growth of transplanted myeloma cells in syngeneic animals90. Conversely, transplanted cells overexpressing PDL1 or PDL2 in syngeneic mice allowed for increased tumour colonization, burden and invasiveness90. Neutralizing the PD1 axis using mAbs89,91 or secreted PD1 extracellular domains92 reversed these effects and enhanced T cell cytotoxicity towards tumour cells90 (Fig. 4). Rescuing CD8+ T cell cytotoxicity by PD1 blockade depends on the expression of CD28 as PD1-mediated immunomodulation is lost in the context of CTLA4Ig, B7 blockade or CD28 conditional-knockout mice92. In addition, reinvigorated T cells in the peripheral blood of patients with lung cancer following PD1 blockade were shown to express CD28 (ref.93). PD1 inhibition not only augments antitumoural immunity but also limits haematogenous seeding of B16 melanoma and CT26 colon carcinoma metastases in mouse models94. Thus, PD1/PDL1 blockade can both enhance tumour cytolysis and limit metastasis. Apart from a role of PD1 and its ligands in cancer treatment, multiple studies have also shown a negative correlation between human tumour expression of proteins involved in the PD1 axis and prognosis, indicating the utility of these proteins as potential biomarkers95–97.
Fig. 4. Mechanisms of PD1 axis inhibition.
Activated T cells express programmed cell death 1 (PD1), which engages with its specific ligand (PDL1 or PDL2) to dampen activation. Blocking of the PD1 axis through the administration of an anti-PD1 (or anti-PDL1 or anti-PDL2) antibody prevents this inhibitory interaction and unleashes antitumoural T lymphocyte activity by promoting increased T cell activation and proliferation, by enhancing their effector functions and by supporting the formation of memory cells. Consequently, more T cells bind to tumour antigens presented on tumour cells by MHC molecules via their T cell receptors (TCRs). This ultimately leads to the release of cytolytic mediators, such as perforin and granzyme, causing enhanced tumour killing. APC, antigen-presenting cell. Adapted from ©2019 Fritz, J. M. & Lenardo, M. J. Originally published in J. Exp. Med. 10.1084/jem.20182395 (ref.135).
Following preclinical success, mAbs designed to counteract negative immunoregulation by the PD1 axis were developed and efficacy was shown in clinical trials98. Development was initiated by Medarex (ultimately purchased by Bristol-Myers Squibb) in 2001 (ref.99). In 2010, a phase I trial demonstrated that PD1 blockade was well tolerated and could promote antitumoural responses100. In 2014, the humanized and fully human anti-PD1 mAbs pembrolizumab and nivolumab (both IgG4) became the first FDA-approved PD1-targeted therapeutics for refractory and unresectable melanoma101–104. In a head-to-head comparison, pembrolizumab showed better 6-month progression-free survival than ipilimumab and conferred an overall survival benefit105,106. Clinical trials of nivolumab demonstrated an overall survival of 72.9% at 1 year compared with 42.1% survival in the group of patients treated with the chemotherapeutic dacarbazine104. In 2015, pembrolizumab was approved for the treatment of PDL1-expressing non-small-cell lung carcinoma because it provided a 4.3-month increase in progression-free survival compared with platinum-based chemotherapeutics and was more effective than the chemotherapeutic paclitaxel107,108. Increased PDL1 expression on the target tumour was associated with improved responses to PD1 axis blockade109. Additional successful clinical trials expanded the use of pembrolizumab to head and neck squamous cell carcinoma110, Hodgkin lymphoma111, urothelial carcinoma112, gastric/gastro-oesophageal junction cancer113 and tissue-agnostic carcinoma with a high degree of microsatellite instability114. Following approval in tissue-agnostic cancers with microsatellite instability, pembrolizumab became the first drug to be approved based on a molecular biomarker rather than by cancer site. However, the immunosuppressive microenvironment of different tissues makes it hard to predict which patients will benefit115,116. Similar to prembrolizumab, the use of nivolumab has since been extended to renal cell carcinoma117, head and neck squamous cell carcinoma118, urothelial carcinoma119, hepatocellular carcinoma120, Hodgkin lymphoma121 and colorectal cancer with a high degree of microsatellite instability122. As was seen with anti-CTLA4 therapy, long-term survival analyses demonstrate a long-lasting immune-mediated survival benefit following PD1 blockade123. However, the reason why PD1 blockade has demonstrated broader clinical utility than anti-CTLA4 treatment has remained elusive. It is hypothesized that the difference may be because the PD1 axis is frequently co-opted by tumours via ligand expression, whereas CTLA4 represents a broader immunoregulatory circuit74,124.
PDL1 is also targetable by specific antibodies that have proven effective treatments in multiple forms of cancer. In 2016, the first PDL1-targeted humanized mAb, atezolizumab (an IgG4 antibody), was approved for treatment of urothelial carcinoma. An overall response rate of 15% was deemed statistically significant based on historical control data, although responses were dependent on tumour PDL1 expression status125. Unfortunately, additional trial data have not demonstrated that atezolizumab has clinical efficacy beyond the standard of care in urothelial carcinoma, although it is less toxic than traditional chemotherapy126. Indications have since expanded to include the treatment of non-small-cell lung carcinoma127, triple-negative breast cancer128 and small-cell lung cancer129. Additional anti-PDL1 human mAbs, avelumab and durvalumab, entered the market in 2017 (ref.98). Avelumab is used for the treatment of Merkel cell carcinoma130, urothelial carcinoma131 and advanced renal cell carcinoma132. Duvalumab is used for urothelial carcinoma133 and non-small-cell lung cancer134. Therefore, similar to PD1, blockade of PDL1 has been effective in difficult-to-treat forms of cancer.
Adverse effects of checkpoint blockade
Blocking a naturally occurring central immune checkpoint unleashes powerful immune effector mechanisms that may not respect the normal boundaries of immune tolerance to self-tissues135. Ctla4- and Pdcd1-knockout mice provided a glimpse into the spectrum of autoimmune responses that occur in humans during immune checkpoint blockade therapy19,77–79. Human loss-of-function mutations in CTLA4 and its interacting regulatory protein, LRBA, also mirror the immune-related side effects observed with anti-CTLA4 therapy13,24. On the basis of a meta-analysis of trial data sets, immune-related adverse events are estimated to occur in 15–90% of patients55. More severe events requiring intervention are observed in 30% and 15% of patients treated with CTLA4 and PD1 axis inhibitors, respectively136. The common immune feature of toxicity is the loss of naive T cells and the accumulation of overactive memory T cells that invade peripheral organs, such as the gastrointestinal tract and lungs, and cause inflammatory damage. Keratinized and non-keratinized mucosa appear to be the most susceptible, as approximately 68% and 40% of treated patients exhibit pruritis and mucositis, respectively137,138. Anti-CTLA4 therapy carries an increased risk of severe autoimmune complications compared with therapies targeting the PD1 axis, as was observed in knockout mice and in clinical studies19,77–80,139. In addition, data from dose-escalation trials support the claim that anti-CTLA4 agents elicit dose-dependent responses not seen with therapies targeted at the PD1 axis107,139. Toxicities affecting the gastrointestinal tract and brain are more common with anti-CTLA4 therapy, whereas patients treated with PD1 axis-targeted therapies are at higher risk of hypothyroidism, hepatoxicity and pneumonitis137. However, as the number of indications treated with checkpoint blockade increases and more patients are treated, rarer side effects in a wider spectrum of organs and heterogeneous responses have manifested137. For example, hyperprogression of disease has been observed in a minority of patients with various tumour types treated with PD1 inhibitors140–142. Most recently, it was shown that the PD1 inhibitor nivolumab can lead to the rapid progression of disease in patients with adult T cell leukaemia/lymphoma, providing evidence for a role of tumour-resident Treg cells in the pathogenesis of this lymphoma143. Multiple immune-related response criteria have been developed to better categorize patient responses to checkpoint blockade. In addition, these criteria aim to distinguish progression from pseudoprogression, a phenomenon in which patients treated with CTLA4 or PD1 inhibitors experience a period of progression followed by rapid tumour clearance144,145. Overall, checkpoint blockade leads to autoimmune toxicities with a therapy-specific pattern of organ involvement, as predicted by the phenotypes of animals genetically deficient for checkpoint molecules.
Interestingly, preclinical immune checkpoint therapy studies did not demonstrate major adverse effects in vivo and, thus, were not great predictors of human toxicities146. This is thought to be due to the short time frame of these studies and the inbred nature of mouse strains146. Recently developed humanized mouse models represent a platform that better recapitulates side effects due to checkpoint therapy146,147. Nevertheless, toxicity associated with immune checkpoint blockade is tolerated better than the toxicities associated with traditional chemotherapeutics, making these therapies attractive for quality of life reasons beyond their survival benefit98,148.
Recent research has aimed to improve the side-effect profiles and clinical response of immune checkpoint blockade through the modification of existing antibodies and the engineering of novel delivery methods. It was recently shown that abnormal CTLA4 recycling and subsequent lysosomal degradation was a mechanism that contributes to toxicities and reduced drug effectiveness. Modified pH-sensitive antibodies that do not interfere with LRBA-mediated CTLA4 recycling were shown to limit adverse events and improve clinical outcomes in established tumours in mouse models, which may ultimately broaden clinical utility149,150. Additional research has focused on developing biomaterials for the localized administration of checkpoint inhibitors151. For example, compared with systemic delivery, transdermal patch delivery of anti-PD1 antibodies was better tolerated and unleashed a more robust antitumoural response in a mouse model of melanoma151. A broad field of research is currently aimed at discovering novel methods to reduce toxicities associated with checkpoint therapy and to increase clinical benefit in a greater variety of tumours.
Clinical management of drug-related toxicities is the same for all checkpoint drugs, and toxicities are graded according to the 2009 National Cancer Institute Common Terminology Criteria for Adverse Events severity scale137,152. Mild (grade 1) toxicities are not typically treated. In the setting of grade 2 or 3 adverse events, checkpoint inhibitors are discontinued until symptoms and laboratory-value abnormalities resolve. Glucocorticoids are also used to effectively control immune hyperactivity. Infliximab and other immunosuppressive agents can be used when glucocorticoids fail. Life-threatening (grade 4) toxicities necessitate the complete discontinuation of therapy and the use of life-saving measures, as required. Active monitoring of symptoms and laboratory parameters is recommended in order to prevent death due to checkpoint blockade (grade 5).
Current research is aimed at identifying predictive biomarkers for organ-specific toxicities due to checkpoint therapy. For example, neutrophil activation, as measured by increased expression of the biliary glycoprotein CEACAM1 and the cell surface glycoprotein CD177, correlates with gastrointestinal-related side effects in patients treated with ipilimumab153. Increases in eosinophil counts and release of the pro-inflammatory cytokine IL-17 are associated with toxicity regardless of the organ affected154,155. Pharmacogenomic profiling (using genetic information to predict responses to drugs) may provide more insight into the relevant genes and pathways mediating toxicity137. Ultimately, the hope is that genetic, biochemical or metabolic profiling could either pre-screen or rapidly detect individuals likely to experience the most severe adverse reactions to checkpoint therapy.
Adoptive T cell transfer therapy
Adoptive T cell (ATC) therapy, in which autologous or allogenic T cells are infused into patients with cancer, has shown considerable promise in recent years. The viability of this type of therapy was first shown by Southam et al. in 1966, when half of the patients with advanced cancer demonstrated tumour regression following co-transplantation with patient-derived leukocytes and autologous tumour cells156. Allogenic haematopoietic stem cell transplants for leukaemia represented the first effective adoptive transfer approach deployed clinically, and clinical improvement was shown to be mediated by a T cell graft versus tumour response157.
ATC with tumour-infiltrating lymphocytes
ATC therapy using tumour-infiltrating lymphocytes (TILs) for the treatment of metastatic melanoma was pioneered at the National Cancer Institute in the late 1980s158. Lymphocytes isolated from a cancer biopsy were greatly expanded with IL-2 and then reinfused intravenously into the same patient with a large bolus of IL-2. The objective response rate was 34%; however, the median duration of response was only 4 months and few patients experienced a complete response159. Later studies incorporating lymphodepletion before ATC therapy in 93 patients with metastatic melanoma were more successful, with complete tumour regression in 20 (22%) patients, 19 of whom were still in complete remission 3 years after treatment160. The screening and enriching for neoantigen-specific TILs, made possible by high-throughput technologies, recently demonstrated promise in a patient with metastatic breast cancer161. In addition, knockdown of the gene encoding cytokine-inducible SH2-containing protein (Cish), a negative regulator of TCR signalling, was shown to boost the antitumoural response of ATC therapy in mouse models162. However, in order for TIL-based ATC therapy to elicit durable responses (Fig. 5), effector T cells with antitumour activity must be present in the tumour, which is not the case for many cancer types163. Other innovative approaches to tweak T cell activity and proliferation may allow for a greater palette of treatments to be developed.
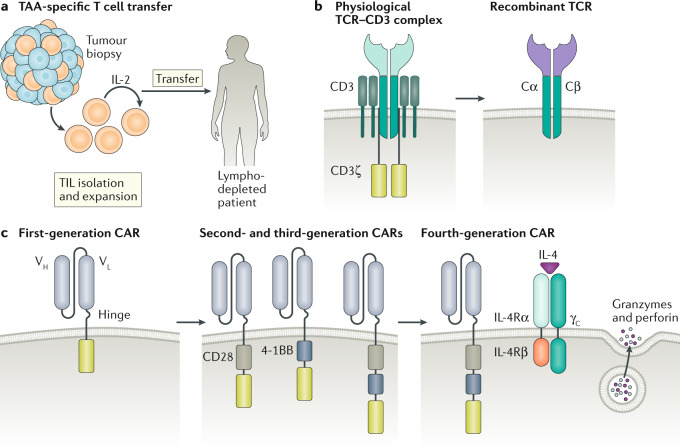
Fig. 5. Adoptive T cell therapy.
a | Tumour-infiltrating lymphocytes (TILs) are isolated from a patient tumour biopsy and expanded ex vivo with IL-2. TILs are then infused into a patient who has undergone lymphodepletion to provide a niche for the transferred TILs to expand, act as effector cells and generate immunological memory. As the T cells were derived from the tumour, it is assumed that a good proportion can recognize tumour-associated antigens (TAAs) or neoantigens. b | The physiological T cell receptor (TCR) complex gains its specificity from polymorphic α- and β-glycoprotein chains that have an antigen-binding portion and a conserved domain that associate with and signal through a group of non-polymorphic proteins, CD3 γ, δ, ε and ζ. Bioengineering of the TCR α- and β-glycoprotein antigen-binding domain (purple), while preserving the conserved domains (Cα and Cβ), allows for the development and expansion of T lymphocytes with specificity to tumour neoantigens. c | Originally, chimeric antigen receptors (CARs) were composed of an extracellular single-chain fragment of an antibody variable region coupled to a CD3 ζ-signalling domain. Poor expansion and functionality of these first-generation CARs led to the development of second and third-generation CARs containing intracellular modules from co-stimulatory molecules (CD28 and/or 4-1BB) that provide additional signals necessary to fully activate the T cell. Subsequent generations of CAR T cells contain further modifications to improve antitumour efficacy. For example, fourth-generation ‘armoured’ CAR T cells have been engineered to secrete pro-inflammatory cytokines, such as IL-12, to overcome immunosuppression in the tumour microenvironment. The chimeric cytokine receptor 4αβ, comprising the ectodomain of IL-4Rα fused to the IL-2/IL-15Rβ chain, signals in response to IL-4, an abundant cytokine in numerous tumour types. VH, variable heavy chain; VL, variable light chain.
Engineered lymphocytes for ATC
The challenges associated with expanding tumour-specific T cells in vitro led to the development of TCR-engineered lymphocytes (Fig. 5). However, these cells are limited to responding to tumour antigens presented by the MHC (also known as human leukocyte antigen (HLA) in humans) rather than surface antigens on tumour cells163. However, synthetic chimeric antigen receptors (CARs) can bypass MHC restriction and direct specific cytotoxicity to a target molecule on the surface of the malignant cell. Isolated T cells from the patient (or allogeneic donor) are genetically modified to express CARs and then expanded and infused into the patient. This overcomes the problem that tumour cells often downregulate MHC molecules, which leaves the cell unable to present antigen to conventional T cells164. CARs comprise an antigen-binding domain, most often from the variable regions of antibodies, linked to signalling domains of the TCR and various co-stimulatory molecules (Fig. 5). Given the domain modularity of cell surface signalling proteins, mixes and matches of extracellular targeting domains and internal signal transduction domains can be assembled using protein engineering. This offers many options to tailor CARs to specific tumours. The first generation of CAR T cells relied only on the CD3 ζ-chain to simulate TCR signalling165, but this design was ineffective in clinical trials owing to limited T cell proliferation and cytokine production166,167. Subsequent generations of CAR T cells have been engineered to include domains from CD28, CD40 ligand and other positive regulators of T cell activation to potentiate activation and cytotoxicity in vivo168–171. An engineered single-chain PD1 blocker has also demonstrated similar enhanced efficacy to second-generation CAR T cells with solely a CD28 domain172. Even though CAR T cells are typically engineered using retroviral transduction, recent work has used CRISPR–Cas9 technology. CRISPR–Cas9 can be used to edit the TCR germline sequence directly, which could lead to more uniform CAR T cell generation and, ultimately, better efficacy173.
A limitation to the development of CAR T cell therapies is the requirement for a distinct tissue-restricted target antigen on the tumour cell surface. For example, CAR T cells designed with specificity for the cell surface molecule CD19, which is expressed by all B cells, have been successful in the treatment of B cell malignancies. The first clinical deployment of second-generation CD19-specific CAR T cells led to durable responses in chronic lymphocytic leukaemia174. Additional clinical trials of CD19-specific second-generation CAR T cells in B cell acute lymphoblastic leukaemia (B-ALL) led to remission in all patients with B-ALL who were tested175. A follow-up report on patients with B-ALL enrolled in this clinical trial showed complete remission of disease in 44 of 53 (83%) patients with a median follow-up of 29 months176. Similar successes were reported for patients with diffuse large B cell lymphoma177, leading to FDA approval for these B cell malignancies in 2017.
The clinical success of CAR T cell therapy for the treatment of B-ALL and diffuse large B cell lymphoma is due, in part, to targeting the CD19 antigen, an ideal candidate owing to its high expression in certain B cell malignancies and specificity to the B cell lineage. Crossover targeting of normal CD19+ B cells does not hamper therapy or cause severe side effects. However, even as an ideal target, CD19 antigen loss is a common cause of treatment failure. CD22 is another antigen commonly expressed by malignant cells in B-ALL and has shown promise as a target for CAR T cell therapy in a phase I trial178. Other targets, especially tumour neoantigens, are currently being investigated for haematological malignancies that do not express CD19, as well as for solid tumours179,180. B cell maturation antigen (BCMA)-targeted CAR T cell therapy is poised for FDA approval for multiple myeloma in 2020 on the basis of promising preclinical and clinical data181,182. However, owing to reported patient relapses, the investigation of additional target antigens continues. A preclinical study recently identified another target antigen, GPRC5D, with comparable efficacy and toxicity to BCMA-targeted CAR T cell therapy183. Thus far, CAR T cell therapy has only been modestly successful for solid tumours184–186 and innovative approaches to improve therapy are underway179. A recently identified pan-cancer target, B7-H3 (also known as CD276), has demonstrated success in multiple paediatric solid tumour models187. In addition to directly acting as cytolytic agents, CAR T cells can also target the unhospitable tumour microenvironment and revive exhausted T cells188,189. For example, a new generation of ‘armoured’ CAR T cells engineered to produce IL-12 can overcome immunosuppression by Treg cells and myeloid cells in the tumour environment, promote CD8+ T cell cytolytic activity190 and enhance myeloid cell recruitment and antigen presentation191,192. Preclinical models using IL-12-expressing CAR T cells that target the conserved extracellular domain of mucin 16 (MUC16ecto) have shown promising results in models of ovarian cancer, a tumour with poor prognosis in advanced stages193,194. A phase I clinical trial is currently in progress for patients with ovarian, fallopian or primary peritoneal cancer195. The efficacy of CAR T cells may also be strengthened through co-expression of a chimeric cytokine receptor (4αβ) that stimulates proliferation in response to IL-4, a cytokine that is usually abundant in the tumour microenvironment. Preliminary studies have shown that this approach works for CAR T cells directed against different tumour-associated antigens (TAAs)196 and clinical trials are underway in head and neck cancer197. In addition, overexpression of the transcription factor JUN was shown to confer resistance to CAR T cell exhaustion198. Overall, CAR T cells have been successful for the treatment of B cell malignancies and it will be exciting to continue research on this new treatment modality for intractable types of cancer.
Limitations and adverse effects of ATCs
Toxicities can arise from CAR T cell therapy and affect many different organ systems with a range of severity199. Patients most commonly experience cytokine release syndrome (CRS) and neurotoxicity200. CRS results from the powerful activation and proliferation of CAR T cells in vivo and typically appears quickly after cell transfer. The symptoms are often mild and flu-like but can also be severe and life-threatening, involving hypotension, high fever, capillary leakage, coagulopathy and multisystem organ failure. Serious neurological events can also occur, such as CAR T cell-related encephalopathy syndrome, typically characterized by confusion and delirium, but sometimes also associated with seizures and cerebral oedema199. Glucocorticoids are the first-line treatment for milder forms of CRS and CAR T cell-related encephalopathy syndrome. Tocilizumab, a humanized anti-IL-6 antibody, is a highly effective second-line treatment for CRS caused by CAR T cell therapy201. Other side effects of CD19-specific CAR T cell therapy include lymphopenia and hypogammaglobulinaemia202, which can be effectively managed with intravenous immunoglobulin therapy, similar to the treatment that patients with primary B cell immunodeficiencies receive203. The mechanisms behind these side effects are unclear and further research may yield ways to avoid or minimize toxicity. Recent development of a novel murine model of CRS demonstrated that it is not mediated by CAR T cell-derived IL-6 but rather by recipient macrophages that secrete IL-6, IL-1 and nitric oxide. Therefore, IL-1 blockade represents a possible novel intervention in the armamentarium against CRS204. Moreover, a clinical study of low-affinity CD19-specific CAR T cells demonstrated reduced toxicity and enhanced efficacy205. Additional efforts to reduce toxicity involve the engineering of CAR T cells with multiple receptor specificities206 and reducing the half-life of cellular toxicity by using mRNA-based methods that allow for transient receptor expression207 or including suicide cassettes that can be activated by exogenous agents to clonally delete the infused cells208.
The ATC approach necessitates a patient-specific therapy design, its cost can be prohibitive, patient access to the treatment is limited and manufacturing is challenging. In the United States, the CAR T cell therapies tisagenlecleucel and axicabtagene ciloleucel have a direct cost of US$475,000 and US$373,000 per patient, respectively209. However, these values do not take into account the additional costs associated with treating the severe adverse effects common to CAR T cell therapy, which are estimated to increase drug-associated costs by US$30,000 or more209. In comparison with CAR T cell therapy, checkpoint blockade has a price tag of approximately US$12,500 per month210. Patient access to CAR T cell therapies also represents a major problem as there are only a few laboratories certified to generate CAR T cells and only a few specialized tertiary care centres able to administer this therapy211. Lastly, variability in the manufacturing of CAR T cells and a lack of standard practices can contribute to heterogeneous outcomes211.
Cancer vaccines
Cancer vaccines prompt the immune system to protect the body from cancer and fall into two categories, prophylactic and therapeutic. Prophylactic vaccines against hepatitis B and human papillomavirus have been instrumental in reducing the incidence of hepatocellular carcinoma and cervical cancer, respectively212. These are classic vaccines used to prevent infection by oncogenic viruses. By contrast, therapeutic vaccines aim to harness the immune system to eliminate disease-causing cells that are already neoplastic212. An early example of this is the use of the bacillus Calmette–Guérin vaccine, comprising attenuated Mycobacterium bovis, which is generally used as a prophylactic tuberculosis vaccine but has also been repurposed as a primitive therapeutic vaccine for bladder cancer213.
Historically, the discovery of TAAs214, which are highly expressed on tumour cells and to a lesser extent on normal tissues, opened the door for further therapeutic vaccine-based approaches. However, as TAAs are often recognized by the immune system as ‘self’, viral antigens and neoantigens that are unique to a malignancy may be more suited as vaccine targets.
Early vaccination approaches in the 1970s were based on autologous tumour vaccines and involved the administration of patient-derived tumour cells together with an adjuvant or virus in order to activate polyclonal immune responses to TAAs215. For example, autologous tumour cells infected with Newcastle disease virus have been used in one type of cancer vaccine that has demonstrated success in preclinical models of metastatic lymphoma and melanoma216,217. Modified Newcastle disease virus-based vaccines have been engineered to express granulocyte–macrophage colony-stimulating factor (GM-CSF) in attempts to enhance efficacy218. Synergism of vaccine approaches with checkpoint blockade agents has also been demonstrated in some preclinical studies of melanoma46,219. Numerous autologous tumour vaccines are being investigated in phase II and phase III trials but have yet to receive FDA approval. This approach suffers from multiple limitations, most notably the difficulty in obtaining patient-derived tumour cells in certain cancer types212. Newer approaches include the development of personalized recombinant cancer vaccines informed by next-generation sequencing of genomic DNA from tumours.
Development of personalized recombinant cancer vaccines
Vaccines that elicit responses to tumour-derived neoantigens should induce more robust immune responses and cause fewer autoimmune-related toxicities than vaccines based on self-derived TAAs, as the T cells that are activated by such a vaccine would not have undergone negative selection during development. These factors, as well as the ability to identify neoantigens through next-generation sequencing of genomic DNA from tumours, has shifted the focus to investigating the clinical feasibility of making personalized recombinant vaccines that target neoantigens. However, although a higher mutational burden in the tumour has been shown to correlate with greater immunogenicity and survival after checkpoint blockade66,220, only a small percentage of neoantigens spontaneously generate immune responses in patients with cancer221. Sahin and colleagues showed that neoantigens identified through next-generation sequencing can generate antitumour responses in vivo; in mice that were vaccinated with 50 different neoantigens, 16 were immunogenic222,223. Interestingly, most neoantigens induced cytokine responses from CD4+ T cells rather than CD8+ T cells, suggesting that neoantigens are selected for MHC class II binding222,223. Other preclinical studies demonstrated effective CD4+ and CD8+ T cell responses to neoantigen vaccines in various cancer types223–227. However, recent preclinical work has also highlighted the non-overlapping role of neoantigen responses mediated by CD4+ and CD8+ T cells228.
To design and manufacture a personalized vaccine for clinical use, computer-based algorithms are used to identify which tumour-derived peptides could potentially form a suitable TAA or tumour neoantigen with the patient’s MHC alleles (Fig. 6). There are several different strategies to formulate neoantigen-based vaccines, including as synthetic peptides, mRNA, viral and DNA plasmids or antigen-loaded dendritic cells, and it is difficult to directly compare how each strategy influences immunogenicity229,230. In one trial that tested a multi-peptide vaccine that included up to 20 personal neoantigens, 4 of 6 patients with melanoma who entered the study with stage III disease experienced complete responses with no recurrence 25 months post vaccination, and the other 2 patients with progressive disease subsequently underwent anti-PD1 therapy that resulted in complete tumour regression231. Further, of the 97 different neoantigens that were tested for immunogenicity in this study, 60% elicited CD4+ T cell responses whereas 15% elicited CD8+ T cell responses. Another clinical trial, which tested an RNA vaccine that encoded 10 peptides representing personalized TAAs in 13 patients with advanced melanoma, achieved similar results232.
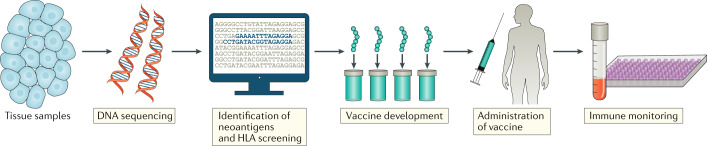
Fig. 6. Personalized vaccine development.
Healthy tissue and tumour tissue from a patient with cancer are submitted for DNA sequencing and bioinformatic analyses to identify gene variants that encode peptides that are specific to the tumour (neoantigens). Prediction algorithms are then used to screen for neoantigens that are likely to stably bind to the patient’s MHC (also known as HLA in human) molecules and their expression is validated by sequencing tumour mRNA. Multiple predicted neoantigens are then formulated into vaccines, which are administered to the patient together with adjuvants. Post treatment, the patient is regularly monitored for neoantigen-specific immune responses and tumour growth.
Pitfalls and adverse effects of cancer vaccines
Although these early cancer vaccine experiments have been promising, challenges remain. An individual tumour can harbour thousands of somatic mutations and predicting which neoantigens can elicit strong antitumour responses remains an imperfect art. However, the current methods, consisting of validating mRNA expression of the mutation in tumour cells and using software/databases to predict peptide–MHC binding, have been surprisingly effective in clinical trials to date229. However, this success has been biased towards MHC class I-specific neoantigens as prediction for MHC class II molecules presents unique challenges. For example, the increased diversity of MHC class II molecules and the structural nature of their open binding pocket make discerning a predictable binding motif difficult233. Taken together, these differences between MHC classes highlight the particular need for new MHC class II prediction algorithms. Other challenging factors to consider are the time and cost associated with developing bespoke vaccines. Currently, development and production of these vaccines takes approximately 4 months, and, although the downtime can be used to initiate other types of treatment, shortening the time span to personalized treatment is critical. For rapidly growing or metastatic tumours, months might matter. Ongoing efforts to improve design and manufacturing could shorten the production time to several weeks229.
Overall, the comprehensive identification of somatic mutations, and the evaluation of peptides derived from these mutations to elicit immune responses, has renewed interest in vaccination strategies for cancer treatment. Even though early clinical trials are promising, extrapolation of these findings could be misleading and advanced clinical trials will ultimately determine the efficacy of personalized vaccine therapy. Nonetheless, cancer vaccines are prototypical ‘single patient and single disease’ precision medications and would have been in the realm of science fiction just a few decades ago. Further research and technological developments will no doubt lead to greater precision and effectiveness and also provide a better understanding of the mechanisms of antitumoural immune responses.
Emerging cancer immunotherapeutics
The molecular diversity of genetic changes that transform cells in human cancers creates a plethora of diseases involving specific tissue types and cancer mechanisms. Given the exciting advances in cancer immunotherapy, various modifications to current immunotherapeutic approaches are being developed and tested to address the complexity of cancer immunopathogenesis and cancer targetability.
Combination therapies
Following the clinical success of checkpoint blockade monotherapy, combination therapies that couple agents with distinct mechanisms of action have augmented treatment success in various cancers. For example, ipilimumab and nivolumab combination therapy conferred a significant survival benefit in patients with metastatic melanoma and advanced renal cell carcinoma, leading to FDA approvals for these conditions234,235. The synergism of anti-CTLA4 and anti-PD1 therapies is not surprising because CTLA4 and PD1 regulate antitumoural immunity in a complementary manner8. Crosstalk between the CTLA4 and PD1 pathways, mediated by CD80 and PDL1 dimerization, provides additional insight into the mechanism behind the success of dual therapy236,237. However, as expected, combination checkpoint therapy also increases the risk of medication-induced toxicities235.
Combining radiation therapy with checkpoint blockade is another treatment option for recalcitrant tumours. The immunomodulatory effect of radiotherapy alone represents a double-edged sword. Mechanistically, radiotherapy increases the diversity of antitumoural T cell responses by exposing novel neoantigens at the same time as blunting the immune response through the induction of PDL1 expression on tumour cells238. Therefore, and on the basis of preclinical data, combining radiotherapy with blockers of the PD1 axis represents an attractive synergistic combination239. Patients with metastatic disease may represent a target population for deploying this combination as abscopal responses to radiotherapy are boosted by checkpoint blockade for many tumour types238,240. Overall, dual checkpoint blockade and radiation–checkpoint polytherapy represent promising avenues for synergistic therapeutic responses because these drug combinations display unique and complementary pharmacodynamics.
New targets for checkpoint blockade
Research is also directed at newly discovered negative regulators of T cell activation, including lymphocyte activation gene 3 (LAG3), T cell immunoglobulin 3 (TIM3), V-domain immunoglobulin suppressor of T cell activation (VISTA), B7-H3 and T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT), as adjuvant cancer drugs241–243. LAG3 is an inhibitory ligand that reduces T cell activation by blocking CD4 contact sites on MHC class II proteins and is expressed on activated T cells and Treg cells. It prevents the overexpansion of the T cell compartment by inducing cell cycle arrest244. Like PD1, LAG3 is a marker of T cell exhaustion, which portends a poorer prognosis when expressed on TILs245. Multiple strategies of blockade have been developed, including a LAG3:Ig fusion protein and LAG3-targeted mAbs246. In clinical trials in patients with renal cell carcinoma and pancreatic adenocarcinoma, these drugs did not succeed as monotherapies even though they increased the frequency of tumour-specific T cells246. However, when combined with paclitaxel for metastatic breast cancer, 50% of patients treated with LAG3:Ig responded to treatment247. Recent research has demonstrated that fibrinogen-like protein 1 (FGL1) activates LAG3 independently of binding MHC class II molecules and interference with this interaction is essential for unleashing potent antitumoural effects248.
TIM3 is another negative regulator of the T cell response. Rather than inhibiting cell cycle progression like LAG3, it regulates apoptosis following galectin 9 binding249. Its upregulation could represent a mechanism of resistance to anti-PD1 therapy, making combination therapy an attractive option to boost the effectiveness of anti-PD1 therapy. In addition, TIM3 expression correlates with poor prognosis in non-small-cell lung cancer and follicular lymphoma, suggesting a role in cancer progression250. Similar to TIM3, VISTA is another molecule shown to be associated with resistance to current checkpoint inhibitors and has demonstrated synergism with anti-PD1 therapy in mouse models251,252.
B7-H3 represents another targetable negative regulator of the T cell response. It is highly expressed in many tumour types, including non-small-cell lung carcinoma, prostate cancer, pancreatic cancer, ovarian cancer and colorectal cancer241,243. Enoblituzumab, a humanized mAb targeting B7-H3, was effective at inducing antitumoural responses in a phase I study of patients with various tumour types253. Dual-affinity retargeting (DART) proteins that bind to B7-H3 and CD3, as well as radioactive iodine-conjugated B7-H3 mAbs, represent additional ways to modulate this pathway and are in early-phase clinical testing254,255.
Lastly, TIGIT, which contains two immunoreceptor tyrosine-based inhibitory motifs in its intracellular domain and dampens T cell hyperactivation, is being investigated as a checkpoint target. It is more robustly expressed in TILs than in peripheral cells, making it an attractive target owing to its increased specificity compared with other checkpoint molecules243. Preclinical evidence demonstrates that TIGIT blockade augments the effect of pre-existing checkpoint inhibitors and reinvigorates tumour-specific exhausted T cells250,256. Currently, blockade of immune checkpoints other than CTLA4 or the PD1 axis have not yet shown major clinical benefits as single agents but rather may increase the effectiveness of pre-existing treatments.
Although the blocking of immune checkpoint molecules releases potent antitumoural responses, the stimulation of T cell co-stimulatory receptors, including inducible co-stimulator (ICOS), tumour necrosis factor receptor superfamily member 4 (TNFRSF4; also known as CD134), tumour necrosis factor receptor superfamily member 9 (TNFRSF9; also known as 4-1BB), glucocorticoid-induced tumour necrosis factor receptor (GITR) and CD27, can also amplify the effect of existing immunotherapies, as shown preclinically and in early-stage clinical studies168,170,171,241–243,257. ICOS is a member of the CD28 family of co-stimulatory molecules that mediates context-dependent cytokine responses with an emphasis on T helper 2 (TH2) cell skewing258. ICOS stimulation by vaccines modified to express ICOS ligand exhibited synergism with treatment with CTLA4-blocking antibodies preclinically259. ICOS upregulation following treatment with currently approved anti-CTLA4 and anti-PD1 therapies may represent a biomarker of active antitumoural responses because it associates with favourable outcomes260.
TNFRSF4 is another co-stimulatory molecule for which preclinical evidence indicates a role in deploying robust antitumoural responses in sarcoma, melanoma and breast cancer261,262. Data suggest that targeting TNFRSF4 amplifies anti-PD1 therapy because TNFRSF4 agonism can upregulate PDL1 expression263. In addition to synergism with checkpoint blockade, TNFRSF4 upregulation within CAR T cells by transfection represents a way to augment tumour cytotoxicity170. Agonism of additional TNFR family members, such as TNFRSF9, GITR and CD27, is being tested as adjuvant therapy in phase I/II trials for various tumour types, with promising results243. Therefore, agonism of positive T cell co-stimulatory signals, in concert with the existing checkpoint inhibitors or CAR T cells, represents a novel therapeutic avenue to boost antitumoural immunity.
Concluding remarks
Cancer immunotherapy focused on T cells has emerged as a powerful tool in the armamentarium against cancer. Nevertheless, it took many years of basic science discoveries and subsequent clinical translation to unequivocally demonstrate the power of modulating the immune system to treat cancer. Further research that investigates the regulation of T cells and other immune cells, for example APCs and natural killer cells, may allow us to enhance the power of this approach. In ‘difficult to treat’ tumours, the effect sizes observed in clinical trials of checkpoint blockade agents, ATC transfer therapies and cancer vaccines have been far higher than the most effective chemotherapeutic agents. Although immune-related adverse effects are common, these innovative immune-targeting therapies are better tolerated than traditional chemotherapeutic agents. The burgeoning field of cancer immunotherapy continues to grow as indications for currently approved therapies expand and the search for novel druggable targets continues. The cancer immunotherapy success stories we have recounted highlight the intrinsic connection between basic science research and clinical practice. They also illustrate how a bench-to-bedside approach, built upon a solid basic science foundation, can be successful in fighting one of humanity’s most dreaded diseases.
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. A.D.W. was supported by the Emory University MD/PhD Program, NIH MD/PhD Partnerships Program and NIH Oxford–Cambridge Scholars Program. J.M.F. was supported by the Postdoctoral Research Associate Training Program of the National Institute of General Medical Sciences. The authors thank R. Kissinger for help with the illustrations. They thank G. De Luca for his support of A.D.W. as a DPhil co-mentor. They also thank Y. Zhang for invaluable editorial and scientific feedback. Lastly, the authors acknowledge and apologize to all researchers in this field who may have authored elegant studies that were not cited owing to space limitations.
Glossary
- Neoantigens
Antigens not expressed by self-tissues under normal conditions that manifest in the context of pathology; in cancer, these could be altered proteins/peptides encoded by mutated genes.
- Immune checkpoint
A mechanism of immune cell inhibition that restrains activation.
- Immunoglobulin superfamily
A group of proteins with genetic and structural similarities to antibodies.
- Antigen-presenting cells
(APCs). Immune cells involved in the uptake and processing of antigens to initiate cellular immune responses.
- CTLA4Ig
Soluble recombinant human cytotoxic T lymphocyte antigen 4 (CTLA4) fused to the immunoglobulin Fc domain that competes with endogenous CD28 for its ligands.
- Immunological synapse
An interface between interacting lymphocytes and antigen-presenting cells that controls antigen-induced signalling.
- Immune conjugate
A biological unit that comprises interacting lymphocytes and antigen-presenting cells.
- IL-2
A cytokine essential for lymphocyte activation, proliferation and tolerance.
- Adaptor protein
An intracytoplasmic protein that facilitates molecular interactions and signal transduction.
- Antibody-dependent cell-mediated cytotoxicity
The process by which antibody-based opsonization of target cells promotes their lysis by immune cytotoxic cells.
- BALB/c
An albino inbred mouse strain commonly used in immunology research.
- Non-obese diabetic
An inbred mouse stain with enhanced susceptibility to spontaneous development of type 1 diabetes mellitus.
- Immunoreceptor tyrosine-based switch motif
A conserved amino acid sequence (TxYxx(V/I)) involved in both activation and inhibition of downstream signalling depending on the cell type and biological context.
- Immunoreceptor tyrosine-based inhibitory motif
A conserved amino acid sequence (S/I/V/LxYxxI/V/L) involved in the recruitment of inhibitory phosphatases to dampen downstream signalling.
- T cell exhaustion
The progressive loss of effector function due to chronic low-affinity antigen stimulation.
- Abscopal responses
A phenomenon in which the therapeutic effect of radiation is extended beyond the boundaries of the tissue that was treated
- T helper 2 (TH2) cell skewing
Biasing of CD4+ T helper cells towards a phenotype essential for humoral immunity.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks J. Oliaro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oiseth SJ, Aziz MSA-E. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017;3:250–261. [Google Scholar]
- 2.Decker WK, Safdar A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley’s legacy revisited. Cytokine Growth Factor. Rev. 2009;20:271–281. doi: 10.1016/j.cytogfr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Decker WK, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front. Immunol. 2017;8:829. doi: 10.3389/fimmu.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res. 1943;3:326. [Google Scholar]
- 5.Smith JL, Jr., Stehlin JS., Jr. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18:1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2012;22:23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RSC. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J. Surg. 1995;19:352–358. doi: 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- 8.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunet JF, et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 10.Dariavach P, Mattei MG, Golstein P, Lefranc MP. Human Ig superfamily CTLA-4 gene: chromosomal localization and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur. J. Immunol. 1988;18:1901–1905. doi: 10.1002/eji.1830181206. [DOI] [PubMed] [Google Scholar]
- 11.Harper K, et al. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J. Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 12.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 13.Lo B, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsley PS, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 16.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 20.Tivol EA, et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 1997;158:5091–5094. [PubMed] [Google Scholar]
- 21.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J. Exp. Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4–/– mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc. Natl Acad. Sci. USA. 2007;104:13756–13761. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, et al. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J. Allergy Clin. Immunol. 2016;137:327–330. doi: 10.1016/j.jaci.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn HS, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darlington PJ, et al. Surface cytotoxic T lymphocyte–associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J. Exp. Med. 2002;195:1337. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 30.Chuang E, et al. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol. 1999;162:1270–1277. [PubMed] [Google Scholar]
- 31.Marengere LE, et al. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 32.Chuang E, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 33.Fraser JH, Rincon M, McCoy KD, Le Gros G. CTLA4 ligation attenuates AP-1, NFAT and NF-κB activity in activated T cells. Eur. J. Immunol. 1999;29:838–844. doi: 10.1002/(SICI)1521-4141(199903)29:03<838::AID-IMMU838>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl Acad. Sci. USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tai X, et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LSK. Treg and CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmunity. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chikuma S, Bluestone JA. Expression of CTLA-4 and FOXP3 in cis protects from lethal lymphoproliferative disease. Eur. J. Immunol. 2007;37:1285–1289. doi: 10.1002/eji.200737159. [DOI] [PubMed] [Google Scholar]
- 40.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedline RH, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sojka DK, Hughson A, Fowell DJ. CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur. J. Immunol. 2009;39:1544–1551. doi: 10.1002/eji.200838603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou TZ, et al. A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J. Immunol. 2015;194:2148–2159. doi: 10.4049/jimmunol.1401876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 46.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 47.Kwon ED, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl Acad. Sci. USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007;13:2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 49.Yang YF, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 50.Mangsbo SM, et al. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J. Immunother. 2010;33:225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 51.Hurwitz AA, Yu TFY, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte–macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc. Natl Acad. Sci. 1998;95:10067. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jure-Kunkel MN, Masters G, Girit E, Dito G, Lee FY. Antitumor activity of anti-CTLA-4 monoclonal antibody (mAb) in combination with ixabepilone in preclinical tumor models. J. Clin. Oncol. 2008;26:3048–3048. [Google Scholar]
- 54.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schadendorf D, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maio M, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. 2015;33:1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch TJ, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 60.Reck M, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann. Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 61.Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribas A, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 2014;35:290–298. doi: 10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 64.He M, et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8:67129–67139. doi: 10.18632/oncotarget.18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin. Oncol. 2015;42:363–377. doi: 10.1053/j.seminoncol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Rooij N, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma N, Vacher J, Allison JP. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion. Proc. Natl Acad. Sci. USA. 2019;116:10453. doi: 10.1073/pnas.1819004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ha D, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc. Natl Acad. Sci. USA. 2019;116:609. doi: 10.1073/pnas.1812186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma A, et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 2019;25:1233. doi: 10.1158/1078-0432.CCR-18-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 74.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 75.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 78.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 79.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, et al. Establishment of NOD-Pdcd1–/– mice as an efficient animal model of type I diabetes. Proc. Natl Acad. Sci. USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei SC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 86.Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 87.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 90.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strome SE, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 92.He YF, et al. Blocking programmed death-1 ligand–PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J. Immunol. 2004;173:4919–4928. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 93.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 95.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl Acad. Sci. USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson RH, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 97.Thompson RH, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 98.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Berman D, et al. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol. Ther. 2015;148:132–153. doi: 10.1016/j.pharmthera.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 100.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber JS, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 103.Weber J, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 104.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 105.Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 106.Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 107.Herbst RS, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 108.Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 109.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 110.Cohen EEW, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 111.Moskowitz CH, et al. Pembrolizumab in relapsed/refractory classical Hodgkin lymphoma: primary end point analysis of the phase 2 KEYNOTE-087 study. Blood. 2016;128:1107. [Google Scholar]
- 112.Bellmunt J, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boyiadzis MM, et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer. 2018;6:35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding L, Chen F. Predicting tumor response to PD-1 blockade. N. Engl. J. Med. 2019;381:477–479. doi: 10.1056/NEJMcibr1906340. [DOI] [PubMed] [Google Scholar]
- 116.Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type—FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol. 2018;4:157–158. doi: 10.1001/jamaoncol.2017.4182. [DOI] [PubMed] [Google Scholar]
- 117.Motzer RJ, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferris RL, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma P, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 120.El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Overman MJ, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Topalian SL, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Powles T, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 127.Rittmeyer A, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 129.Horn L, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 130.Kaufman HL, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patel MR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Motzer RJ, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Powles T, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3:e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Antonia SJ, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 135.Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019;216:1244–1254. doi: 10.1084/jem.20182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Michot JM, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 137.Kumar V, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front. Pharmacol. 2017;8:49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 139.Wang P-F, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front. Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Champiat S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 141.Saada-Bouzid E, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017;28:1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 142.Ferrara R, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rauch DA, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood. 2019;134:1406–1414. doi: 10.1182/blood.2019002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tazdait M, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur. J. Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 145.Champiat S, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat. Rev. Clin. Oncol. 2018;15:748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 146.Liu J, Blake SJ, Smyth MJ, Teng MW. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin. Transl Immunol. 2014;3:e22. doi: 10.1038/cti.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Du X, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018;28:416–432. doi: 10.1038/s41422-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist. 2017;22:470–479. doi: 10.1634/theoncologist.2016-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 2019;29:609–627. doi: 10.1038/s41422-019-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y, Zheng P. Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol. Sci. 2020;41:4–12. doi: 10.1016/j.tips.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug. Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 153.Shahabi V, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J. Transl Med. 2013;11:75. doi: 10.1186/1479-5876-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Callahan MK, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J. Clin. Oncol. 2011;29:2505–2505. [Google Scholar]
- 155.Schindler K, et al. Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab. J. Clin. Oncol. 2014;32:9096. [Google Scholar]
- 156.Southam CM, Brunschwig A, Levin AG, Dizon QS. Effect of leukocytes on transplantability of human cancer. Cancer. 1966;19:1743–1753. doi: 10.1002/1097-0142(196611)19:11<1743::aid-cncr2820191143>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 157.Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 158.Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 159.Rosenberg SA, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 160.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zacharakis N, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018;24:724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palmer DC, et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med. 2015;212:2095. doi: 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 2015;6:e0004. doi: 10.5041/RMMJ.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuwana Y, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 166.Brocker T. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 167.Jensen MC, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transpl. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 169.Kuhn NF, et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 2019;35:473–488.e6. doi: 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR ζ chain. J. Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 171.Imai C, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 172.Rafiq S, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eyquem J, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016;13:370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat. Med. 2019;25:1488–1499. doi: 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 181.Raje N, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cohen AD, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Smith EL, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl Med. 2019;11:eaau7746. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beatty GL, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 187.Majzner RG, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Batchu RB, et al. Inhibition of interleukin-10 in the tumor microenvironment can restore mesothelin chimeric antigen receptor T cell activity in pancreatic cancer in vitro. Surgery. 2018;163:627–632. doi: 10.1016/j.surg.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 189.Chang ZL, et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao J, Zhao J, Perlman S. Differential effects of IL-12 on Tregs and non-Treg T cells: roles of IFN-γ, IL-2 and IL-2R. PLoS One. 2012;7:e46241. doi: 10.1371/journal.pone.0046241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 192.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017;7:10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilkie S, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van Schalkwyk MC, et al. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum. Gene Ther. Clin. Dev. 2013;24:134–142. doi: 10.1089/humc.2013.144. [DOI] [PubMed] [Google Scholar]
- 198.Lynn RC, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Neelapu SS, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shimabukuro-Vornhagen A, et al. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Conley ME, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu. Rev. Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 204.Giavridis T, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ghorashian S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 206.Bielamowicz K, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hung CF, et al. Development of anti-human mesothelin-targeted chimeric antigen receptor messenger RNA-transfected peripheral blood lymphocytes for ovarian cancer therapy. Hum. Gene Ther. 2018;29:614–625. doi: 10.1089/hum.2017.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 2014;5:254–254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4:994–996. doi: 10.1001/jamaoncol.2018.0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moon EK, Langer CJ, Albelda SM. The era of checkpoint blockade in lung cancer: taking the brakes off the immune system. Ann. Am. Thorac. Soc. 2017;14:1248–1260. doi: 10.1513/AnnalsATS.201702-152FR. [DOI] [PubMed] [Google Scholar]
- 211.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 212.Guo C, et al. Therapeutic cancer vaccines: past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 214.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 215.Hanna MG, Jr., Peters LC. Immunotherapy of established micrometastases with Bacillus Calmette–Guerin tumor cell vaccine. Cancer Res. 1978;38:204–209. [PubMed] [Google Scholar]
- 216.Heicappell R, Schirrmacher V, von Hoegen P, Ahlert T, Appelhans B. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. Int. J. Cancer. 1986;37:569–577. doi: 10.1002/ijc.2910370416. [DOI] [PubMed] [Google Scholar]
- 217.Plaksin D, et al. Effective anti-metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle disease virus (NDV) Int. J. Cancer. 1994;59:796–801. doi: 10.1002/ijc.2910590615. [DOI] [PubMed] [Google Scholar]
- 218.Burke, S. et al. Oncolytic Newcastle disease virus activation of the innate immune response and priming of antitumor adaptive responses in vitro. Cancer Immunol. Immunother. 10.1007/s00262-020-02495-x (2020). [DOI] [PMC free article] [PubMed]
- 219.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tran E, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Castle JC, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 223.Kreiter S, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duan F, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J. Exp. Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 228.Alspach E, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 230.Zhang X, Sharma PK, Peter Goedegebuure S, Gillanders WE. Personalized cancer vaccines: targeting the cancer mutanome. Vaccine. 2017;35:1094–1100. doi: 10.1016/j.vaccine.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ott PA, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 233.Nielsen M, Lund O, Buus S, Lundegaard C. MHC class II epitope predictive algorithms. Immunology. 2010;130:319–328. doi: 10.1111/j.1365-2567.2010.03268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Motzer RJ, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wolchok JD, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhao Y, et al. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51:1059–1073.e9. doi: 10.1016/j.immuni.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ngwa W, et al. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2018;52:39–52. doi: 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 242.Donini C, D’Ambrosio L, Grignani G, Aglietta M, Sangiolo D. Next generation immune-checkpoints for cancer therapy. J. Thorac. Dis. 2018;10:S1581–S1601. doi: 10.21037/jtd.2018.02.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Marin-Acevedo JA, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 2018;11:39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.He Y, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107:1193–1197. doi: 10.1111/cas.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Deng WW, et al. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology. 2016;5:e1239005. doi: 10.1080/2162402X.2016.1239005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 247.Brignone C, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J. Transl Med. 2010;8:71. doi: 10.1186/1479-5876-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–347.e12. doi: 10.1016/j.cell.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 250.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu J, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl Acad. Sci. USA. 2015;112:6682–6687. doi: 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gao J, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Powderly J, et al. Interim results of an ongoing phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J. Immunother. Cancer. 2015;3:O8. [Google Scholar]
- 254.Kramer K, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tolcher AW, et al. Phase 1, first-in-human, open label, dose escalation ctudy of MGD009, a humanized B7-H3 × CD3 dual-affinity re-targeting (DART) protein in patients with B7-H3-expressing neoplasms or B7-H3 expressing tumor vasculature. J. Clin. Oncol. 2016;34:TPS3105. [Google Scholar]
- 256.Chauvin JM, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J. Clin. Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kowolik CM, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 258.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 259.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 2014;211:715. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vonderheide RH, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 261.Kjaergaard J, et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 262.Weinberg AD, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 263.Valzasina B, et al. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 264.Parrott DMV, de Sousa MAB, East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J. Exp. Med. 1966;123:191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Miller JF, Mitchell GF. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J. Exp. Med. 1968;128:801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.van den Broek T, Borghans JAM, van Wijk F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 2018;18:363–373. doi: 10.1038/s41577-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 268.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 271.Wilson IA, Garcia KC. T-cell receptor structure and TCR complexes. Curr. Opin. Struct. Biol. 1997;7:839–848. doi: 10.1016/s0959-440x(97)80156-x. [DOI] [PubMed] [Google Scholar]
- 272.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 273.Martin PJ, et al. A 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J. Immunol. 1986;136:3282–3287. [PubMed] [Google Scholar]
- 274.Gmunder H, Lesslauer W. A 45-kDa human T-cell membrane glycoprotein functions in the regulation of cell proliferative responses. Eur. J. Biochem. 1984;142:153–160. doi: 10.1111/j.1432-1033.1984.tb08263.x. [DOI] [PubMed] [Google Scholar]
- 275.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol. Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 276.Lesslauer W, et al. T90/44 (9.3 antigen). A cell surface molecule with a function in human T cell activation. Eur. J. Immunol. 1986;16:1289–1296. doi: 10.1002/eji.1830161017. [DOI] [PubMed] [Google Scholar]
- 277.Hansen JA, Martin PJ, Nowinski RC. Monoclonal antibodies identifying a novel T-cell antigen and Ia antigens of human lymphocytes. Immunogenetics. 1980;10:247–260. [Google Scholar]
- 278.Hara T, Fu SM, Hansen JA. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen) J. Exp. Med. 1985;161:1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 281.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cantrell DA. T-cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 284.Alexander DR. The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin. Immunol. 2000;12:349–359. doi: 10.1006/smim.2000.0218. [DOI] [PubMed] [Google Scholar]
- 285.Rossy J, Williamson DJ, Gaus K. How does the kinase Lck phosphorylate the T cell receptor? Spatial organization as a regulatory mechanism. Front. Immunol. 2012;3:167. doi: 10.3389/fimmu.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Macian F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 287.Miller JF, Morahan G. Peripheral T cell tolerance. Annu. Rev. Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 288.Lenardo M, et al. Mature T lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 289.Zheng L, Li J, Lenardo M. Restimulation-induced cell death: new medical and research perspectives. Immunol. Rev. 2017;277:44–60. doi: 10.1111/imr.12535. [DOI] [PubMed] [Google Scholar]
- 290.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 291.Sade-Feldman M, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Azizi E, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pauken KE, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gide TN, et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell. 2019;35:238–255.e6. doi: 10.1016/j.ccell.2019.01.003. [DOI] [PubMed] [Google Scholar]