Abstract
The spread of COVID-19 is accelerating. At present, there is no specific antiviral drugs for COVID-19 outbreak. This is a multicenter retrospective cohort study of patients with laboratory-confirmed COVID-19 infection pneumonia from 3 hospitals in Hubei and Guangdong province, 141 adults (aged ≥18 years) without ventilation were included. Combined group patients were given Arbidol and IFN-α2b, monotherapy group patients inhaled IFN-α2b for 10–14 days. Of 141 COVID-19 patients, baseline clinical and laboratory characteristics were similar between combined group and monotherapy group, that 30% of the patients leucocytes counts were below the normal range and 36.4% of the patients experienced lymphocytopenia. The duration of viral RNA of respiratory tract in the monotherapy group was not longer than that in the combined therapy group. There was no significant differences between two groups. The absorption of pneumonia in the combined group was faster than that in the monotherapy group. We inferred that Arbidol/IFN - 2 b therapy can be used as an effective method to improve the COVID-19 pneumonia of mild patients, although it helpless with accelerating the virus clearance. These results should be verified in a larger prospective randomized environment.
Keywords: COVID-19, 2019-nCoV, Pneumonia, Arbidol, IFN-α2b, Treatment, RNA
In December 2019, the outbreak of the new coronavirus infections (COVID-19) caused by in Wuhan in the Hubei province of China has quickly become a global health emergency [1]. The virus is highly infectious and pathogenic, On 11 March 2020, the World Health Organization (WHO) classified the outbreak as a pandemic. By Mar 31, 2020, 718685cases of COVID-19 have been reported from 189 countries with 14,510 deaths, This corresponds to a 4.7% case fatality rate. At present, there is no vaccine or specific antiviral therapies for human and animal coronavirus (COV) [2].
The current management of COVID-19 is mainly supportive, some anti-coronavirus drugs are possibly effective of human pathogen coronavirus. Several drugs therapeutic interventions for coronavirus were investigated during the Middle East Respiratory Syndrome (MERS), caused by a novel coronavirus (MERS-CoV). Reviews of the available literature suggest that interferon alpha and beta might be of benefit in patients with severe MERS-CoV infection [3,4]. Interferon (IFN) is an immune active protein with broad-spectrum antiviral effect in vivo, IFN-α is a broad spectrum antiviral drug, different preparations of recombinant rIFNs (rIFN-α2a, rIFN-α2b, rIFN-β1a, and rIFN-β1b) were active against MERS-CoV in vitro [5]. Interferon-α treatment strongly inhibite severe in vitro cytopathology induced by MERS-coronavirus replication. Furthermore, MERS-CoV was found to be 50–100 times more sensitive to alpha interferon (IFN-α) treatment than SARS-CoV in vitro. These findings highlight relevant differences between these distantly related CoVs in terms of their interaction with and evasion of the cellular innate immune response [6]. Arbidol was used to treat for influenza infection [7]. Arbidol play an antiviral role not only directly inhibiting virus but also inducing interferon and immune cells producing [8], After taking arbidol, there was a significant increase of serum immunoglobulin levels of CD4+ and CD8+ lymphocytes in patients with hypoimmunity [9]. It was reported that Arbidol was effective of COVID-19 at a concentration range of 10–30 μM in vitro.
According to the guidelines, IFN-α combine Arbidol or other antiviral drugs less than 10 days are recommended as anti COVID-19 therapy [10], However, clinical data and study on Arbidol/IFN-α in COVID-19 haven’t been reported, the efficacy and toxic effects of these drugs for COVID-19 remains uncertain, and that combining IFN-α with Arbidol to create a new drug classes that would sufficiently increase efficacy need to be further confirmed by clinical experiments.
This study aimed to evaluate the effects of Arbidol when combined with IFN-α in reducing hospitalization days and shortening time of virus RNA clearance. Treatment outcomes would be useful in providing more evidence for the clinical management of patients with COVID-19.
1. Materials and methods
1.1. Patients
Initially, 221 hospitalized patients laboratory-confirmed COVID-19 infection pneumonia from 3 hospitals in Hubei and Shenzhen diagnosed between January 2019 and Mar 2020 were screened. After excluding ineligible patients, A total of 141 patients were included in this retrospective multicentre cohort study. All patients was diagnosed COVID-19 infection by Fluorescence PCR (polymerase chain reaction) testing of respiratory tract specimens for 2019-nCoV ORF 1 ab and N genes. The study inclusion criteria were: (1) adult (aged ≥18 years) (2) without ventilation (3) laboratory-confirmed COVID-19 with PCR detection of the virus in samples taken from the respiratory tract of the patient (4) chest CT scan showed the characteristics of viral pneumonia, which was defined as new, otherwise unexplained, in addition to new or progressive pulmonary infiltrates on lung. The exclusion criteria were: (1) current pregnancy (2) patients who were enrolled a clinical trials treated antiviral therapy for COVID-19 besides IFN-α2b or Arbidol (3) Invasive ventilation for the aggravation of the disease, or death. (4) can’t tolerate drug adverse effects. The study was approved by the Ethics Committee of Jianli People Hospital, Jianli Traditional Chinese medicine Hospital and Peking University Shenzhen.
1.2. Data collection
Epidemiological, clinical, laboratory, imaging data, management, and clinical outcomes data were collected through a review of medical records. According to distribution of the affected lung parenchyma, the severity of total lung lobes was evaluated and classified score according to CT scan [12]:0 as normal, 1 as < 25% abnormality, 2 as 25–50% abnormality, 3 as 50–75% abnormality, and 4 as > 75% abnormality.
1.3. Procedures
Treatment protocols was Arbidol/IFN-α2b combination, IFN-α2b alone. Monotherapy group patients inhaled IFN-α2b (twice per day, inhale, 5 × 10 (5) IU, for 10–14days). Combined group patients oral take Arbidol (200 mg, oral, third times per day, for 7–10 days) and IFN-α2b, dosed as above. All patients received appropriate supportive care such as supplementary oxygen, control of blood pressure, blood sugar and other conventional treatment and regular clinical and laboratory monitoring as needed. IFN- α2b (Changchun Biopharmaceutical, Changchun, Jilin, China); Arbidol (Shijiazhuang Pharmaceutical Company, Shijiazhuang, Hebei, China).
For patients, respiratory samples were collected for 2019-nCoV PCR detection approximately 1–3 times per week and chest CT taken per 5–10 day at the discretion of the treating teams for assessing and infection control purposes. All confirmed cases were admitted in isolation rooms and were followed until in-hospital death or need invasive ventilation or hospital discharge.
The exposure variable was the treatment regimen of Arbidol plus IFN-α2b. The outcome variable was duration of hospital stay, duration of COVID-19 RNA and CT absorption time. The covariates were age, sex, vital signs on admission, pre-existing comorbid conditions on admission (diabetes, hypertension, hepatitis B, and chronic heart disease), Laboratory examinations (blood oxygen saturation, leukocyte and lymphocyte values and liver function etc.). The primary composite end point was the time from symptom onset to discharged. Discharge standard was defined as survival with guidelines [10]. Temperature returned to normal for more than 3 days; 2. Respiratory symptoms improved significantly; 3. Pulmonary imaging showed that acute exudative lesions improved significantly; 4. Nucleic acid test of respiratory samples was negative for two consecutive times (sampling time at least 1 day interval). Other secondary outcomes was virus RNA clearance, was defined as the time from admission until the test was negative on 2 occasions, without a positive test afterward. To assess safety profile, white blood cell (WBC) count, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin and creatinine.
1.4. Statistical analysis
Descriptive statistics were used for demographics. Categorical variables were expressed as n (%), Mean (Range) were used for normally-distributed data, and Median (IQR) were used for abnormally distributed data, using χ2 test or Fisher exact test for categorical variables and Student t test or Mann–Whitney U test for continuous variables. We used Cox proportional-hazards regression to analyze time-to-event end points. Two-sided P values of 0.05 were considered statistically significant. Data were analysed using IBM SPSS Statistics, version 25.0 (IBM Corp., USA).
2. Results
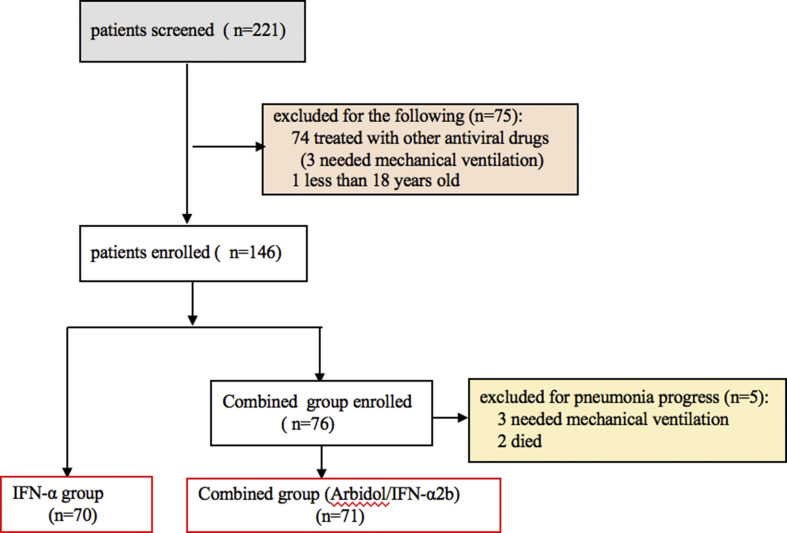
Between January 2019 and Mar 2020, a total of 221 hospitalized patients, with laboratory-confirmed COVID-19 infection pneumonia without invasive ventilation were screened. All patients treated with Arbidol/IFN-α2b or IFN-α2b. We excluded the ones that did not meet the eligibility criteria. 74 cases among them were treated with other antiviral drugs, such as ribavirin, zanamivir, chloroquine, lopinavir/ritonavir, 3 cases of them developed ARDS. 1 patient was less than 18 years old, 5 patients, who taked Arbidol/IFN-α2b for 2–4 days, failed to complete the drug treatment course (3 patients needed invasive mechanical ventilation and 2 patients died for pneumonia progress), so 5 patients were excluded, 141patients were included in the multicenter cohort study. As shown in Fig. 1 . After excluding ineligible patients, 141 patients with COVID-19included in the remaining cohort.
-
2.1
In total, 74 men (52.4%) and 67 (47.6%) women with a median age of 51.9 (24.0–83.0) were analyzed, of whom 70 (49.6%) received IFN-α2b therapy, 71 (50.4%) received Arbidol/IFN-α2b therapy. Patients in the 2 groups were similar in age, sex, comorbidities, blood oxygen saturation, leukocyte and lymphocyte values and lung severity score (Table 1). The most common symptoms were fever (96/141, 68.6%), followed by dry cough (76/141, 53.9%), Shortness of breath (16/141, 11.3%), fatigue (16/141,11.3%), Other symptoms were Diarrhea (5/141, 3.5x%) and abdominal distension (15/141, 10.6%). 78 patients (36/70, 52.% in IFN-α2b group, 42/71, 59.2%in combined group) needed nasal catheter for oxygen, 2 patients required non-invasive ventilator.
-
2.2
On admission, laboratory findings revealed most patients have normal liver function, creatinine, white blood cells and lymphocytes, that 30% of the patients leucocytes counts were below the normal range and 36.4% of the patients experienced lymphocytopenia (Table 1). Chest imaging showed ground-glass opacity, multiple patchs-like shadows and consolidation in the lungs. There was no significant difference in the degree of lung injury between two groups (P = 0.124).
-
2.3
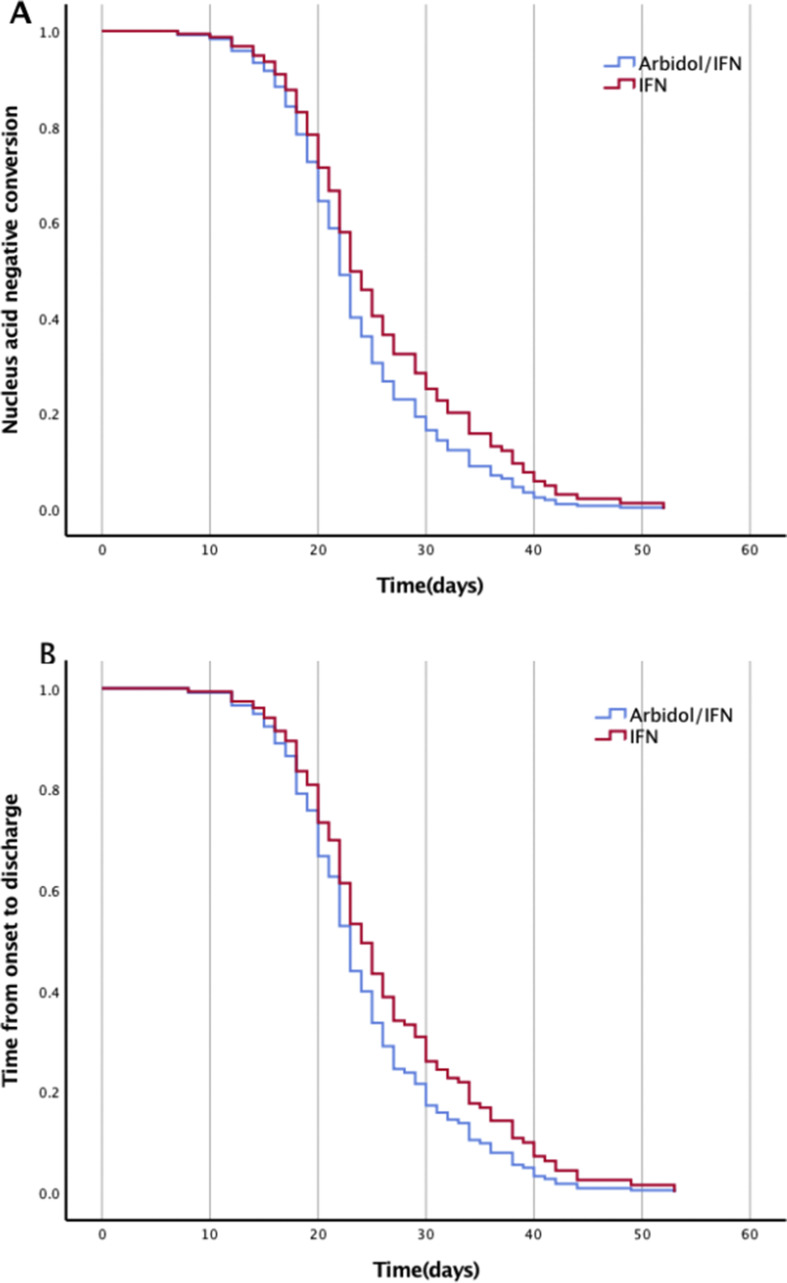
In this study, there were no significant differences between hospitalization and RNA clearance days with respect to age, sex, symptoms After treatment for 7–14 days, there was no statistically differences of the viral RNA clearance days between two group (Fig. 2A), The duration of viral RNA detected from oropharyngeal/nasopharynxswabs/sputum samples in the monotherapy group was not longer than that in the combined therapy group (27.4 days vs.23.8days, respectively; P = 0.057). Furthermore, we found there are great individual differences in the persistence and clearance of viral RNA. the duration of RNA positive from the onset of symptoms to 2 occasions negative RT-PCR results was 8–53 days in monotherapy group, 11–53 days in combined group respectly (Table 1).
Fig. 1.
Flow chart of patients screening and selection process.
Table 1.
Baseline characteristics and physiological parameters and laboratory features of patients with COVID-19 pneumonia who received Arbidol/IFN-α2b.
| Characteristics | IFN-α2b (n = 70) | Abidol/IFN-α2b (n = 71) | P |
|---|---|---|---|
| Age(y), mean (Range) | 53.2 (26–83) | 50.9 (24–75) | 0.543 |
| Sex (Male/Female), n (%) | 33(47.1)/37(52.9) | 41(57.7)/30(42.3) | 0.293 |
| Comorbidities | |||
| Hypertension, n (%) | 8 (11.4) | 7 (9.9) | 0.740 |
| Diabetes, n (%) | 7 (10.0) | 5 (7.0) | 0.113 |
| Othersa, n (%) | 5 (7.1) | 1 (1.4) | 0.113 |
| Clinical symptoms | |||
| Fever, n (%) | 40 (57.1) | 56 (81.2) | 0.003 |
| Cough, n (%) | 44 (62.9) | 32 (46.4) | 0.028 |
| Fatigue, n (%) | 9 (12.9) | 7 (10.1) | 0.998 |
| Shortness of breath, n (%) | 9 (12.9) | 7 (10.1) | 0.595 |
| Abdominal distension | 8 (11.4) | 7 (9.9) | 0.740 |
| Diarrhea, n (%) | 1 (1.4) | 4 (5.8) | 0.366 |
| Laboratory examinations | |||
| Decline in white blood cells, n (%) | 22 (31.4) | 20 (28.2) | 0.632 |
| Decline in lymphocytes, n (%) | 29 (41.4) | 22 (31.0) | 0.175 |
| Increase in aminotransferase, n (%) | 4 (5.7) | 11 (15.5) | 0.064 |
| Increase in creatine kinase, n (%) | 1 (1.4) | 6 (8.5) | 0.116 |
| Lung: total severity score | |||
| 1, <25% abnormality, n (%) | 29 (41.4) | 27 (38.0) | 0.124 |
| 2, 25%–50% abnormality, n (%) | 40 (57.1) | 44 (62.0) | |
| 3, 50%–75% abnormality, n (%) | 1 (1.4) | 2 (2.8) | |
| 4, ≥75% abnormality, n (%) | 0 (0) | 0 (0) | |
| SpO2, median (IQR) | 97.3 (93–99) | 97.3 (94–99) | 0.539 |
| Time from onset to be discharge, median (IQR) | 27.1 (8–53) | 24.2 (11–53) | 0.056 |
| Nucleus acid negative conversion time, median (IQR) | 23.8 (10–52) | 27.4 (7–52) | 0.057 |
| CT absorption time, mean (Range) | 16.7 (7–33) | 19.8 (10–36) | 0.037 |
Data are presented as no (%). For continuous variables, Mann–Whitney U test was used to calculate the P value. For categorical variables, χ2 test was used to calculate the P value.
Others included chronic heart diseases, arthrolithiasis, chronic hepatitis B.
Fig. 2.
Nucleus acid negative conversion time (Fig. 2A) and time from onset to be discharge (Fig. 2B).
The median hospitalization days was 27.1 vs. 24.2 days in two group (P = 0.056). There was no significant differences between two groups (Fig. 2 A). Among 141 patients, 39 patients had a long hospitalization days more than 30 days (20/70 in monotherapy group, 19/71 in combined group, for most patients (36/39), the time of CT and symptom improvement was significantly shorter than that of nucleic acid conversion negative. CT absorption time was16.7 days vs.19.8 days, respectively; P = 0.037 (Table 1 ), the improvement rate of CT and symptoms was faster than that of nucleic acid clearance. The absorption of pneumonia in the combined group was faster than that in the monotherapy group. Only 3 patients in IFN-α2b group, the time of virus turning negative is shorter than that of clinical symptoms and CT improved. These symptoms were neurogenic bladder caused dysuria and exertional dyspnea.
-
2.4
There were no differences between the 2 groups in hemoglobin, WBC count, platelet count, ALT, AST, or creatinine during or after treatment. 13 patients (18.8%) treated with Arbidol demonstrated mild nausea, stomachache, but all patients could tolerate without giving up treatment.
3. Discussion
Recently, the COVID-19 infections epidemic broke out all over the world. The virus is highly infectious. We observation that COVID-19 generally induced mild respiratory or intestinal infections (213/221,96.38%), but can also cause serious audlt respiratory distress syndrome (ARDS) even fatal outcomes (8/221, 3.62%) which is very similar to what reported in previous study [12].
Antiviral drug therapy is an important treatment for COVID-19 infections, but until now there are no approved antiviral medication with proven efficacy for the treatment of COVID-19, nor are there any prospective randomized, controlled trials of potentially useful anti-adenovirus therapies. Apparent clinical success is limited to a few case reports and small series [13].
Most of the drug options come from experience treating SARS, MERS or some other new influenza virus previously [2,14], The combination of Arbidol and other antiviral drugs, such as interferon, is currently a recommended antivirus regimen in the Diagnosis and Treatment of Pneumonia Caused by 2019-nCoV (version 5) issued by National Health Commission of the People’s Republic of China [10]. While benefit of Arbidol was suggested by preclinical studies. Arbidol is a synthetic antiviral drug to combat seasonal infuenza virus [15]. Arbidol has been shown to inhibit viruses from many different families [16], Arbidol were shown to have antiviral effect in early viral replication in vitro for SARS-CoV [17], Arbidol have been found to be effective for COVID-19 in vitro [18], previous studies reported that a combination of Arbidol and LPV/r achieved further therapeutic effect than LPV/r only [19].
Because Arbidol can induce interferon and immune cells, we hypothesized that Arbidol combined with interferon has synergistic therapeutic effect in against coronavirus. To test our hypothesis, we conducted this observational study. Our retrospective multicenter cohort study analysis 141 general type patients [11] without invasive ventilation, treated with Arbidol/IFN-α2b, or IFN-α2b. Effective drug combination should be more effective than single drug in accelerating virus clearance and improving CT and clinical symptoms, and shortening hospitalization duration, we evaluated the RNA negative conversion time day, time from onset to be discharged, therapeutic response of Arbidol. But it is regreat the study demonstrates that Arbidol/IFN-α2b combination was not associated with decreased hospitalization day or accelerate COVID-19 RNA clearance, when compared with IFN-α2b monotherapy.
Discharge standard in China is not only the improvement of clinical symptoms and CT, but also RNA is negative in two consecutive tests. In our study, specimens taken from oropharyngeal/nasopharynxswabs/sputum for RT-PCR RNA test, the median time from the onset of symptoms to 2 occasions negative RT-PCR results was 23.8 (8–53) days in IFN-α2b group, 27.4 (11–53) days in combined group respectly, presenting notable individual differences in patients regardless age, sex, underly disease, It is suggested that it will take a long time for nucleic acids to be removed from the respiratory tract. During this period, patients who expel the virus may become a potential source of infection, the quantity and activity of virus are important infectious factors.
For most patients in our study, CT improvement more sooner than the nucleic acid clearance. The median CT improvement days of combination group less than that of monotherapy group (19.8 days of monotherapy group vs.16.7days of combined group, P = 0.037), The data suggested that Arbidol combined IFN-α2b versus IFN-α2b alone could have potential benefits on inhibiting COVID-19 lung inflammation of mild cases without invasive ventilation, but no benefits on RNA clearance, and we find Abidol and IFN-α2b therapy were well tolerated by the treatment group with no premature discontinuation secondary to adverse effects.
Our study is limited by its retrospective non-randomised control, there may be a potential selection bias. It should be noted that there were 5 patients received IFN-α2b alone treatment for 2–4 days were excluded because of the invasive ventilation or death due to deterioration. It was difficult to assess whether the aggravation is related to the difference of drug treatment. So we removed the five individuals to avoid potentially detrimental to clinical outcome, but it is difficult to completely avoid results biased.
In conclusion, Arbidol in combination with IFN-α2b was no effective in COVID-19 RNA clearance and hospitalization than IFN-α2b monotherapy in this cohort, but accelerate pneumonia absorption, but these results should be verified in a larger prospective randomized setting.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank the Jianli People Hospital, Jianli Traditional Chinese medicine Hospital and Peking University Shenzhen Hospital for its support in the database construction. This work was supported by the Natural Science Foundation of Guangdong Province (No 2017030313830).
Contributor Information
Weidong Song, Email: songwd123@hotmail.com.
Li Yi, Email: pkusz123@sina.com.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 3.Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A. Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the kingdom of Saudi arabia. BMC Infect Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wilde A.H., Raj V.S., Oudshoorn D., Bestebroer T.M., van Nieuwkoop S., Limpens R.W.A.L. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J Gen Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Ding Y., Yang C., Li R., Du Q., Hao Y. Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret) Biomed Pharmacother. 2017;91:393–401. doi: 10.1016/j.biopha.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 8.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Q., Gu Z., Leneva I., Jiang H., Li R., Deng L. The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis. Int Immunopharm. 2019;68:58–67. doi: 10.1016/j.intimp.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L., Li T.S. [Interpretation of "guidelines for the Diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (trial version 5)"] Zhonghua Yixue Zazhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]
- 11.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 14.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pécheur E.I., Borisevich V., Halfmann P., Morrey J.D., Smee D.F., Prichard M. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90:3086–3092. doi: 10.1128/JVI.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnard D.L., Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6:615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman C.M., Sisk J.M., Mingo R.M., Nelson E.A., White J.M., Frieman M.B. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J Virol. 2016;90:8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L., Li C., Zeng Q., Li X., Zhang H., Hong Z. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]