Abstract
Background
A non-fatal opioid overdose (NFOO) increases the risk of another overdose and identifies high-risk patients. We estimated the risk of repeat opioid overdose for patients with and without substance use disorder (SUD) diagnoses and the change in substance use treatment utilization rates associated with the first NFOO.
Methods
We selected patients (>18 years of age) from Kaiser Permanente Northern California with a NFOO between 2009–2016 (n=3,992). Cox Proportional Hazards models estimated the 1-year risk of opioid overdose associated with SUD diagnoses (opioid, alcohol, cannabis, amphetamine, sedative, and cocaine), controlling for patient characteristics. Among patients with an index NFOO, we calculated monthly utilization rates for outpatient substance use services and buprenorphine before and after the index overdose. Interrupted time series models estimated the change in level and trend in utilization rates associated with the index overdose.
Results
Approximately 7.2% of patients had a repeat opioid overdose during the year after the index NFOO. The only SUD diagnosis significantly associated with greater risk of repeat overdose was opioid use disorder (OUD) (aHR: 1.51; 95% CI: 1.13–2.01).
Before the index overdose, 4.16% of patients received outpatient substance use services and 1.32% received buprenorphine. The index overdose was associated with a 5.94% (standard error: 0.77%) absolute increase in outpatient substance use services and a 1.29% (standard error: 0.15%) increase in buprenorphine.
Conclusion
Patients with a NFOO and OUD are vulnerable to another overdose. Low initiation rates for substance use treatment after a NFOO indicate a need to address patient, provider, and system barriers.
Keywords: opioid overdose, substance use treatment, substance use disorder
1. INTRODUCTION
In 2017, over 47,000 people in the United States died from an opioid overdose and about 36% of the deaths involved prescription opioids (Scholl et al., 2019). Along with a nearly 20% annual increase from 2013 to 2016 in the number opioid overdose deaths, the rates of opioid-related hospitalizations and emergency department visits have increased (Centers for Disease Control and Prevention, 2018; Weiss et al., 2016).
For health systems, overdose prevention efforts rely on identifying patients with the highest risk for opioid overdose. Risk factors for an opioid overdose include opioid dose, mental health disorders, and substance use disorders (SUDs) (Brady et al., 2017; Park et al., 2016). Many initial opioid overdoses are not fatal, and depending on the number of non-fatal opioid overdoses (NFOO), the risk of a fatal overdose is up to five time greater for individuals who experienced at least one overdose compared to individuals who did not have an overdose (Caudarella et al., 2016). Filling an opioid prescription before a NFOO and prescribed dose greater than 50 Morphine Milligrams Equivalent (MME) after a NFOO increase the risk of repeat overdose (Larochelle et al., 2016; Olfson et al., 2018).
The risk factors for repeat opioid overdose, which may differ from risk factors of initial opioid overdose, have been understudied. Common risk factors for opioid overdose and death include SUDs (e.g. opioid use disorder (OUD), alcohol use disorder, and cannabis use disorder) (Bohnert et al., 2012; Park et al., 2016). Among patients with a NFOO, the association between specific SUDs and risk of a subsequent overdose is inconsistent. Some studies have found that SUDs, such as alcohol, opioid, and other drug use disorders, are associated with greater risk of repeat overdose, and other studies have not found an association (Boscarino et al., 2016; Larochelle et al., 2016; Olfson et al., 2018). The evidence on risk factors for repeat overdose focuses on select patient populations and may not generalize to all patients with a NFOO. For example, one study examined risk factors for patients who had been prescribed opioids for more than 90 days, which excludes patients who may use opioids without a prescription or for fewer than 90 days (Larochelle et al., 2016). Results from a study on Medicaid recipients may not generalize to other insured populations because the risk of overdose and prevalence of SUDs may differ for commercially insured and Medicaid populations (Larochelle et al., 2016; Olfson et al., 2018). Without knowing the risk factors for repeat overdoses and how they may differ among distinct populations, overdose prevention efforts may have a limited impact.
Medications for opioid use disorder (MOUD) (e.g., buprenorphine and methadone) reduce the risk of opioid overdose and mortality (Morgan et al., 2019; Sordo et al., 2017). Among patients with a NFOO, methadone and buprenorphine reduce the risk of opioid-related mortality, but almost 2/3 never receive MOUD after a NFOO (Larochelle et al., 2018). Utilization rates of substance use services and MOUDs increased modestly directly after a NFOO for select patient populations (Frazier et al., 2017; Koyawala et al., 2019; Larochelle et al., 2018). In subsequent months after an overdose, MOUD utilization slightly increased, and substance use treatment service (e.g., inpatient or residential detoxification and treatment services) utilization declined (Larochelle et al., 2018). Previous studies on substance use treatment rates were descriptive and focused on select populations; however, little is known about whether a NFOO predicts changes in utilization rates of substance use services and MOUDs (Frazier et al., 2017; Koyawala et al., 2019; Larochelle et al., 2018). This study adds to the generalizability of previous research by focusing on a population seeking care in an integrated care system. Understanding the changes in immediate and long-term treatment patterns attributed to a NFOO in an insured population could inform care delivery strategies to engage patients and promote retention in substance use treatment (e.g. initiating buprenorphine in the emergency room or primary care) (Herring et al., 2019; Korthuis et al., 2017).
This study sought to understand the risk factors of repeat opioid overdose in an insured patient population, including patients with and without opioid prescriptions before the index opioid overdose. We examined which specific SUDs (e.g., opioid, alcohol, cannabis, cocaine, amphetamine, barbiturate, and hallucinogen) are associated with the one-year risk of repeat opioid overdose and adjusted for prescribed opioid dose before and after the index NFOO and substance use treatment use after the index NFOO. We hypothesized that SUDs were associated with greater risk of a repeat opioid overdose, and that OUD was associated with the highest risk of repeat opioid overdose compared to the other SUDs. We examined the differences in level and trend of utilization rates for outpatient substance use services and buprenorphine before and after the index NFOO. We hypothesized that a NFOO was associated with higher utilization rates of outpatient substance use services and buprenorphine.
2. METHODS
2.1. Setting
Kaiser Permanente Northern California (KPNC) is a nonprofit, integrated health care delivery system providing comprehensive health services to approximately 4.2 million members. The membership reflects the region’s general population, although people with low levels of education and income are under-represented (Gordon, 2012). Membership includes commercial, Medicare, Medicaid, and the State Health Insurance Assistance Program enrollees.
2.2. Data Sources
KPNC’s electronic health record (EHR) includes data on health plan membership, outpatient pharmacy, and inpatient and outpatient medical encounters. We extracted age, race/ethnicity, sex, residence, membership status, health service utilization, International Classification of Diseases (ICD)-9 and 10 diagnoses, and pharmacy data from the EHR (Ross et al., 2014). Diagnoses were identified from encounters observed during the study period. We extracted mortality data (2009–2016), including date of death and underlying cause of death from the EHR and state death certificates; data on the underlying cause (e.g. opioid-related overdose) of death was available from 2009–2015. To identify patients with cancer, we used the KPNC tumor registry which contains information on all new cancers (excluding basal cell and squamous cell carcinomas of the skin) for KPNC members diagnosed after January 1, 1997.
2.3. Cohort Selection
We identified patients >18 years of age with at least one diagnosis of opioid-related overdose (ICD-9: 965.X, E85.X; ICD-10: T400-T404, X42, X62, Y12) between 2009 and 2016 (Campbell et al., 2018). We used 2008 as the lookback year, and excluded patients with a diagnosis of an overdose in 2008 if they had a NFOO in 2009. The index overdose was the first non-fatal, opioid-related overdose following a year without an opioid overdose. The date of the index overdose was the index date. Eligible index NFOOs occurred during 2009 to 2016. We excluded patients without continuous KPNC membership in the year before the index overdose or patients who were diagnosed with a new cancer between January 1, 1997 and their index overdose. Patients were followed for one year after the index overdose or until one of the following censoring events: a fatal or non-fatal opioid overdose or poisoning, death from other causes, disenrollment from KPNC, or end of study follow-up as December 31, 2016.
To examine the trends in substance use treatment, we selected a subset of the cohort with an index overdose between January 1, 2009 and December 31, 2015. This allowed for a follow up period of 360 days after the index overdose to observe the use of substance use treatment; patients who died during that period were censored.
2.4. Measures
The outcome of interest was the first fatal or non-fatal opioid-related overdose in the year after the index overdose. The primary independent variables were diagnoses of the following SUDs which were given for an encounter in the year before the index overdose using the ICD-9/10 codes: opioid, alcohol, cannabis, cocaine, amphetamine, barbiturate, hallucinogens, multi-drug, and unspecified (Supplemental Table 1).
We selected individual characteristics that confound the association between diagnoses of SUDs and risk of repeat opioid overdose based on literature on the predictors of opioid-related overdose (Campbell et al., 2018; Larochelle et al., 2018; Larochelle et al., 2016; Park et al., 2016; Ranapurwala et al., 2019; Shiels et al., 2019). We accounted for comorbidities by using the Deyo-Charlson comorbidity index which represents 17 comorbidities diagnosed in the year before the index overdose (Deyo et al., 1992). Because the Deyo-Charlson score has a skewed distribution, we categorized patients into four groups: 0, 1, 2, and ≥3. We identified mental health disorder diagnoses in the year before the index overdose: anxiety, bipolar disorder, depression, developmental disorder, eating disorder, obsessive compulsive disorder, panic disorder, and schizophrenia. Using annual screening data, we categorized patients’ tobacco smoking status as current, former, or never smokers. The neighborhood deprivation index (NDI), which) was categorized into quartiles, was a proxy for the patient’s socioeconomic status based on residence and census tract variables (e.g. households in poverty) from the U.S. Census Bureau’s 2006–2010 American Community Survey (Messer et al., 2006; U.S.Census Bureau, 2008).
We extracted data for prescription opioid fills in the year before and after the index overdose. Using the quantity filled, the drug strength, the MME conversion factor, and the days’ supply, we calculated the dose in MMEs for specified time periods before and after the index date (National Center for Injury Prevention and Control, 2017 ; Von Korff et al., 2008). For the 30 days before the index overdose, we calculated the mean daily MME, and identified whether the patient used benzodiazepines in that period.
For each day after the index overdose, we calculated the mean daily MME in the prior 30 days by dividing total MMEs used in the prior 30 days by 30. Based on cutoffs from previous studies, we categorized the mean daily dose into five groups: 0, 1-<20, 20-<50, 50-<100, or ≥100 MME (Campbell et al., 2018; Larochelle et al., 2016). We determined benzodiazepine use on each day after the index overdose, and categorized patients into two groups: no use in the prior 30 days or any use. We calculated change before and after the index overdose by subtracting the mean daily MME in the 30 days before the index overdose from the mean daily MME in the 30 days after the index overdose. We created a categorical variable based on the change in dose: a positive value indicated a dose escalation, a negative value indicated a dose reduction, and zero indicated no change in dose.
We identified outpatient substance use service use and buprenorphine prescriptions filled after the index overdose. Outpatient substance use services are specialty substance use services delivered in an outpatient addiction medicine facility. Both measures were binary indicating any use in the 30 days prior. Although both methadone and naltrexone are also indicated to treat OUD, these medications were not used at KPNC during the full study period and thus were excluded from the analysis (Dowell et al., 2016).
The utilization analysis estimated the differences in level and trend of the utilization rates for outpatient substance use services and buprenorphine medication associated with the index overdose. We created an aggregated monthly panel data set. First, we identified 12 months (30-day time periods) before and after the index overdose. For each month, we created binary variables to indicate any use of outpatient substance use services and buprenorphine. Utilization rates were calculated by dividing the number of people with any use of substance use treatment by the total number of patients in that time period. We did not require complete follow-up after the index overdose for patients. Patients who died during the year after the index date had less than 12 months of follow-up after the index date. At baseline, 2,847 patients were included, and 318 patients (~11%) were censored by the end of the study period, for a total of 2,529 patients.
2.5. Statistical Analysis
We calculated means and proportions for baseline characteristics. T-tests and chi-square tests were used to compare baseline characteristics for members with and without a repeat opioid-related overdose during the year follow up. Stata version 15.0 (StataCorp) was used for bivariate tests (StataCorp LP, 2018).
Fatal and non-fatal overdose outcomes were analyzed together because there were not enough fatal overdoses for a separate analysis. For each SUD diagnosis, we estimated unadjusted hazard ratios of repeat opioid-related overdose. We estimated adjusted hazard ratios associated with opioid, alcohol, cannabis, cocaine, amphetamine, barbiturate, multi-drug, and unspecified drug use disorders using an extended Cox hazard model, with days since index date as the time scale. The model adjusted for time invariant characteristics: age at index overdose, sex, race, NDI quartiles, Charlson score, mental health disorder diagnosis one year before the index overdose (anxiety, depression, and other mental health disorder (bipolar disorder, developmental disorder, eating disorder, obsessive compulsive disorder, panic disorder, and schizophrenia)), smoking status, average daily dose of prescription opioids 30 days before the index overdose, and any benzodiazepine use 30 days before the index date. The model also included time varying characteristics defined after the index overdose: average daily dose, any benzodiazepine use, any buprenorphine use, and any substance use service use in the prior 30 days. Hallucinogen use disorder was excluded from the adjusted model because estimates were unstable. This analysis used the PHREG procedure in SAS for Windows software version 9.4 (Cary, NC).
For the utilization analysis, we calculated the proportion of the cohort that used outpatient substance use services and buprenorphine per month. We assessed changes in the utilization rates and differences in trend before and after the NFOO using an interrupted time series model, which assumes that the slope and trend of the utilization rates before the NFOO represent the counterfactual, or what would happen to the utilization rates without a NFOO (Penfold and Zhang, 2013). The linear regression model included a month variable for each month (30-day period), a post variable for time after the index overdose, and the interaction between the month and post variable. The level of change after the overdose is the coefficient of the post variable. The change in trend after the overdose is the coefficient of the interaction term. The Durbin-Watson statistic for the models ranged from 1.17 to 1.58, which were less than 2 and indicated serial correlation of the errors. We used the Yule-Walker method to adjust for serial correlation (Gallant and Goebel, 1976). After applying the Yule-Walker method, the Durbin-Watson statistic was 1.64–1.77. We plotted the observed and predicted utilization rates before and after the index NFOO. This analysis used the AUTOREG procedure in SAS (Cary, NC) to account for the autoregressive form of the error (Penfold and Zhang, 2013). This study was approved by the Institutional Review Board at Kaiser Permanente Northern California.
3. RESULTS
We identified 7,596 patients who experienced a NFOO during 2008 to 2016. To ensure that we identified patients who did not have an overdose in the year before the index overdose, we excluded patients with an opioid overdose in 2008 (N=662). We also excluded: patients with cancer (N=545); patients with less than one year of continuous membership prior to the NFOO (N=2,460); patients younger than 18 years of age (N=247); those with missing values for the NDI (N=19); and 259 patients who met multiple exclusion criteria. The final cohort had 3,922 patients who experienced a NFOO during 2009 to 2016. The mean follow-up time was 280.44 days.
Approximately 58.2% of the cohort were women, 63.6% were White, and 41.0% were between 18–44 years old (Table 1). Seven percent of the cohort experienced a repeat opioid overdose at some point during the year after the index overdose. Before the index overdose, 47.3% had at least one SUD, the most common of which was OUD (26.7%). Nearly 76.6% of patients had a mental health disorder, and anxiety (40.2%) and depression (57.6%) were the most common. Most patients had an opioid prescription 30 days before the index overdose (54.5%), and the average dose was 55.91 MME.
Table 1:
Characteristics of adults with a non-fatal opioid overdose during 2009 to 2016.
| Characteristics | N (%) | Total N=3,922 | No second overdose N=3,639 | Second overdose N=283 | P-value |
|---|---|---|---|---|---|
| Sex | Female | 2283 (58.2%) | 2135 (58.7%) | 148 (52.3%) | 0.036 |
| Male | 1639 (41.8%) | 1504 (41.3%) | 135 (47.7%) | ||
| Race | Black | 370 (9.4%) | 353 (9.7%) | 17 (6.0%) | 0.14 |
| Hispanic | 581 (14.8%) | 537 (14.8%) | 44 (15.5%) | ||
| Other | 478 (12.2%) | 448 (12.3%) | 30 (10.6%) | ||
| White | 2493 (63.6%) | 2301 (63.2%) | 192 (67.8%) | ||
| Neighborhood deprivation index | Quartile 1 (least deprived) | 905 (23.1%) | 835 (22.9%) | 70 (24.7%) | 0.39 |
| Quartile 2 | 1049 (26.7%) | 975 (26.8%) | 74 (26.1%) | ||
| Quartile 3 | 1161 (29.6%) | 1070 (29.4%) | 91 (32.2%) | ||
| Quartile 4 (most deprived) | 807 (20.6%) | 759 (20.9%) | 48 (17.0%) | ||
| Deyo-Charlson score | 0 | 2993 (76.3%) | 2787 (76.6%) | 206 (72.8%) | 0.29 |
| 1 | 292 (7.4%) | 264 (7.3%) | 28 (9.9%) | ||
| 2 | 171 (4.4%) | 160 (4.4%) | 11 (3.9%) | ||
| 3+ | 466 (11.9%) | 428 (11.8%) | 38 (13.4%) | ||
| Tobacco smoking status | Current | 1116 (28.6%) | 1000 (27.6%) | 116 (41.3%) | <0.001 |
| Former | 1014 (26.0%) | 953 (26.3%) | 61 (21.7%) | ||
| Never | 1337 (34.3%) | 1266 (35.0%) | 71 (25.3%) | ||
| Unknown | 432 (11.1%) | 399 (11.0%) | 33 (11.7%) | ||
| Age | 18–44 years | 1607 (41.0%) | 1499 (41.2%) | 108 (38.2%) | 0.019 |
| 45–64 years | 1363 (34.8%) | 1246 (34.2%) | 117 (41.3%) | ||
| 65–74 years | 496 (12.6%) | 458 (12.6%) | 38 (13.4%) | ||
| >75 years | 456 (11.6%) | 436 (12.0%) | 20 (7.1%) | ||
| Any mental health condition | 3006 (76.6%) | 2785 (76.5%) | 221 (78.1%) | 0.55 | |
| Anxiety | 1571 (40.1%) | 1438 (39.5%) | 133 (47.0%) | 0.013 | |
| Bipolar | 601 (15.3%) | 538 (14.8%) | 63 (22.3%) | <0.001 | |
| Depression | 2259 (57.6%) | 2090 (57.4%) | 169 (59.7%) | 0.45 | |
| Developmental disorder | -- | -- | -- | -- | |
| Eating disorder | 34 (0.9%) | -- | -- | -- | |
| Obsessive compulsive disorder | 46 (1.2%) | -- | -- | -- | |
| Panic disorder | 241 (6.1%) | 216 (5.9%) | 25 (8.8%) | 0.051 | |
| Schizophrenia | 98 (2.5%) | -- | -- | -- | |
| Any substance use disorder | 1856 (47.3%) | 1682 (46.2%) | 174 (61.5%) | <0.001 | |
| Opioid use disorder | 1049 (26.7%) | 930 (25.6%) | 119 (42.0%) | <0.001 | |
| Alcohol use disorder | 739 (18.8%) | 682 (18.7%) | 57 (20.1%) | 0.56 | |
| Cannabis use disorder | 352 (9.0%) | 317 (8.7%) | 35 (12.4%) | 0.038 | |
| Amphetamine use disorder | 252 (6.4%) | 220 (6.0%) | 32 (11.3%) | <0.001 | |
| Barbiturate use disorder | 207 (5.3%) | 180 (4.9%) | 27 (9.5%) | <0.001 | |
| Cocaine use disorder | 141 (3.6%) | 126 (3.5%) | 15 (5.3%) | 0.11 | |
| Hallucinogen use disorder | 11 (0.3%) | -- | -- | -- | |
| Multidrug use disorder | 161 (4.1%) | 139 (3.8%) | 22 (7.8%) | 0.001 | |
| Unspecified drug use disorder | 696 (17.7%) | 615 (16.9%) | 81 (28.6%) | <0.001 | |
| Mean daily prescription opioid dose 30 days before first non-fatal overdose (mean/standard deviation) | 55.91 (132.99) | 54.20 (131.99) | 77.94 (143.60) | 0.004 | |
| Mean daily prescription opioid dose 30 days after first non-fatal overdose (mean/standard deviation) | 19.81 (65.91) | 19.78 (66.90) | 20.23 (51.65) | 0.91 | |
| Mean daily prescription opioid dose 30 days before first non-fatal overdose | 0 MME | 1785 (45.5%) | 1673 (46.0%) | 112 (39.6%) | <0.001 |
| 1–<20 MME | 683 (17.4%) | 642 (17.6%) | 41 (14.5%) | ||
| 20–<50 MME | 444 (11.3%) | 414 (11.4%) | 30 (10.6%) | ||
| 50–<100 MME | 400 (10.2%) | 370 (10.2%) | 30 (10.6%) | ||
| ≥100 MME | 610 (15.6%) | 540 (14.8%) | 70 (24.7%) | ||
| Mean daily prescription opioid dose 30 days after first non-fatal overdose | 0 MME | 2426 (61.9%) | 2258 (62.1%) | 168 (59.4%) | 0.80 |
| 1–<20 MME | 671 (17.1%) | 622 (17.1%) | 49 (17.3%) | ||
| 20–<50 MME | 405 (10.3%) | 372 (10.2%) | 33 (11.7%) | ||
| 50–<100 MME | 229 (5.8%) | 213 (5.9%) | 16 (5.7%) | ||
| ≥100 MME | 191 (4.9%) | 174 (4.8%) | 17 (6.0%) | ||
| Prescription opioid dose change based on 30 days before and after first non-fatal overdose | Escalated dose | 432 (11.0%) | 407 (11.2%) | 25 (8.8%) | 0.073 |
| No change in dose | 1593 (40.6%) | 1490 (40.9%) | 103 (36.4%) | ||
| Lowered dose | 1897 (48.4%) | 1742 (47.9%) | 155 (54.8%) | ||
| At least one benzodiazepine prescription 30 days before first non-fatal overdose | 1187 (30.3%) | 1080 (29.7%) | 107 (37.8%) | 0.004 | |
| At least one buprenorphine prescription 30 days before first non-fatal overdose | 48 (1.2%) | -- | -- | -- | |
| At least one benzodiazepine prescription 30 days after first non-fatal overdose | 794 (20.2%) | 723 (19.9%) | 71 (25.1%) | 0.035 | |
| At least one buprenorphine prescription 30 days after first non-fatal overdose | 87 (2.2%) | 76 (2.1%) | 11 (3.9%) | 0.048 | |
| Any outpatient substance use service use 90 days before first non-fatal overdose | 175 (4.5%) | 406 (11.2%) | 38 (13.4%) | 0.25 | |
| Any outpatient substance use service use 90 days after first non-fatal overdose | 444 (11.3%) | 398 (11.2%) | 38 (13.4%) | 0.26 |
-- Indicates cell size ≤10
Patients with a repeat opioid overdose were more likely to be male, between 45–64 years old, and tobacco smokers in the year before the overdose compared to patients without a repeat opioid overdose (Table 1). Patients with a repeat overdose were more likely to have opioid, cannabis, amphetamine, and barbiturate use disorders compared to patients without a repeat overdose. About two-thirds of the patients with a repeat opioid overdose had at least one SUD, and 42.0% had OUD. The mean daily opioid dose in the 30 days before the index overdose was significantly greater for patients who had a repeat opioid overdose compared to patients without a repeat overdose (77.94 MME vs 54.20 MME).
3.1. Factors associated with the risk of repeat opioid-related overdose
In unadjusted models, opioid, cannabis, amphetamine, barbiturate, multidrug use, and unspecified disorders were associated with greater risk of repeat opioid overdose [opioid Hazard Ratio (HR): 2.00, 95% confidence interval (CI): 1.58–2.54; cannabis HR: 1.47, 95% CI: 1.03–2.10; amphetamine HR: 1.93, 95% CI: 1.33–2.80; barbiturate HR: 1.89, 95% CI: 1.27–2.82; multi-drug HR: 2.84, 95% CI: 1.40–3.39; unspecified HR: 1.84, 95% CI: 1.42–2.39] (Table 2). After controlling for demographics, prescription opioid use, benzodiazepine use, and substance use treatment utilization, patients with OUD had a significantly higher risk of a repeat opioid overdose compared to patients without OUD (adjusted HR (aHR): 1.51; 95% CI: 1.13–2.01) (Table 2). Patients with an unspecified drug use disorder had a significantly higher risk of a repeat opioid overdose compared to patients without an unspecified drug use disorder (aHR: 1.41, 95% CI: 1.04–1.91). Alcohol, cannabis, barbiturate, cocaine, and multidrug substance use disorders were not significantly associated with the one-year risk of a repeat opioid overdose.
Table 2.
Unadjusted and Adjusted Hazard Ratios for the second opioid related overdose one year after a non-fatal opioid-related overdose.
| Unadjusted Hazard Ratio | 95% Confidence Interval | P-value | Adjusted Hazard Ratio | 95% Confidence Interval | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Opioid use disorder | 2.00 | 1.58 | 2.54 | <.0001 | 1.51 | 1.13 | 2.01 | 0.0054 |
| Alcohol use disorder | 1.10 | 0.82 | 1.47 | 0.54 | 0.86 | 0.61 | 1.20 | 0.37 |
| Cannabis use disorder | 1.47 | 1.03 | 2.10 | 0.034 | 1.08 | 0.74 | 1.59 | 0.69 |
| Amphetamine use disorder | 1.93 | 1.33 | 2.80 | 0.0006 | 1.29 | 0.83 | 1.99 | 0.26 |
| Barbiturate use disorder | 1.89 | 1.27 | 2.82 | 0.0018 | 1.11 | 0.71 | 1.76 | 0.64 |
| Cocaine use disorder | 1.48 | 0.88 | 2.51 | 0.14 | 1.02 | 0.58 | 1.81 | 0.95 |
| Hallucinogen use disorder | 1.22 | 0.17 | 8.89 | 0.84 | -- | -- | -- | -- |
| Multi drug use disorder | 2.18 | 1.40 | 3.39 | 0.0006 | 1.47 | 0.90 | 2.40 | 0.12 |
| Unspecified drug use disorder | 1.84 | 1.42 | 2.39 | <.0001 | 1.41 | 1.04 | 1.91 | 0.028 |
| Buprenorphine use after index overdose | 1.28 | 0.60 | 2.72 | 0.52 | 0.71 | 0.30 | 1.72 | 0.45 |
| Outpatient substance use service use after index overdose | 1.75 | 1.20 | 2.55 | 0.0039 | 1.54 | 0.97 | 2.45 | 0.068 |
Adjusted for age, race, sex, neighborhood deprivation index, Charlson comorbidity score, anxiety, depression, other mental health disorder (bipolar disorder, developmental disorder, eating disorder, obsessive compulsive disorder, panic disorder, and schizophrenia) daily opioid dose before and after index overdose, dose change, benzodiazepine use before and after index overdose
3.2. Substance use treatment utilization before and after the index overdose
In the month before the index overdose, 4.42% of the cohort had at least one outpatient substance use service visit (Figure 1). In the first month after the index overdose, the utilization rate of outpatient substance use services increased to 11.28%. Similarly, 1.23% of the cohort received at least one day of buprenorphine in the month before the index overdose (Figure 1). After the index overdose, the monthly rate of any buprenorphine use increased to 2.32%. The peak buprenorphine rate (2.84%) after the index overdose occurred at month 3.
Figure 1: Observed and predicted rate of substance treatment utilization before and after a non-fatal opioid-related overdose.
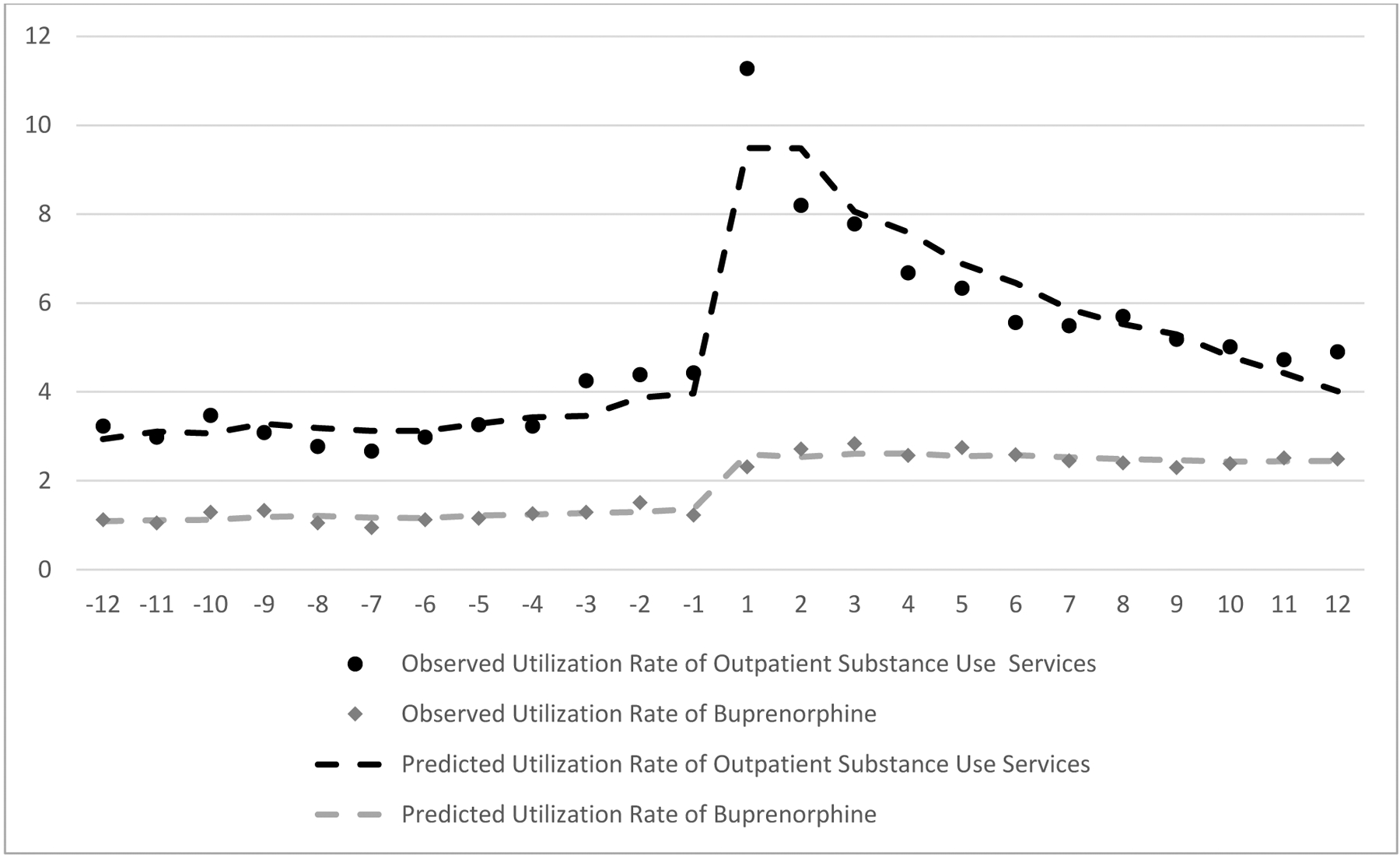
Negative months indicate months before the index opioid overdose.
Positive months indicate months after the index opioid overdose.
Zero indicates date of the index overdose
At baseline, 3.76% patients used outpatient substance use services and 1.34% received buprenorphine (Table 3). Compared to baseline, a NFOO was significantly associated with a 5.94% [Standard error (SE): 0.77%] absolute increase in the outpatient substance use service utilization rate and a 1.29% [SE: 0.15%] absolute increase in the buprenorphine utilization rate. A NFOO was associated with a significant downward trend of 0.55% [SE: 0.12%] in the utilization rate of outpatient substance use services. The trend of buprenorphine utilization after the overdose was not significantly associated with a NFOO.
Table 3.
Change in level and trend of substance use treatment utilization associated with a non-fatal opioid overdose
| Outpatient substance use services | Buprenorphine | |||||
|---|---|---|---|---|---|---|
| Slope | Standard Error | P-value | Slope | Standard Error | P-value | |
| Baseline | 3.76 | 0.61 | <.0001 | 1.34 | 0.11 | <.0001 |
| Month | 0.069 | 0.081 | 0.41 | 0.021 | 0.015 | 0.19 |
| Post | 5.94 | 0.77 | <.0001 | 1.29 | 0.15 | <.0001 |
| Post * month | −0.55 | 0.12 | 0.0002 | −0.037 | 0.022 | 0.12 |
Coefficients are from the interrupted time series analysis. The baseline indicates the intercept, the month coefficient is the slope of pre-overdose trend in the utilization rate, the coefficient of the post variable is the level of change in the utilization rate after the overdose, and coefficient of the interaction between the month and post variable is the change in trend of the utilization rate after the overdose.
4. DISCUSSION
SUDs, especially OUD, were common among patients with a NFOO. The prevalence of SUDs in our study population was slightly higher than the prevalence of SUDs in a national cohort of privately insured patients with more than 90 days of prescription opioid use and a NFOO (Larochelle et al., 2016). The higher prevalence may be because our definition of SUDs was more inclusive, using a year for the lookback period, rather than 90 days (Larochelle et al., 2016). The higher prevalence of SUDs may also reflect use of substance use screening practices in integrated health systems, which may provide more opportunities to diagnose patients. Many health systems, including this one, have begun to implement strategies to support screening for hazardous substance use (Richards et al., 2019; Williams et al., 2010), and increasing provider training and sensitivity to such problems may result in more discussions about substance misuse, including opioid use (Williams et al., 2015; Williams et al., 2016).
OUD specifically was associated with higher risk of a repeat opioid overdose, even after controlling for opioid use, benzodiazepine use, substance use treatment, comorbidities, and the NDI. This is consistent with findings from study of Medicaid recipients (Olfson et al., 2018), providing additional evidence that OUD is a significant risk factor for repeat opioid-related overdose. This finding aligns with studies that found that OUD is a risk factor for an initial opioid-related overdose (Campbell et al., 2018; Zedler et al., 2014). The reasons underlying an OUD diagnosis are unavailable in EHR data but documenting the events or patient behaviors that trigger an OUD diagnosis is important for overdose prevention strategies.
While OUD had the strongest association with the risk of repeat opioid overdose, interestingly there were no significant associations between alcohol, cannabis, amphetamine, barbiturate, and cocaine use disorders and risk of repeat opioid overdose. This is inconsistent with previous studies that found SUDs are associated with greater risk of initial and repeat opioid-related overdose (Campbell et al., 2018; Olfson et al., 2018). The differences may be attributed to the type of study population since we focused on a high-risk subset of commercially insured patients who had already experienced a non-fatal, opioid-related overdose (Campbell et al., 2018; Olfson et al., 2018). Among this population, when risk is already high, other SUDs may not show a significant association. Questions remain about how individual SUDs may contribute to repeat overdose in other populations, especially given the significant associations observed for other SUDs with an initial opioid-related overdose (Campbell et al., 2018).
The higher risk of repeat opioid overdose associated with an unspecified drug use disorder may indicate use of multiple, unrecognized drugs; patients with this diagnosis had other drug use diagnoses. Many opioid-related overdose deaths involve multiple substances (Barocas et al., 2019; Yarborough et al., 2016). About 36% of opioid-related overdose deaths involved opioids and a stimulant (e.g., cocaine or amphetamines), and 47% involved opioids and other substances (e.g. cannabis, benzodiazepines, gabapentin, and alcohol) (Barocas et al., 2019). Given the lack of specificity of the drug use disorder diagnosis and the frequency of polysubstance overdose, health systems should continue to improve screening processes for drug use disorders and for substances involved in opioid overdoses (Dowell et al., 2016; Herring et al., 2019).
A NFOO represents an opportunity to intervene with patients who need treatment by improving care processes. Like other studies in different populations, we found low substance use treatment utilization and retention rates in this high-risk group seeking care in an integrated health system (Frazier et al., 2017; Larochelle et al., 2018; Larochelle et al., 2016; Morgan et al., 2018). Encouragingly, we found that the highest rates for outpatient substance use services occurred one month after the index overdose (11.28%), and for buprenorphine treatment, 3 months after the overdose (2.84%), although rates for both subsequently decreased. Longer retention in treatment and on medication is associated with improved outcomes, and thus the decrease in treatment utilization over time raises the critical question of how to keep patients engaged in treatment (Fareed et al., 2012; Ward et al., 1999). While patients in integrated systems may be less likely to encounter barriers to accessing substance use treatment such as lack of insurance coverage or access to providers, additional barriers such as cost, stigma, and patient treatment preferences, persist and may account for low substance use treatment utilization. (Gordon et al., 2011; Hutchinson et al., 2014).
In addition to substance use treatment for OUD following a NFOO, a NFOO may present an opportunity to provide patients with naloxone, a medication used to reverse an opioid overdose and prevent a fatality (Dowell et al., 2016). Naloxone has been under prescribed, and previous research showed only 1.5% of commercially insured patients with opioid misuse, dependence, or overdose received naloxone prescriptions (Follman et al., 2019). We were unable to determine naloxone use during the study period because naloxone had not been widely distributed. Recent legislation in California now requires a prescription for naloxone for patients with an opioid overdose (Medical Board of California) but lack of knowledge about naloxone, cost, perception of risk of overdose, and fear of changes to current opioid treatment may still impede access to naloxone (Mueller et al., 2017). Therefore, a NFOO may be an important opportunity to identify patients who need naloxone.
Finally, a NFOO may be an opportunity to consider tapering prescription opioid therapy. We found that 38.1% of patients with a NFOO had opioid prescriptions after the overdose, which is considerably lower than estimates from the literature, and may reflect better care coordination in an integrated health care system. A previous study of patients with chronic opioid therapy (>90 days) indicated that 78% continued to receive opioid prescriptions after a NFOO (Larochelle et al., 2016). In the study cohort, nearly half of the patients with a NFOO had a lower dose after the index overdose. Prescription opioid use after an opioid overdose is common and not unexpected given the complexities and time required to appropriately taper patients with chronic opioid treatment. We note that our measure of opioid use post-overdose was brief (30 days), and patients on chronic opioid therapy often require longer periods to be tapered appropriately. A taper that is too rapid can be associated with psychological distress and suicidality (Dowell et al., 2016; Kroenke et al., 2019; US Food and Drug Administration, 2019)
This observational study had several limitations. Unobserved variables (e.g., income, patient preferences, lifetime history of opioid-related overdose, and prescriber knowledge and attitudes towards SUDs) could confound the relationship between SUD diagnoses and risk of repeat opioid overdose. SUDs may be underdiagnosed, and patients who may meet diagnostic criteria before the index date may not actually be diagnosed until after the index overdose. Underdiagnosis could make it less likely to observe the true association between SUDs and risk of repeat opioid overdose. In addition, misclassification could occur since we could not determine the reasons for an OUD diagnosis, which may include multiple behaviors regarding opioid use and symptoms. To assess the population level risk of opioid overdose, all patients identified with an opioid-related overdose were included in this study. Nearly half of the cohort did not have a recent prescription for an opioid dispensed through the health system at the time of the overdose, which increases the likelihood that these patients used opioids that were stockpiled and used later, or illicitly obtained, rather than dispensed through the health system. We did not have the underlying cause of death for patients who died in 2016 which could result in undercounting the number of fatal opioid-related overdoses. Results may not be generalizable to populations that are not commercially insured or in an integrated care setting, although KPNC has a diverse patient population.
5. CONCLUSION
As the rate of NFOO increases, preventing opioid overdoses is a priority for health care systems and policymakers. The high prevalence of SUDs among patients with NFOO underscores their complex treatment needs. Of the SUDs, we found that only OUD is associated with a significant risk of repeat opioid overdose. Future research should examine the events and/or behaviors that trigger an OUD diagnosis and how these may be associated with opioid overdoses. Utilization rates for outpatient substance use services and buprenorphine were low before and after a NFOO. Many patient, provider, and health care system barriers may contribute to low utilization rates despite better access and insurance coverage of treatment in integrated health systems. Patients who survive a NFOO may need substance use treatment and naloxone to prevent future overdoses. Future research should explore care coordination efforts for substance use treatment and naloxone to improve outcomes for patients with NFOO.
Supplementary Material
Highlights.
Patients with opioid use disorder had a higher 1-year risk of repeat overdose.
Substance use service and buprenorphine use rates were low before the index overdose.
Overdose was associated with higher substance use treatment rates post-overdose.
After the index overdose, substance use treatment rates increased and then declined.
Acknowledgements:
This work was funded by The Permanente Medical Group and the National Institute on Drug Abuse, Clinical Trials Network, UG1DA040314-01S1.
List of abbreviations:
- aHR
Adjusted Hazard Ratio
- CI
95% Confidence Interval
- EHR
Electronic Health Record
- ICD-9
International Classification of Disease, 9th Revision
- ICD-10
International Classification of Disease, 10th Revision, Clinical Modification
- KPNC
Kaiser Permanente of Northern California
- MME
morphine milligrams equivalent
- MOUD
Medications for opioid use disorder
- NDC
National Drug Code
- NFOO
Non-fatal opioid overdose
- OUD
opioid use disorder
- SD
Standard Deviation
- SUD
substance use disorders
- VDW
Virtual Data Warehouse
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Ruchir Karmali received funding from The Permanente Medical Group (TPMG) Delivery Science Fellowship Program. Thomas Ray has received research support on grants to the Kaiser Permanente Division of Research in the past 3 years from Pfizer. Both he and Cynthia Campbell have received research support through their institution from the Industry PMR Consortium, a consortium of companies working together to conduct post-marketing studies required by the Food and Drug Administration that assess known risks related to opioid analgesic use. The remaining authors report no conflict of interest.
REFERENCES
- Barocas JA, Wang J, Marshall BD, Larochelle MR, Bettano A, Bernson D, Beckwith CG, Linas BP, Walley AY, 2019. Sociodemographic factors and social determinants associated with toxicology confirmed polysubstance opioid-related deaths. Drug Alcohol Depend. 200, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC, 2012. Risk of Death From Accidental Overdose Associated With Psychiatric and Substance Use Disorders. Am. J. Psychiat(169), 64–70. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Kirchner HL, Pitcavage JM, Nadipelli VR, Ronquest NA, Fitzpatrick MH, Han JJ, 2016. Factors associated with opioid overdose: a 10-year retrospective study of patients in a large integrated health care system. Subst. Abuse. Rehabil 7, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JE, Giglio R, Keyes KM, DiMaggio C, Li G, 2017. Risk markers for fatal and non-fatal prescription drug overdose: a meta-analysis. Inj. Epidemiol 4(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CI, Bahorik AL, VanVeldhuisen P, Weisner C, Rubinstein AL, Ray GT, 2018. Use of a prescription opioid registry to examine opioid misuse and overdose in an integrated health system. Prev. Med 110, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K, 2016. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 162, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Annual Surveillance Report of Drug-Related Risks and Outcomes - United States. Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published August 31, 2018. Accessed 3/2/2019 from https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf.
- Deyo RA, Cherkin DC, Ciol MA, 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 45(6), 613–619. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm. Rep 65(1). [DOI] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Casarella J, Drexler K, 2012. Effect of buprenorphine dose on treatment outcome. J. Addict. Dis 31(1), 8–18. [DOI] [PubMed] [Google Scholar]
- Follman S, Arora VM, Lyttle C, Moore PQ, Pho MT, 2019. Naloxone Prescriptions Among Commercially Insured Individuals at High Risk of Opioid Overdose. JAMA Netw. Open 2(5), e193209–e193209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W, Cochran G, Lo-Ciganic W-H, Gellad WF, Gordon AJ, Chang C-CH, Donohue JM, 2017. Medication-Assisted Treatment and Opioid Use Before and After Overdose in Pennsylvania Medicaid. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant AR, Goebel JJ, 1976. Nonlinear regression with autocorrelated errors. JASA. 71(356), 961–967. [Google Scholar]
- Gordon AJ, Kavanagh G, Krumm M, Ramgopal R, Paidisetty S, Aghevli M, Goodman F, Trafton J, Liberto J, 2011. Facilitators and barriers in implementing buprenorphine in the Veterans Health Administration. Psychol. Addict. Behav 25(2), 215. [DOI] [PubMed] [Google Scholar]
- Gordon N, 2012. Statistics from the 2007 California Health Interview Survey. Kaiser Permanente Division of Research. (Accessed 3/26/2018, 2018, at https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2007.pdf.).
- Herring AA, Perrone J, Nelson LS, 2019. Managing Opioid Withdrawal in the Emergency Department With Buprenorphine. Ann. Emerg. Med 73(5), 481–487. [DOI] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CHA, Baldwin L-M, Rosenblatt RA, 2014. Barriers to primary care physicians prescribing buprenorphine. Ann. Fam. Med 12(2), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, Grusing S, Devine B, Chou R, 2017. Primary care-based models for the treatment of opioid use disorder: A scoping review. Ann. Intern. Med 166(4), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyawala N, Landis R, Barry CL, Stein BD, Saloner B, 2019. Changes in Outpatient Services and Medication Use Following a Non-fatal Opioid Overdose in the West Virginia Medicaid Program. J. Gen. Intern. Med 34(6), 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, Haake KJ, Hanling S, Hooten WM, Kertesz SG, 2019. Challenges with implementing the centers for disease control and prevention opioid guideline: a consensus panel report. Pain Med. 20(4), 724–735. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann. Intern. Med 169(3), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF, 2016. Opioid Prescribing After Nonfatal Overdose and Association With Repeated Overdose: A Cohort Study. Ann. Intern. Med 164(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Medical Board of California, AB 2760 (Wood, Chapter 324, Statutes of 2018) - Frequently Asked Questions https://www.mbc.ca.gov/Download/Documents/AB2760FAQs.pdf. (Accessed 2/10/2020).
- Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, Elo I, Burke JG, O’Campo P, 2006. The development of a standardized neighborhood deprivation index. J. Urban. Health 83(6), 1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY, 2018. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J. Subst. Abuse Treat 85, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP, 2019. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 200, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SR, Koester S, Glanz JM, Gardner EM, Binswanger IA, 2017. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J. Gen. Intern. Med 32(3), 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control, 2017. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2017 version. Atlanta, GA: Centers for Disease Control and Prevention; ; [Accessed February 26, 2018]. https://www.cdc.gov/drugoverdose/resources/data.html [Google Scholar]
- Olfson M, Wall M, Wang S, Crystal S, Blanco C, 2018. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug Alcohol Depend. 190, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TW, Lin LA, Hosanagar A, Kogowski A, Paige K, Bohnert AS, 2016. Understanding Risk Factors for Opioid Overdose in Clinical Populations to Inform Treatment and Policy. J. Addict. Med Nov/Dec(10), 6. [DOI] [PubMed] [Google Scholar]
- Penfold RB, Zhang F, 2013. Use of interrupted time series analysis in evaluating health care quality improvements. Acad. Pediatr 13(6), S38–S44. [DOI] [PubMed] [Google Scholar]
- Ranapurwala SI, Naumann RB, Austin AE, Dasgupta N, Marshall SW, 2019. Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiology and drug safety 28(1), 4–12. [DOI] [PubMed] [Google Scholar]
- Richards JE, Bobb JF, Lee AK, Lapham GT, Williams EC, Glass JE, Ludman EJ, Achtmeyer C, Caldeiro RM, Oliver M, 2019. Integration of screening, assessment, and treatment for cannabis and other drug use disorders in primary care: an evaluation in three pilot sites. Drug Alcohol Depend. Aug 1(201), 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF, 2014. The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. EGEMS (Wash DC). 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2019. Drug and opioid-involved overdose deaths-United States, 2013–2017. MMWR. 67(5152), 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, de González AB, Best AF, Chen Y, Chernyavskiy P, Hartge P, Khan SQ, Pérez-Stable EJ, Rodriquez EJ, Spillane S, 2019. Premature mortality from all causes and drug poisonings in the USA according to socioeconomic status and rurality: an analysis of death certificate data by county from 2000–15. Lancet Public Health 4(2), e97–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 357, j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp LP, 2018. Stata. College Station, TX. [Google Scholar]
- U.S.Census Burea, 2008. A Compass for Understanding and Using American Community Survey Data: What General Data Users Need to Know. https://www.census.gov/content/dam/Census/library/publications/2008/acs/ACSGeneralHandbook.pdf. (Accessed 1/28/2016 2016).
- US Food and Drug Administration, 2019. FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. https://www.fda.gov/Drugs/DrugSafety/ucm635038.htm. (Accessed December 20 2019).
- Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C, 2008. De facto long-term opioid therapy for noncancer pain. Clin. J. Pain 24(6), 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Hall W, Mattick RP, 1999. Role of maintenance treatment in opioid dependence. Lancet 353(9148), 221–226. [DOI] [PubMed] [Google Scholar]
- Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L, 2016. Opioid-related inpatient stays and emergency department visits by state, 2009–2014: statistical brief# 219. HCUP Statistical Brief. [Google Scholar]
- Williams EC, Achtmeyer CE, Kivlahan DR, Greenberg D, Merrill JO, Wickizer TM, Koepsell TD, Heagerty PJ, Bradley KA, 2010. Evaluation of an electronic clinical reminder to facilitate brief alcohol-counseling interventions in primary care. J. Stud. Alcohol Drugs 71(5), 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, Bradley KA, 2015. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J. Gen. Intern Med 30(8), 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Young JP, Rittmueller SE, Ludman EJ, Lapham GT, Lee AK, Chavez LJ, Berger D, Bradley KA, 2016. Local implementation of alcohol screening and brief intervention at five Veterans Health Administration primary care clinics: perspectives of clinical and administrative staff. J. Subst. Abuse Treat 60, 27–35. [DOI] [PubMed] [Google Scholar]
- Yarborough BJH, Stumbo SP, Janoff SL, Yarborough MT, McCarty D, Chilcoat HD, Coplan PM, Green CA, 2016. Understanding opioid overdose characteristics involving prescription and illicit opioids: A mixed methods analysis. Drug Alcohol Depend. 167, 49–56. [DOI] [PubMed] [Google Scholar]
- Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, Baser O, Murrelle L, 2014. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 15(11), 1911–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


