Graphical abstract
Keywords: Deep learning, COVID-19, Disease, Classification, Chest CT images
Abstract
The COVID-19 infection is increasing at a rapid rate, with the availability of limited number of testing kits. Therefore, the development of COVID-19 testing kits is still an open area of research. Recently, many studies have shown that chest Computed Tomography (CT) images can be used for COVID-19 testing, as chest CT images show a bilateral change in COVID-19 infected patients. However, the classification of COVID-19 patients from chest CT images is not an easy task as predicting the bilateral change is defined as an ill-posed problem. Therefore, in this paper, a deep transfer learning technique is used to classify COVID-19 infected patients. Additionally, a top-2 smooth loss function with cost-sensitive attributes is also utilized to handle noisy and imbalanced COVID-19 dataset kind of problems. Experimental results reveal that the proposed deep transfer learning-based COVID-19 classification model provides efficient results as compared to the other supervised learning models.
1. Introduction
The first case of novel coronavirus disease (COVID-19) was reported in Wuhan, China at the end of 2019. Thereafter, it turned out to be a COVID-19 outbreak in the entire world. The main objective of this paper is to automatically classify COVID-19 infected persons from their chest CT images [1]. Chest CT images can be used to classify COVID-19 patients and can be used for COVID-19 testing. As all hospitals have CT imaging machines, thus, CT based COVID-19 classification can be implemented to test COVID-19 infected patients at a good speed. However, it requires an expert doctor and additional time to predict COVID-19 infected patients from chest CT images. Thus, supervised learning models can be used for COVID-19 patients classification from their respective chest CT images [2].
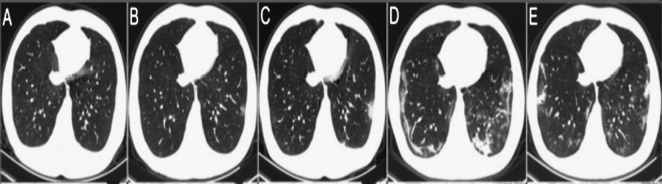
Fig. 1 shows the changes in chest CT of COVID-19 affected patients within six days. Initially, there is no change in the chest CT image and its normal behavior. But as infection increases day by day, it is shown that the bilateral changes take place. For an increase in the number of days, chest CT images show the progression of pneumonia with mixed ground-glass opacities and linear opacities in the subpleural area.
Fig. 1.
Chest CT image of COVID-19 infected patient: (a) When the patient come for checkup and (b) to (d) Chest CT images taken on day 2, 3, 4, and 5, respectively.
COVID-19 is a challenging and serious worldwide issue. There exist several open online databases of chest CT and chest X-ray datasets. These datasets can be utilized for building classification models. Due to the high amount of pandemics dataset, it becomes more challenging of a selection of appropriate datasets for building the classification models. Therefore, in this paper, the COVID-19 dataset of chest CT images is selected as these images show bilateral change if the given patient is infected from COVID-19 infection.
As already discussed, the chest CT images may be used to diagnose COVID-19 infection. The chest CT images have high specificity and low sensitivity for classifying the COVID-19 related lung opacities [3]. However, the main objective is to understand the diagnostic accuracy and limitations of chest CT images in COVID-19 to improve the quality of patient management by ensuring an appropriate balance between the practicality of chest CT images versus better diagnostic performance of CT scans [3].
In this paper, a COVID-19 classification model is implemented. The main novelty of this paper is:
-
1.
The deep transfer learning model is used to classify COVID-19 infected patients by considering their chest CT images.
-
2.
The cost-sensitive top-2 smooth loss function is also utilized to enhance the results further.
-
3.
The deep transfer learning model is trained on a benchmark open dataset of chest CT images.
-
4.
Comparisons are also drawn by considering some well-known supervised learning models.
The remaining paper is organized as Sec. 2 discusses the various supervised learning techniques which can be used to classify the COVID-19 infected patients. The proposed deep transfer learning-based COVID-19 infected model is discussed in Sec. 3. Experimental results are depicted in Sec. 4. Conclusions are presented in Sec. 5.
2. Related work
With the advancement in medical image processing techniques, the development of intelligent prediction and diagnosis tools increased at a rapid rate [4]. Machine learning techniques are widely accepted as a prominent tool to improve the prediction and diagnosis of many diseases [5], [6]. However, efficient feature extraction techniques are required to obtain better machine learning models. Therefore, deep learning models are widely accepted in medical imaging systems because they can extract features automatically or by using some pre-trained networks such as ResNet [7].
Li et al. used the conventional neural network to classify the COVID-19 infected patients form chest CT images. Nardelli et al. [8] used 3-D CNN to classify the pulmonary artery–vein from chest CT images. Shin et al. [9] used deep CNN to classify the interstitial lung disease in CT images. Xie et al. [10] classified the benign-malignant lung nodule using knowledge-based collaborative deep learning on chest CT. It achieves higher accuracy for lung nodule classification.
Hagerty et al. [11] classified the melanoma dermoscopy images using deep learning with remarkable accuracy. Gerard et al. [12] detected the pulmonary fissure in CT using a supervised discriminative learning framework. Setio et al. [13] used multi-view convolutional networks to detect the pulmonary nodules in CT images. Xia et al. [14] used deep adversarial networks to perform segmentation on abdominal CT images. Pezeshk et al. [15] used 3-D CNN to detect the pulmonary nodules in Chest CT.
Zreik et al. [16] implemented a classification technique using recurrent CNN to classify the Coronary Artery Plaque and Stenosis in Coronary CT. Li et al. utilized 3D fully CNN to fuse multimodality information for tumor segmentation in CT. Bhandary et al. [17] proposed a technique to detect the lung abnormality using deep learning framework. Gao et al. [18] used 3D block-based residual deep learning network to predict the severity levels of tuberculosis in CT pulmonary images.
Singh et al. [19], [20] designed particle swarm optimization based Adaptive Neuro-Fuzzy Inferences Systems (ANFIS) to improve the classification rate. Zeng et al. implemented gated bi-directional convolutional neural networks (GCNN) [21]. GCNN can be used to classify COVID-19 infected patients.
From the extensive review, it is found that the deep learning model may achieve significant results for COVID-19 disease classification from chest CT images. The deep learning models may achieve significant results, but results can be improved further by using efficient feature extraction techniques such as variants of ResNet [22]. Additionally, hyper-tuning of the deep learning models can be achieved using transfer learning. Therefore, the development of novel Deep Transfer Learning (DTL) based COVID-19 infected patient classification model is the main motivation for this work.
3. Proposed model
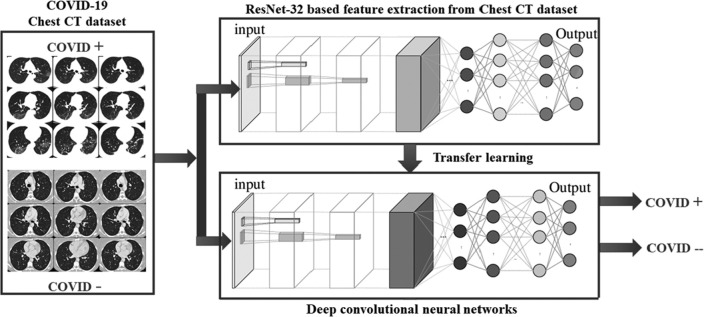
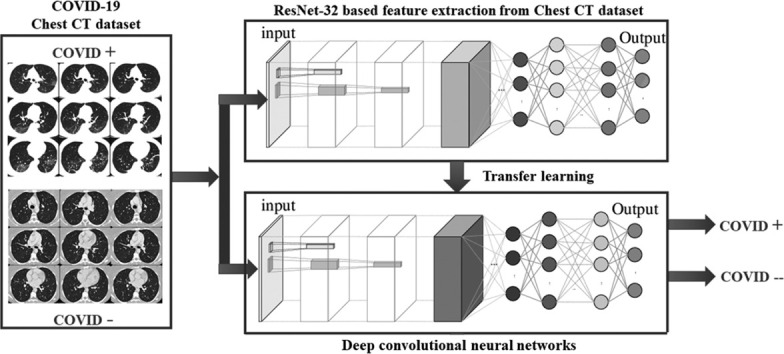
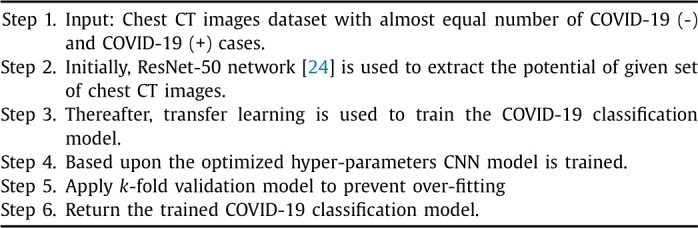
In this paper, deep transfer learning implemented in [23] is considered. Fig. 2 shows the diagrammatic flow of the proposed model. The major steps of the proposed model is shown in Algorithm 1 .
Fig. 2.
Proposed deep transfer learning (DTL) based COVID-19 classification model.
Algorithm 1.
Deep transfer learning based COVID-19 classification [24].
3.1. Transfer learning with deep residual networks
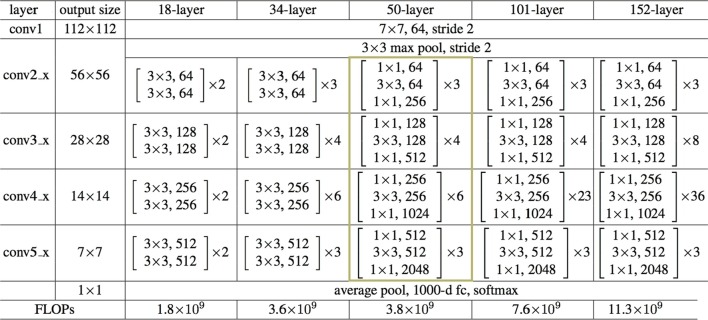
In this work, a ResNet-50 network is discussed. Fig. 3 shows the architectural requirement so ResNet-50. It can extract potential features of chest CT images. Transfer learning is used to tune the initial parameter of deep layers. The ImageNet pre-trained model is popular as a transferred source. Deep Transfer Learning (DTL) [23] is used to train the COVID-19 classification model.
Fig. 3.
Architectural requirements of ResNet-50.
3.2. Convolutional neural networks
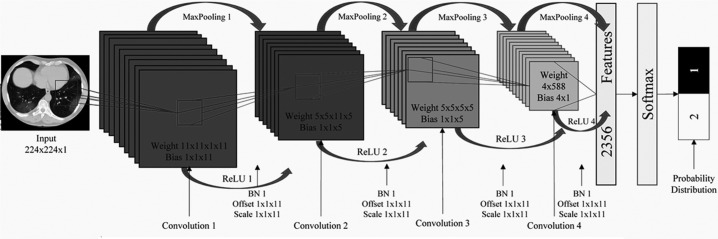
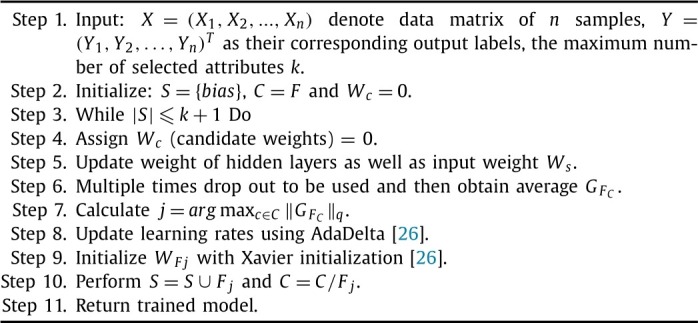
To classify the patients as COVID-19 (+) or COVID-19 (-), a Convolutional Neural Network (CNN) is used. The complete structure of the utilized CNN is shown in Table 1 and step by step working is depicted in Algorithm 2 .
Table 1.
Proposed architecture of convolutional neural network.
Algorithm 2.
Convolutional neural networks.
In this paper, Softmax is used as an activation function. The remaining parameters are set to be bias initialization = 1.0, normal distribution is used as a distribution function, learning rate is set to be 0.2, bias learning rate is set to be 0.02, momentum = 0.8, gradient normalization threshold [25] is set to be 1.0, loss MCXENT is used as loss function [26], [27].
Where S states selected set. C defines candidate set. . defines input weights. Elected input weights is evaluated using . defines selected candidate weights. shows gradient to . define to elect single feature such as jth from C. and define that S and C need to be updated by aggregation or removal of j, respectively (for more details please see [26]).
4. Performance analyses
This section provides comparative analyses of the proposed COVID-19 classification model over the existing models. A 10-fold cross-validation in all the models is used to prevent overfitting issues. The training and testing ratio of the dataset is set to be 60% and 40%, respectively. Out of 60% training data, 10% of data is used for validation purposes. The Deep Transfer Learning (DTL) is used to build a COVID-19 infected patients classification model. Epochs of classification models are set to be 110.
4.1. Chest CT images dataset
In this paper, images are collected from various datasets such as from [28] and [2], there are 413 COVID-19 (+) images and 439 images of normal or pneumonia infected patients images.
4.2. Comparative analyses
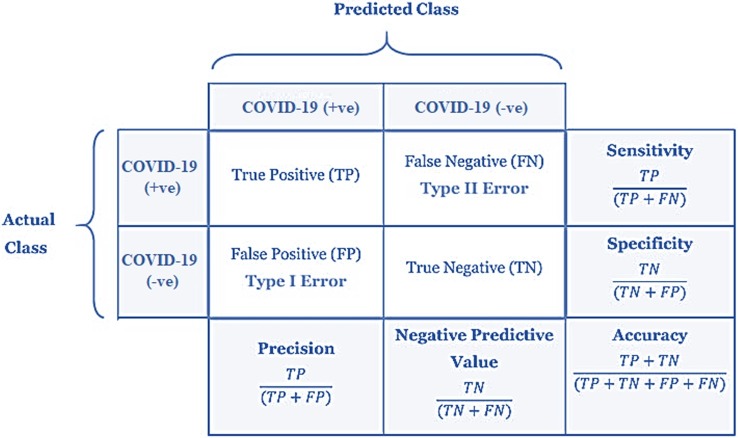
Confusion matrix-based performance metrics are used for evaluating the significant improvement of the proposed COVID-19 classification model over the competitive supervised COVID-19 classification models (see Fig. 4 ). These metrics include accuracy, sensitivity, specificity, precision, and negative predictive value.
Fig. 4.
Confusion matrix based performance metrics used for comparative analyses.
The training and validation analyses of the proposed DTL and GCNN models are shown in Fig. 5 . It clearly shows that the proposed DTL based COVID-19 classification model achieves good training and validation accuracy as compared to the GCNN model.
Fig. 5.
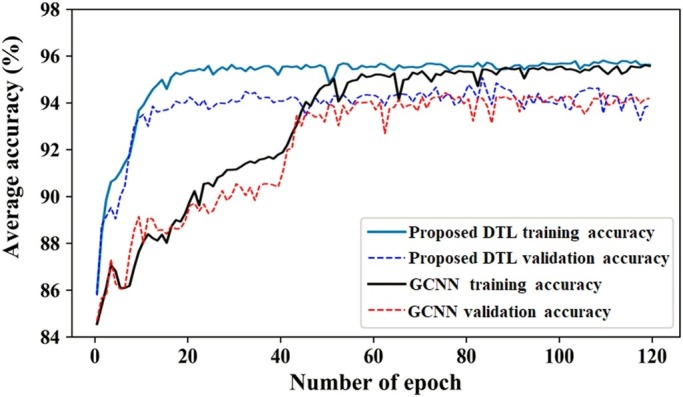
Training and validation accuracy analyses of deep transfer learning (DTL) and gated bi-directional convolutional neural networks (GCNN) models.
Table 2, Table 3 represent the training and validation based comparative analyses between the proposed and existing classification models when to the COVID-19 dataset. Accuracy, sensitivity, specificity, precision, and negative predictive value measures are used to compute the significant performance of the proposed COVID-19 classification model. It is found that the proposed models achieve significantly good values as compared to the existing supervised models. Also, there is not much difference between the training and validation analyses, therefore, the COVID-19 classification models are trained efficiently. Therefore, the proposed model can be more reliable and can be used as an alternative to the various COVID-19 testing kits. Also, since the CT imaging systems are already established in the hospitals, it can provide results at rapid speed.
Table 2.
Training analyses of the COVID-19 classification models by considering confusion matrix and various performance metrics.
| Model | TP | FP | TN | FN | Precision | NPV | Sn | Sp | Acc |
|---|---|---|---|---|---|---|---|---|---|
| ANN | 234 | 36 | 239 | 21 | 0.866667 | 0.919231 | 0.917647 | 0.869091 | 0.892453 |
| ANFIS | 245 | 25 | 241 | 19 | 0.907407 | 0.926923 | 0.92803 | 0.906015 | 0.916981 |
| CNN | 247 | 23 | 235 | 25 | 0.914815 | 0.903846 | 0.908088 | 0.910853 | 0.909434 |
| DTL | 256 | 14 | 238 | 22 | 0.948148 | 0.915385 | 0.920863 | 0.944444 | 0.932075 |
| Proposed | 264 | 6 | 246 | 14 | 0.977778 | 0.946154 | 0.94964 | 0.97619 | 0.962264 |
Table 3.
Validation analyses of the COVID-19 classification models by considering confusion matrix and various performance metrics.
| Model | TP | FP | TN | FN | Precision | NPV | Sn | Sp | Acc |
|---|---|---|---|---|---|---|---|---|---|
| ANN | 223 | 47 | 228 | 32 | 0.825925926 | 0.876923077 | 0.874509804 | 0.829090909 | 0.850943396 |
| ANFIS | 238 | 32 | 229 | 31 | 0.881481481 | 0.880769231 | 0.884758364 | 0.877394636 | 0.881132075 |
| CNN | 236 | 34 | 227 | 33 | 0.874074074 | 0.873076923 | 0.877323420 | 0.869731801 | 0.873584906 |
| DTL | 250 | 20 | 231 | 29 | 0.925925926 | 0.888461538 | 0.896057348 | 0.920318725 | 0.907547170 |
| Proposed | 257 | 13 | 236 | 24 | 0.951851852 | 0.907692308 | 0.914590747 | 0.947791165 | 0.930188679 |
5. Conclusion
In this paper, a Deep Transfer Learning (DTL) technique is used to build a COVID-19 infected patient's classification model. 10-fold cross-validation was used to prevent overfitting issues. The training and testing ratio of the dataset was set as 60% and 40%, respectively. Out of 60% training data, 10% of data was utilized for validation purposes. Experimental results have shown that the proposed deep transfer learning-based COVID-19 classification model achieves efficient results as compared to the other supervised learning models. The proposed model achieves training and testing accuracy up to 96.2264% and 93.0189%, respectively. Therefore, the proposed model can be used as an alternative to COVID-19 testing kits. Further, a novel and lightweight deep learning model that can more effectively reduce network attributes can be designed. In this paper, the optimal selection of hyper-parameters is not considered, therefore, in near future various algorithms such as genetic algorithm [29], non-dominated sorting genetic algorithm-III [30], [31], parallel Strength Pareto Evolutionary Algorithm-II [32], memetic differential evolution, [33], etc., can be used to tune the hyper-parameters of the proposed model.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
CRediT authorship contribution statement
Y. Pathak: Conceptualization, Methodology, Software. P.K. Shukla: Data curation, Writing – original draft. A. Tiwari: Investigation, Visualization. S. Stalin: Supervision. S. Singh: Software, Validation. P.K. Shukla: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial or personal relationships that could be viewed as influencing the work reported in this paper.
Acknowledgements
We would like to acknowledge the authors (Dr K.V. Arya, Dr Prashant Singh Rana, Dr Shailendra Tiwari), whose work motivated us and shows a road map for the current research summarized in this paper.
References
- 1.Hemdan E.E.-D., Shouman M.A., Karar M.E. Covidx-net: a framework of deep learning classifiers to diagnose covid-19 in x-ray images. 2020. arXiv:2003.11055 arXiv preprint.
- 2.Dilbag, Kumar V., Vaishali, Kaur M. Classification of covid-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks. Eur J Clin Microbiol Infect Dis. 2020:1–15. doi: 10.1007/s10096-020-03901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi H., Qi X., Yoon S.H., Park S.J., Lee K.H., Kim J.Y., et al. Extension of coronavirus disease 2019 (covid-19) on chest CT and implications for chest radiograph interpretation. Radiol Card Imaging. 2020;2(2) doi: 10.1148/ryct.2020204001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur M., Singh D. Fusion of medical images using deep belief networks. Clust Comput. 2019:1–15. doi: 10.1007/s10586-019-02999-x. [DOI] [Google Scholar]
- 5.Shukla P.K., Shukla P.K., Sharma P., Rawat P., Samar J., Moriwal R., et al. Efficient prediction of drug-drug interaction using deep learning models. IET Syst Biol. 2020 doi: 10.1049/iet-syb.2019.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur M., Gianey H.K., Singh D., Sabharwal M. Multi-objective differential evolution based random forest for e-health applications. Mod Phys Lett B. 2019;33(05) [Google Scholar]
- 7.Yu Y., Lin H., Meng J., Wei X., Guo H., Zhao Z. Deep transfer learning for modality classification of medical images. Information. 2017;8(3):91. [Google Scholar]
- 8.Nardelli P., Jimenez-Carretero D., Bermejo-Pelaez D., Washko G.R., Rahaghi F.N., Ledesma-Carbayo M.J., et al. Pulmonary artery–vein classification in CT images using deep learning. IEEE Trans Med Imaging. 2018;37(11):2428–2440. doi: 10.1109/TMI.2018.2833385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin H., Roth H.R., Gao M., Lu L., Xu Z., Nogues I., et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285–1298. doi: 10.1109/TMI.2016.2528162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Xia Y., Zhang J., Song Y., Feng D., Fulham M., et al. Knowledge-based collaborative deep learning for benign-malignant lung nodule classification on chest CT. IEEE Trans Med Imaging. 2019;38(4):991–1004. doi: 10.1109/TMI.2018.2876510. [DOI] [PubMed] [Google Scholar]
- 11.Hagerty J.R., Stanley R.J., Almubarak H.A., Lama N., Kasmi R., Guo P., et al. Deep learning and handcrafted method fusion: higher diagnostic accuracy for melanoma dermoscopy images. IEEE J Biomed Health Inform. 2019;23(4):1385–1391. doi: 10.1109/JBHI.2019.2891049. [DOI] [PubMed] [Google Scholar]
- 12.Gerard S.E., Patton T.J., Christensen G.E., Bayouth J.E., Reinhardt J.M. Fissurenet: a deep learning approach for pulmonary fissure detection in CT images. IEEE Trans Med Imaging. 2019;38(1):156–166. doi: 10.1109/TMI.2018.2858202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setio A.A.A., Ciompi F., Litjens G., Gerke P., Jacobs C., van Riel S.J., et al. Pulmonary nodule detection in CT images: false positive reduction using multi-view convolutional networks. IEEE Trans Med Imaging. 2016;35(5):1160–1169. doi: 10.1109/TMI.2016.2536809. [DOI] [PubMed] [Google Scholar]
- 14.Xia K., Yin H., Qian P., Jiang Y., Wang S. Liver semantic segmentation algorithm based on improved deep adversarial networks in combination of weighted loss function on abdominal CT images. IEEE Access. 2019;7:96349–96358. [Google Scholar]
- 15.Pezeshk A., Hamidian S., Petrick N., Sahiner B. 3-d convolutional neural networks for automatic detection of pulmonary nodules in chest CT. IEEE J Biomed Health Inform. 2019;23(5):2080–2090. doi: 10.1109/JBHI.2018.2879449. [DOI] [PubMed] [Google Scholar]
- 16.Zreik M., van Hamersvelt R.W., Wolterink J.M., Leiner T., Viergever M.A., Išgum I. A recurrent CNN for automatic detection and classification of coronary artery plaque and stenosis in coronary CT angiography. IEEE Trans Med Imaging. 2019;38(7):1588–1598. doi: 10.1109/TMI.2018.2883807. [DOI] [PubMed] [Google Scholar]
- 17.Bhandary A., Prabhu G.A., Rajinikanth V., Thanaraj K.P., Satapathy S.C., Robbins D.E., et al. Deep-learning framework to detect lung abnormality – a study with chest x-ray and lung CT scan images. Pattern Recognit Lett. 2020;129:271–278. [Google Scholar]
- 18.Gao X.W., James-Reynolds C., Currie E. Analysis of tuberculosis severity levels from CT pulmonary images based on enhanced residual deep learning architecture. Neurocomputing. 2019 [Google Scholar]
- 19.Pannu H.S., Singh D., Malhi A.K. Improved particle swarm optimization based adaptive neuro-fuzzy inference system for benzene detection. CLEAN - Soil, Air, Water. 2018;46(5) [Google Scholar]
- 20.Pannu H.S., Singh D., Malhi A.K. Multi-objective particle swarm optimization-based adaptive neuro-fuzzy inference system for benzene monitoring. Neural Comput Appl. 2019;31:2195–2205. [Google Scholar]
- 21.Zeng X., Ouyang W., Yang B., Yan J., Wang X. European conference on computer vision. Springer; 2016. Gated bi-directional CNN for object detection; pp. 354–369. [Google Scholar]
- 22.Saha S., Khabir K.M., Abir S.S., Islam A. Real-time image processing and deep learning 2019. vol. 10996. International Society for Optics; 2019. A newly proposed object detection method using faster R-CNN inception with ResNet based on tensorflow; p. 109960X. [Google Scholar]
- 23.Huang Z., Dumitru C.O., Pan Z., Lei B., Datcu M. Classification of large-scale high-resolution SAR images with deep transfer learning. IEEE Geosci Remote Sens Lett. 2020:1–5. [Google Scholar]
- 24.Rezende E., Ruppert G., Carvalho T., Ramos F., De Geus P. Malicious software classification using transfer learning of ResNet-50 deep neural network. 2017 16th IEEE international conference on machine learning and applications; ICMLA; IEEE; 2017. pp. 1011–1014. [Google Scholar]
- 25.Ghosh S., Shivakumara P., Roy P., Pal U., Lu T. Graphology based handwritten character analysis for human behaviour identification. CAAI Trans Intell Syst. 2020;5(1):55–65. [Google Scholar]
- 26.Panda M. Elephant search optimization combined with deep neural network for microarray data analysis. J King Saud Univ, Comput Inf Sci. 2017 [Google Scholar]
- 27.Qi G., Wang H., Haner M., Weng C., Chen S., Zhu Z. Convolutional neural network based detection and judgement of environmental obstacle in vehicle operation. CAAI Trans Intell Syst. 2019;4(2):80–91. [Google Scholar]
- 28.Chowdhury M.E., Rahman T., Khandakar A., Mazhar R., Kadir M.A., Mahbub Z.B., et al. Can AI help in screening viral and covid-19 pneumonia? 2020. arXiv:2003.13145 arXiv preprint.
- 29.Kaur M., Kumar V. Beta chaotic map based image encryption using genetic algorithm. Int J Bifurc Chaos. 2018;28(11) [Google Scholar]
- 30.Gupta A., Singh D., Kaur M. An efficient image encryption using non-dominated sorting genetic algorithm-iii based 4-d chaotic maps. J Ambient Intell Humaniz Comput. 2020;11(3):1309–1324. [Google Scholar]
- 31.Kaur M., Singh D., Sun K., Rawat U. Color image encryption using non-dominated sorting genetic algorithm with local chaotic search based 5d chaotic map. Future Gener Comput Syst. 2020;107:333–350. [Google Scholar]
- 32.Kaur M., Singh D., Singh Uppal R. Parallel strength Pareto evolutionary algorithm-ii based image encryption. IET Image Process. 2020;14(6):1015–1026. [Google Scholar]
- 33.Kaur M., Kumar V., Li L. Color image encryption approach based on memetic differential evolution. Neural Comput Appl. 2019;31(11):7975–7987. [Google Scholar]