Graphical abstract
Keywords: Apabetalone, C-reactive protein, Inflammation, Hypertension, Microbiome, Nutraceuticals
Abstract
Inflammation is an obligatory marker of arterial disease, both stemming from the inflammatory activity of cholesterol itself and from well-established molecular mechanisms. Raised progenitor cell recruitment after major events and clonal hematopoiesis related mechanisms have provided an improved understanding of factors regulating inflammatory phenomena. Trials with inflammation antagonists have led to an extensive evaluation of biomarkers such as the high sensitivity C reactive protein (hsCRP), not exerting a causative role, but frequently indicative of the individual cardiovascular (CV) risk. Aim of this review is to provide indication on the anti-inflammatory profile of agents of general use in CV prevention, i.e. affecting lipids, blood pressure, diabetes as well nutraceuticals such as n-3 fatty acids. A crucial issue in the evaluation of the benefit of the anti-inflammatory activity is the frequent discordance between a beneficial activity on a major risk factor and associated changes of hsCRP, as in the case of statins vs PCSK9 antagonists. In hypertension, angiotensin converting enzyme inhibitors exert an optimal anti-inflammatory activity, vs the case of sartans. The remarkable preventive activity of SLGT-2 inhibitors in heart failure is not associated with a clear anti-inflammatory mechanism. Finally, icosapent ethyl has been shown to reduce the CV risk in hypertriglyceridemia, with a 27 % reduction of hsCRP. The inflammation-based approach to arterial disease has considerably gained from an improved understanding of the clinical diagnostic strategy and from a better knowledge on the mode of action of numerous agents, including nutraceuticals.
1. Introduction
Inflammation has been historically considered as an obligatory marker of arterial disease. Cholesterol itself is an inflammatory mediator, being a crystalline product [1] and, by its physical presence, activating NLRP3 (NACHT-, LRR- and pyrin domain-containing 3), a general mediator of arterial tissue inflammation [2]. NLRP3 nucleates the assembly of an inflammasome, leading to caspase 1-mediated activation of the interleukin-1β (IL-1β) family of cytokines, thus inducing an inflammatory pyroptotic cell death [3]. This molecular mechanism is the final development of the seminal idea by Ross and Glomset, who postulated endothelial injury as the inducer of cell proliferation and expansion of smooth muscle cells (SMCs) [4,5]. The association between local inflammation, elevated levels of low-density lipoproteins (LDL) and noxious life habits brought forward the concept of structural lipoprotein changes allowing aggregation and/or oxidation [6]. The presently established role of enhanced myelopoiesis in the development of arterial inflammatory changes and the identification of newer mediators from both inflammatory and immune systems can provide novel mechanisms underlying the development of arterial disease.
As a lipid-driven inflammatory disease, a balance of proinflammatory and inflammation-resolving mechanisms is responsible for the final outcomes [7]. While bone marrow (BM) and spleen were not considered to play a significant role in atheroma formation, it is now well established that BM is responsible for the enhanced myelopoiesis, allowing recruitment of inflammatory cells, particularly monocytes, to the arterial intima [8,9]. The rise of hematopoietic and progenitor cells (HSPCs) occurring after myocardial infarction (MI) [10] can well explain the increased growth of plaques and the associated higher protease activity. Clonal hematopoiesis (CH), in addition to eliciting effects through inflammatory mediators, reduces the epigenetic modifier enzyme ten-eleven translocation 2 (TET2) raising atherosclerotic risk [11]. TET2 deficient cells, when clonally expanded, markedly increase plaque size and NLRP3 inflammasome mediated IL-1β secretion [12]. Further, toll-like receptor 4 (TLR4) [13] by interacting with myeloid differentiation factor-88 (MyD88) can lead to cellular signaling, resulting in hematopoietic and stromal cell development [14]. Hypercholesterolemia causes HSPCs to proliferate, leading to leukocytosis and enhanced atherosclerosis both in animal models and humans [15]. As very recently described by Gu and colleagues [16], in the zebrafish deficient in the cholesterol efflux pathway mediated by apolipoprotein binding protein 2 there is a loss of capacity of HDL to accept cholesterol and increased hematopoiesis by way of NOTCH signaling, hypothesizing a cholesterol metabolic pathway controlling emergence of HSPCs. These findings have postulated a role of the NOTCH family in the expansion of hematopoietic stem cells [17]. Further, the reported novel roles of apolipoprotein binding protein 2 and of the sterol regulatory element-binding protein 2 (SREBP2) [17] have clearly indicated the presence of SREBP2 binding DNA sequences in Notch as well as in genes regulating cholesterol synthesis, most likely relevant in adult hematopoiesis [16].
Hematopoietic cells are also characterized by the Akt (protein kinase B) pathway, i.e. a serine/threonine-specific protein kinase playing multiple roles in processes, such as glucose metabolism, apoptosis, cell migration and proliferation, with three isoforms, Akt1, Akt2 and Akt3. Loss of Akt1 in apo E−/− mice leads to severe atherosclerosis [18], whereas loss of Akt1 and Akt2 in hematopoietic cells (Akt3only) provides arterial protection. The presence of only the Akt1 isoform is detrimental for the viability of monocytes/macrophages, eventually leading to the development of smaller atherosclerotic lesions.
LDL-associated inflammatory changes may thus be linked to enhanced hematopoiesis although the role of TET2 mutations has not been confirmed in all studies [19] but also to direct activities such as: a) enhanced cholesterol crystal deposition, raising the vascular inflammasome NLRP3; b) rise of different T lymphocyte subtypes leading to plaque proliferation and potential rupture [20]; c) development of tissue inflammatory changes, mainly linked to raised cytokines and hsCRP.
A link between the first two mechanisms, i.e. direct inflammatory effects and T cell proliferation, has been provided by the identification of a novel regulator i.e. the NLRC5 (NLRF family CARD Domain Containing 5) previously known as a major regulator of innate immunity [21]. NLRC5 has an essential role in determining vascular intimal hyperplasia [22] and NLRC5 knock-out (Nlrc5−/) mice exhibit more severe intimal hyperplasia compared to wild-type mice after carotid ligation. NLRC5 deficiency leads to increased proliferation and migration of human vascular SMC. NLRC5 appears to bind to peroxisome proliferator-activated receptor (PPAR)γ inhibiting dedifferentiation of human SMC. These damaging mechanisms are antagonized by the PPARγ agonist pioglitazone [22].
Recently Lee et al [23] investigated the contribution of SMC to the foam cell abundance in apo E−/− mice. By evaluating expression of leukocyte specific markers, non-leukocyte foam cells increased from 37 % in 27-week-old to 75 % in 57-week-old male apo E-/- mice fed a chow diet compared to about 70 % in male and female apo E-/- after 6 weeks on a Western diet. This finding provides an important link between the observation of foam cells in human and mouse atheromas, suggesting an upregulated role of SMCs in foam cell formation [24]. It indicates the need for more advanced lineage tracing models on mice and proteomic evaluations in man, in order to accurately define the origin and functions of cell types in lesions and to design improved therapeutic approaches to enhance plaque stability.
A novel and poorly explored immune-related area of vascular inflammation is that of serum factor H, a regulator of the alternative complement pathway [25]. The central function of factor H related protein 1 (FHR1) has remained unclear. FHR1 competes with factor H in the regulation of its inhibitory activity [26]. Studies on the role of factor H and FHR1 in cytoplasmic antibody associated vasculitis and atherosclerosis have provided exciting openings on the role of FHR1 in the activation of monocyte inflammasomes. FHR1 appears to bind necrotic cells in plaques, in addition inducing inflammasome NLRP3 in blood derived monocytes that subsequently secrete interleukin (IL)1β, tumor necrosis factor (TNF)α, IL-18 and IL-6 [27]. FHR1 concentrations in patients with antibody-associated vasculitis are associated with levels of inflammation and progressive disease. This unexpected role for FHR1 during inflammation may explain the protection by FHR1 deficiency, indicating it as potential target for treatment of arterial inflammatory diseases [27].
Finally, a potential regulator of the proinflammatory signaling from neutrophils is the dimeric S100 A8/A9, a proinflammatory alarmin, highly expressed in neutrophils and rapidly released into the circulation after myocardial ischemia [28]. This potent activator of the receptor of advanced glycation end products [29] was found to be associated with reduced ventricular ejection fraction one-year after the MI [30], with a raised incidence of hospitalizations from heart failure.
In view of the diversified tissue responses to inflammatory stimuli and the need for a better understanding of diagnostic procedures and possible therapeutic approaches, the aim of this review article is manifold. It will examine present day evidence of the most appropriate diagnostic approaches and will evaluate in detail the pharmacological strategies, examining the possible anti-inflammatory profile of drugs of major use in cardiovascular prevention. This in-depth scrutiny will lead at times to apparent discrepancies between efficacy on a major therapeutic target and presence or absence of anti-inflammatory activity. These observations may be of crucial value in the selection of the most appropriate therapeutic agent.
2. Clinical evaluation of inflammation-associated cardiovascular risk
The need for an easier access to the evaluation of cardiovascular (CV) risk associated to inflammation has led to the wide use of C-reactive protein (CRP), a mediator well known in the monitoring of systemic inflammatory diseases, such as rheumatoid arthritis and other articular disorders. CRP is a member of the short pentraxin family, expression being stimulated by proinflammatory cytokines, i.e. in particular IL-6, but also IL-1β, TNFα and others [31]. CRP binds microbial polysaccharides and ligands on damaged cells. This leads to activation of the classical complement mediated pathway, thus removing damaged or apoptotic cells, in turn activating monocytes [32].
While there is general agreement that CRP is a relevant risk biomarker for CVD [33,34], there has been no consensus on a causative role. Mendelian randomization studies failed to prove a causal link between genetic variants of CRP affecting protein level and risk of coronary heart disease [35], in contrast to the case of IL-6 [36], with, however, impractical diagnostic application due to a short half-life and circadian variations [37]. The same conclusion applies to IL-1β, since there is no available technology allowing a reliable measurement. The difficulty in determining these cytokines contrasts with the commercially robust and standardized high-sensitivity (hs)CRP immunoassay.
The association of plaque morphology/tendency to rupture with hsCRP levels has been evaluated by appropriate studies [38]. The prognostic value of hsCRP in post-acute coronary syndrome (ACS) patients on optimal medical treatment was found to be weak [39]. A higher sensitivity was found in a cross-sectional study [40], indicating significant correlations with the dynamic changes in plaque structure. In the small IBIS Study a reduction of necrotic core volume with lower hsCRP, not LDL-C, was described [41]. In a more extensive serial intravascular imaging, after 12 months of rosuvastatin treatment, changes of hsCRP showed highly significant positive correlations with necrotic core and dense calcium volumes and negative correlations with fibrous and fibrofatty plaque volumes, not with LDL-cholesterol changes [42].
There is thus as present a high interest in evaluating risk of CV disease (CVD) and other diseases in individuals with low LDL-C [43], a frequent finding in coronary patients, associated with an increased risk of all-cause mortality [44]. Low LDL-C may be a chance finding in random series of individuals or it may be dependent upon mutations in proprotein convertase subtilisin/kexin type 9 (PCSK9), angiopoietin-like 3, microsomal triglyceride transfer protein or apolipoprotein (apo) B [45]. In order to assess the critical value of LDL-C vs hsCRP, a crucial study was that by Penson et al on Reasons for Geographical and Racial Differences in Stroke (REGARDS) [46]. In this, the relationship between low LDL-C (<70 mg/dL) compared to >70 mg/dl and hsCRP < 2 compared with ≥2 mg/L was evaluated. The Authors concluded that participants with high LDL-C and low hsCRP have a lower risk of stroke, CHD and CHD deaths compared to those in the same LDL-C category but with high hsCRP. Participants with high hsCRP (≥ 2 mg/L) and low LDL-C (<70 mg/dL) did not show any additional risk reduction but a significant rise of all-cause mortality [46].
These findings are in line with those more recently provided by Guedenay et al evaluating residual inflammatory risk (RIR) in 3,013 patients undergoing percutaneous interventions with low baseline LDL-C (≤ 70 mg/dL) [47] and serial hsCRP assessments. They were characterized as persistent low RIR, attenuated RIR (first high then low hsCRP), increased RIR (first low then high hsCRP), or persistent high RIR. There was a stepwise raised incidence rate of major adverse cardiac and cerebrovascular accidents in these four risk categories within one year of the second hsCRP measurement.
3. Drug approaches to the inflammatory cardiovascular residual risk
A number of drug approaches have been guided by knowledge of the inflammatory basis of CVD (Table 1 ). The CV preventive activity of the most widely used inhibitor of cyclooxygenase (COX)-1 antagonist, aspirin, has not been recently supported by a number of large controlled intervention studies [48,49], although possibly the drug may be effective in individuals homozygous for the soluble guanylate cyclase (sGC) gene variant GUCY1A3 risk(G) allele [50]. Selective COX-2 inhibitors have led to CV harm, in particular rofecoxib, although the later large PRECISION study comparing celecoxib with ibuprofen and naproxen in over 27,000 subjects with arthritis did not identify a clear CV harm [51]. In a follow-up study celecoxib proved actually safer than ibuprofen and naproxen when given with aspirin [52] but there was no evidence of CV benefit.
Table 1.
Percentage changes of hsCRP upon treatments with Canakinumab, Metotrexate and Colchicine.
| Clinical study | hsCRP (mg/L) in the active group |
Primary Endpoint | ||
|---|---|---|---|---|
| pre | post | |||
| Canakinumab [57] | CANTOS* | 4.25 | 2.10 | HR 0.85; 95 % CI 0.74–0.98; p = 0.021 |
| Methotrexate [63] | CIRT** | 1.45 (0.73, 3.40) | 1.56 (0.77, 3.53) | HR 1.01; 95 % CI 0.82–1.25; p = 0.91 |
| Colchicine [68] | COLCOT*** | 4.27 (2.12, 7.22) | 1.37 (0.75, 2.13) | HR 0.77; 95 % CI, 0.61 to 0.96; p = 0.02 |
CANTOS, Canakinumab Antiinflammatory Thrombosis Outcome Study; CIRT, Cardiovascular Inflammation Reduction Trial; COLCOT, Colchicine Cardiovascular Outcomes Trial. hsCRP, high-sensitivity C-reactive protein.
values are referred to all doses (50 mg, 150 mg and 300 mg). Post = after 48 months.
Pre = from enrollment; Post = 8 months post randomization.
Pre = at randomization; Post = 6 months post randomization.
Aside from the COX-inhibitors, agents modulating pathways in downstream response to injury, i.e. secretory and lipoprotein associated phospholipase A2 [53], P-selectin [54] and the IL-1 β selective inhibitor anakinra [55], all failed to provide evidence of potential activity on CV endpoints.
The CANTOS trial with the anti-IL-1-β monoclonal antibody canakinumab [56], enrolled patients with previous MI and hsCRP ≥ 2 mg/L and provided a proof-of-concept for the hypothesis of residual inflammatory risk. Canakinumab reduced hsCRP by 35 % and did not affect, at any dose, LDL-C, HDL-C and TG levels [57]. After a median follow-up of 3.7 years, doses of 150 and 300 mg were superior to placebo in meeting the primary endpoint, i.e. a composite of nonfatal MI, nonfatal stroke, or CV death. The hazard ratio (HR) = 0.85 (95 %CI: 0.74-0.98), translated to a number-needed to treat (NNT) of 156 for 1 year to prevent an event; NNT becomes 189 when the analysis is confined to changes in MI (HR: 0.76). CV deaths were not significantly reduced (HR: 0.88) [58]. No reduction in the incidence of new-onset diabetes was found. Canakinumab reduced MACE rates to a similar extent in patients with or without diabetes [59] and was associated with a significant reduction in deaths from lung cancer [60]. Deaths from infection were instead significantly raised.
Among patients on canakinumab, those achieving on-treatment hsCRP ≤ 2 mg/L had a higher benefit in terms of reduction of major CV events (HR: 0.75; 95 % CI: 0.66-0.85), CV mortality (HR: 0.69; 95 % CI: 0.56-0.85) and all-cause mortality (HR: 0.69; 95 % CI: 0.58-0.81) regardless of canakinumab dose (Fig. 1 ) [61]. Reaching this hsCRP threshold translated into a 5-year NNT estimate of 16 for MI, stroke, coronary revascularization or all-cause death. For participants who did not achieve on-treatment hsCRP < 2 mg/dL the 5-year NNT estimate was 57 [61]. Among chronic kidney disease patients, canakinumab reduced the risk of MACEs by 18 % with the highest benefit for those achieving on-treatment hsCRP levels below 2 mg/L (-32 %) [62]. Canakinumab reduced in a dose dependent manner the composite of hospitalizations for heart failure or heart failure-related mortality: HR = 1.00 (50 mg, reference value), 0.88 (150 mg) and 0.78 (300 mg) [62].
Fig. 1.
A secondary analysis of CANTOS study showed that patients achieving on-treatment hsCRP concentrations, at 3 months, ≤ 2 mg/L had a higher benefit in terms of reduction in major CV events, CV mortality and all-cause mortality. The dash represents the reference value. CV, cardiovascular; MI, myocardial infarction (reproduced with permission of Taylor & Francis) [76].
A further trial investigating efficacy of anti-inflammatory treatments in coronary patients was the Cardiovascular Inflammation Reduction Trial (CIRT), designed to evaluate the effects of low dose methotrexate (15–20 mg per week) vs placebo. Patients had to have, in addition to prior MI or multivessel coronary disease, either type 2 diabetes or the metabolic syndrome. The trial was stopped since methotrexate did not result in the lowering of IL-1β, IL-6 or hsCRP vs placebo. The primary endpoint occurred in 201 patients in the methotrexate and 207 in the placebo group (HR 0.96, 95 % CI 0.79–1.16). Methotrexate treatment was associated with raised liver enzymes, reduction in leukocytes and hematocrit and a higher incidence of non-basal cell skin cancer vs placebo [63]. The different patient selection may explain the divergent results: in the CANTOS study patients were selected based on elevated hsCRP levels, whereas CIRT included patients with relatively normal inflammatory biomarkers (hsCRP = 1.6 mg/L), poorly modified by treatment [64].
Colchicine, of general use in the treatment of gouty attacks and pericarditis, by inhibiting neutrophil motility and with marked anti-inflammatory effects, particularly in reducing local cytokine production [65], and improving endothelial function [66] was the object of a large outcome trial, the Colchicine Cardiovascular Outcomes Trial (COLCOT). This examined the effects of low dose colchicine (0.5 mg/day) or placebo on CV events in 4,745 patients with ACS on optimal medical treatment, randomized within 30 days of the event [67]. The primary end-point occurred in 5.5 % of patients given colchicine vs 7.5 % in the placebo group (HR 0.77; 95 % CI 0.62-0.96) [68]. The effect was predominantly driven by lower risk of angina and stroke, without a significant effect on death from CV causes or MI incidence. No changes in hsCRP were detected, thus casting doubt on the postulated anti-inflammatory protective mechanism [69]. A number of other inflammation antagonists including those targeting L-6 or TNF-α have been also tested without success. TNF-α (etanercept) and IL-6 blockade (tociluzumab) tend to increase pro-atherogenic apo B containing lipoproteins [70,71].
A protective role of HDL in reducing plaque inflammation has been finally described recently [72] in diabetic mice, reporting a promoted atherosclerosis regression by raising HDL levels, a benefit linked to suppressed hyperglycemia induced hematopoiesis, monocytosis and neutrophilia. The improvement in cholesterol efflux from bone marrow progenitors led to a suppression of proliferation as well as to polarization of plaque macrophages to the atherosclerosis-resolving M2.
4. Lipid lowering agents and inflammation
4.1. Statins
The beneficial effect of statins on coronary events is generally assumed to be linked to their hypocholesterolemic properties [73]. Since mevalonic acid is the precursor of numerous metabolites, inhibition of HMG-CoA reductase potentially results in pleiotropic effects [74]. However, statins can decrease the number of LDL particles infiltrating the vessel wall and thus potentially reducing CRP. Statins do indeed display significant anti-inflammatory effects and this has led to some non-lipid indications, e.g. the treatment of periodontal inflammation associated with reduced carotid inflammation [75].
The role of hsCRP reduction in the large number of statin trials has been the object of a prior review article [76] and data are thus summarized in Table 2 . The early CARE (Cholesterol and Recurrent Events) trial showed that the relative CV risk reduction was larger in patients with elevated hsCRP [77]. This observation was confirmed in other studies, such as the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering), PROVE IT–TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22) and A to Z (Aggrastat-to-Zocor) [[78], [79], [80], [81]]. In all these, the largest benefit was found in patients achieving LDL-C < 70 mg/dL and hsCRP < 2 mg/L [82]. This was confirmed in the primary prevention JUPITER trial on 18,000 patients with median LDL-C 108 mg/dL and elevated hsCRP (> 2.0 mg/L) [83], where the largest reduction in CV events (-65 %), occurred upon achieving both LDL-C < 70 mg/dL and hsCRP < 2 mg/dL [84].
Table 2.
Percentage changes of hsCRP and LDL-C upon treatments with statins, ezetimibe and bempedoic acid.
| Clinical study | hsCRP (mg/L) |
LDL-C (mg/dL) |
|||||
|---|---|---|---|---|---|---|---|
| pre | post | Δ | pre | post | Δ | ||
| Statins | |||||||
| Pravastatin [247,248] | CARE | 2.3 | 1.9 | −17.4 % vs baseline | 139.2 | 98.0 | −32 % vs baseline |
| Pravastatin [249] | PRINCE | 2.4 | 2.0 | −16.6 % vs baseline | 142.9 | 97.5 | −31.8 % vs baseline |
| Lovastatin [250] | AFCAPS/TexCAPS | 1.6 | 1.3 | −14.8 % vs baseline | 156.0 | 115.0 | −27 % vs baseline |
| Atorvastatin [251,252] | MIRACL | 11.5 | 2.9 | −75.0 % vs placebo | 135.0 | 72.0 | −40 % vs placebo |
| Pravastatin [253] | REVERSAL | 3.0 | 2.9 | −5.2 % vs baseline | 150.2 | 110.4 | −25.2 % vs baseline |
| Atorvastatin [253] | REVERSAL | 3.0 | 1.8 | −36.4 % vs baseline | 150.2 | 78.9 | −46.3 % vs baseline |
| Pravastatin [80,254] | PROVE IT–TIMI 22 | 11.9 | 2.1 | −82.4 % vs baseline | 106.0 | 95.0 | −10.4 % vs baseline |
| Atorvastatin [80,254] | PROVE IT–TIMI 22 | 12.2 | 1.3 | −89.3 % vs baseline | 106.0 | 62.0 | −41.5 % vs baseline |
| Simvastatin [255] | A-to-Z Trial | 2.01 | 0.17 | −91.5 % vs baseline | 112.0 | 62.0 | −44.6 % vs baseline |
| Rosuvastatin [83] | JUPITER | 4.2 | 2.2 | −47.6 % vs baseline | 108.0 | 55.0 | −49.1 % vs baseline |
| Simvastatin [256] | Heart Protection Study | 3.07 | 2.24 | −27 % vs baseline | 127.9 | 95.9 | −25 % vs baseline |
| Atorvastatin [257] | ASCOT | 2.4 | 1.8 | −25.8 % vs baseline | 136.8 | 85.6 | −38.7 % vs baseline |
| Atorvastatin [258] | CARDS | 1.3 | 1.2 | −9.8 % vs baseline | 121.0 | 60.0 | −50.4 % vs baseline |
| Ezetimibe | |||||||
| Ezetimibe + atorvastatin [259] | 2.19 | 1.98 | −10 % vs atorvastatin | 101.8 | 89.5 | −12.1 % vs atorvastatin | |
| Ezetimibe + rosuvastatin [260] | EXPLORER | 1.7 | 1.2 | −17.8 % vs rosuvastatin | 81.5 | 56.9 | −30.2 % vs rosuvastatin |
| Ezetimibe + Simvastatin [90] | SHARP | 1.1 | 0.99 | −21 % vs placebo | 106.0 | 68.9 | −35 % vs placebo |
| Ezetimibe + Simvastatin [89] | IMPROVE-IT | 1.9 | 1.6 | −14 % vs simvastatin | 67.7 | 49.9 | −20 % vs simvastatin |
| Bempedoic acid | |||||||
| Bempedoic acid [93] | CLEAR Tranquility* | 2.21 | 1.35 | −32.5 % vs baseline | 129.8 | 96.2 | −23.5 % vs baseline |
| Bempedoic acid [94] | CLEAR Harmony*** | 1.49 | 1.25 | −14.4 % vs baseline | 103.6 | 88.9 | −12.6 % vs baseline |
| Bempedoic acid [95] | CLEAR Wisdom** | 1.61 | – | −24.1 % vs baseline | 119.4 | 99.7 | −12.1 % vs baseline |
| Bempedoic acid [96] | CLER Serenity** | 2.92 | 2.47 | −25.1 % vs baseline | 158.5 | 121.5 | −21.2 % vs baseline |
| Bempedoic acid plus ezetimibe* [97] | 3.1 | – | −35.1 % vs baseline | 154 | – | −36.2 % vs baseline | |
hsCRP, high-sensitivity c-reactive protein; LDL-C, low-density lipoprotein cholesterol; -, not available. Adapted from [76] with permission of Taylor & Francis.
Post = 12 weeks.
Post = 24 weeks.
Post = 52 weeks.
4.2. Ezetimibe
Ezetimibe, an inhibitor of the cholesterol transport protein NPC1-like 1, reduces intestinal cholesterol absorption [85]. When added to statins, it leads to an incremental lowering of LDL-C levels and improved cardiovascular outcomes [86].
In a study on rabbits on a high fat diet and atherosclerosis, ezetimibe led to both reduced progression of atheromas and plaque stabilization. CRP levels were significantly reduced with no further reduction occurring by the addition of simvastatin [87]. Clinical studies did not fully confirm this conclusion, i.e. a pooled analysis of trials in hypercholesterolemic patients showed no significant hsCRP changes. Conversely, when ezetimibe was added to baseline statin therapy, a -10 % reduction of hsCRP was observed [88]. In the recent IMPROVE-IT trial, adding ezetimibe to simvastatin gave a further 20 % and 14 % reduction of LDL-C and hsCRP, respectively [89]. In chronic kidney disease patients (SHARP trial), the combination simvastatin/ezetimibe resulted in a 35 % reduction of LDL-C vs placebo and a 21 % reduction of hsCRP [90] (Table 2).
4.3. Bempedoic acid
This prodrug, targeting ATP-citrate lyase (ACL), is primarily responsible for the production of extra-mitochondrial acetyl-CoA [91], the carbon precursor for cholesterol and fatty acid biosynthesis. Bempedoic acid is metabolized to the active form only in the liver, not in muscle.
Phase 3 studies have indicated a stable LDL-C reduction in the range of 25–35 %, additional to that of statins, with minimal muscular side effects. Clinical studies have consistently shown a marked hsCRP lowering activity, the highest efficacy being reported in diabetics [92], i.e. -41 % (median changes). In the CLEAR Tranquility trial on statin intolerant patients, bempedoic acid (180 mg) added to background lipid-modifying therapy reduced LDL-C and hsCRP by -28.5 % and -34.6 %, respectively (vs placebo) [93]. In the CLEAR Harmony trial, enrolling patients with LDL-C of at least 70 mg/dL despite maximum tolerated statin therapy, bempedoic acid treatment resulted in greater LDL-C lowering vs placebo at week 12 (difference, -18.1 %), 24 (difference, -16.1 %) and 52 (difference, -13.6 %). Relative to hsCRP, the absolute difference was -25 %, -19.1 % and -16.2 % at weeks 12, 24, and 52 respectively [94] (Table 2).
In the CLEAR Wisdom trial, enrolling patients with atherosclerotic cardiovascular diseases (ASCVD), heterozygous familial hypercholesterolemia, or both, on optimal statin treatment, bempedoic acid was superior to placebo in reducing hsCRP at weeks 12 and 24 with an absolute difference of -8.7 % and -21.3 %, respectively. At week 52, the difference between groups was not statistically significant (-7.6 %; p = 0.10). At enrolment, hsCRP levels were 1.61 (mg/L) in the bempedoic and 1.88 (mg/L) in the placebo group [95] (Table 2).
The CLEAR Serenity trial evaluated the LDL lowering activity and tolerability of bempedoic acid in statin intolerant patients with ASCVD and inadequately controlled LDL-C over a period of 24 weeks. After a 12-week treatment, in patients on very low dose statin, other lipid-modifying therapy or no therapy, hsCRP fell by 28 % (vs placebo). This effect was maintained at week 24 (-27.1 %) [96].
Combination of bempedoic acid with ezetimibe dramatically reduced LDL-C up to -41 %. Bempedoic acid plus ezetimibe reduced hsCRP by 35.1 % compared to a rise of 21.6 % in the placebo group and an 8.2 % reduction with ezetimibe, given as a monotherapy [97]. The combination therapy was not superior to bempedoic acid alone, probably due to the considerable hsCRP lowering achieved in monotherapy (-31.9 %). In each treatment group, baseline levels of hsCRP were 3.1 mg/L (bempedoic acid + ezetimibe), 2.9 mg/L (bempedoic acid), 2.8 mg/L (ezetimibe), and 3.0 mg/L (placebo) [97,98] (Table 2).
4.4. Nicotinic acid
Nicotinic acid (niacin or vitamin B3), at pharmacological doses, i.e. about 1–2 g/day exerts a powerful antilipolytic activity, reducing free fatty acid (FFA) release from the adipose tissue to the liver, where triglycerides (TG) re-synthesis occurs [99]. Some Authors believe that nicotinic acid, similar to fibrates, may exert a moderate stimulation of peroxisomal proliferation as a PPARα agonist [100]. In this way nicotinic acid may activate lipoprotein lipase reducing TG, to a lesser extent LDL-C, and raising HDL-C levels [101].
The profile of nicotinic acid, particularly the significant positive impact on HDL-C levels, has led to large placebo controlled studies in ASCVD patients [102,103] given extended release nicotinic acid. These studies did not result in a significant reduction of major coronary events. In metabolic syndrome [104], nicotinic acid reduced hsCRP by -40 % (from 2.7 ± 0.55 mg/L to 1.7 ± 0.25 mg/L), and similar, albeit lesser reductions, were noted for TNF-α, Plasminogen activator inhibitor-1 (probably reflecting the reduction of TGs) and IL-7, not IL-6. In a comparative evaluation of nicotinic acid and fenofibrate, the former was more effective at lowering hsCRP, though fenofibrate led to a more beneficial lipoprotein profile [105].
4.5. Fibrates
These activators of the PPARα system have not shown clear effects on hsCRP. A 5-year treatment with fenofibrate significantly reduced LDL-C (14.2 %) but not hsCRP (+ 0.8 mg/L) [106], a finding similar to the data described in a sub-analysis of the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) trial in type 2 diabetics [107]. In patients with mixed dyslipidemia, rosuvastatin (40 mg), rosuvastatin (10 mg) plus fenofibrate (200 mg) or rosuvastatin (10 mg) plus omega 3 (2 g) led to 53 %, 28 % and 23 % reductions in hsCRP, respectively, vs baseline [108]. In patients with the metabolic syndrome, however, a highly significant hsCRP reduction of 49.5 % was reported [109].
4.6. Proprotein convertase subtilisin/kexin 9 (PCSK9) antagonists
PCSK9 is a liver-secreted plasma protein that regulates the number of cell-surface LDL receptors, thus inhibiting LDL uptake [110,111]. Two fully human mAbs, alirocumab and evolocumab, have been approved for human use in August 2015, whereas bococizumab has been discontinued in November 2016 [112]. Other approaches to inhibit PCSK9 are being developed, including small molecule inhibitors, antisense oligonucleotides, RNA interference therapies (inclisiran) and a vaccine [113,114].
Raised PCSK9 liver expression is positively linked to TNF-α and interferon γ levels [115,116]], but no clear relationship has been observed between PCSK9 levels and hsCRP. This supports the observation that PCSK9 antagonists do not exert a significant anti-inflammatory activity. As previously reviewed by our group [117], although there is no impact on hsCRP, the application of this biomarker identifies patients with a high CV risk achieving better ASCVD prevention after PCSK9 inhibition. In the FOURIER study, even in patients with extremely low levels of LDL-C, there was a stepwise risk increment according to baseline hsCRP: +9 % (<1 mg/L), +10.8 % (1–3 mg/L) and +13.1 % (>3 mg/L) [118]. The ODYSSEY OUTCOMES with alirocumab showed no absolute changes for hsCRP although baseline hsCRP levels did identify subjects at higher risk [119]. The debate on whether or not LDL-C and hsCRP are inseparable risk markers of risk was addressed in a post hoc analysis of the SPIRE-1 and -2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events) trials, with bococizumab. This latter led to a dramatic reduction in LDL-C, but when patients were stratified according to on-treatment hsCRP levels, i.e. <1 (reference value), 1–3, and >3 mg/L, HRs were 1, 1.16 (95 % CI 0.81–1.66) and 1.62 (95 % CI 1.14–2.30) [120] (Table 3 ).
Table 3.
Percentage changes of hsCRP and LDL-C upon drug treatments with PCSK9 inhibitors.
| Clinical study | hsCRP (mg/L) |
LDL-C (mg/dL) |
|||||
|---|---|---|---|---|---|---|---|
| Pre | post | Δ | pre | post | Δ | ||
| Evolocumab (PCSK9 fully human mAb) | FOURIER [118] | 1.7 | 1.4 | 0 % vs placebo | 92.0 | 30.0 | −59 % vs placebo |
| Bococizumab (PCSK9 humanized mAb) | SPIRE-1 and -2 [118] | 1.88 | 1.84 | at week 14: mean change was +6.6 % vs placebo (median change 0 %); at week 52: +6.7 % | 96.5 | 34.7 | −60.5 % vs placebo (week 14) |
| Alirocumab (PCSK9 fully human mAb) | ODYSSEY OUTCOMES [261] | 1.6 (0.8-3.9) | – | – | 87.0 | 53.0 | −54.7 % vs placebo |
FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; SPIRE, Studies of PCSK9 Inhibition and the Reduction of vascular Events; ODYSSEY OUTCOMES, Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. mAb, monoclonal antibody; -, not available.
The lack of effect on hsCRP was also a common feature of inclisiran, a small-interfering RNA (siRNA) targeting PCSK9 [117]. Recently, no significant effects on leucocytes, monocytes or neutrophils were reported, as well as no significant decrement in IL-6 (-21.3) and TNF-α (-12.3) at the two-dose regimens of 100 mg, 200 mg or 300 mg (at 1 and 90 days) [121].
4.7. Lomitapide
This powerful inhibitor of the microsomal transfer protein (MTP) system was developed for the treatment of homozygous type II patients. While clinical studies have not been addressed to the evaluation of inflammatory/anti-inflammatory markers, of special interest is the very long study on efficacy/safety in homozygous patients [122]. In addition to a very effective LDL-C reduction (-45.5 %), the evaluation of data after 246 weeks of treatment reported a moderate rise in liver fat (from 0.7 to 10.2 %) and, most interestingly, a progressive reduction of hsCRP, with a dramatic change, i.e. -60 % from baseline to week 246. This finding, certainly of high clinical interest, may be also linked to the drug’s activity on the human ether-a-go-go-related gene (hERG) channel currents, observed only at high concentrations (>1.7 μM) [123].
4.8. Apabetalone
Among lesser evaluated pathways underlying vascular disease and particularly vascular calcification are the bromo- and extra-terminal (BET) proteins (BRD2, BRD3 and BRD4). These are epigenetic readers that bind acetylated lysines on histone tails transcription factors via bromodomains (BD 1 and 2) [124]. BET proteins play a role as molecular scaffolds between acetylation dependent binding sites and transcriptional machinery. Interest in this pathway has been raised after the observation that thienotriazolodiazepines, reportedly increasing plasma levels of apo AI and macrophage reverse cholesterol transport in mice [125], inhibit the BET proteins (BRTD, BRD2, BRD3 and BRD4), this being apparently the main molecular mechanism for the apo AI change.
In view of the non-optimal activity of the thienotriazolodiazepines, affecting essentially all BET associated proteins, a compound that has entered clinical trial is apabetalone, preferentially targeting BRD4 and the bromodomain BD2 [126]. Apabetalone (RVX208) has been shown to raise HDL-C from 3.2 to 8.3 %, particularly the larger HDL particles in humans [127].
Besides the HDL-C raising capacity, apabetalone is able to reduce factors and pathways associated with vascular calcification, i.e. by inhibiting extracellular calcium deposition and reducing differentiation markers in coronary artery vascular SMCs in osteogenic conditions [128]. The mechanism of reduced calcification of VSMCs appears to be a true epigenetic mechanism involving BDR4. Apabetalone also appeared to reduce alkaline phosphatase (ALP) levels and also hsCRP, the two being correlated, although reduced ALP predicted CV risk independent of hsCRP, thus possibly providing a novel mechanism of CV protection [129].
While the numerous early studies on the CV preventive activity of apabetalone had failed to provide convincing evidence of significant effectiveness [130], a pooled analysis of these studies [131] had shown, in contrast, a close to 40 % reduction of events following drug treatment. This encouraged planning a secondary prevention trial in type 2 diabetics with low HDL-C (<40 mg/dL in males and <45 mg/dL in females) on optimal lipid-lowering therapy. They were randomized to apabetalone (100 mg b.i.d.) or a matching placebo [132]. After a median follow-up of 26.5 months, 124 primary endpoints (non-fatal MI, CV death or stroke), i.e. 10.3 %, occurred in drug-treated, vs 149 (12.4 %) in placebo-treated patients (HR 0.82: 95 % CI, 0.64–1.04). More patients allocated to apabetalone interrupted treatment (9.4 vs 5.7 % for the placebo group) for reasons including elevation of liver enzyme activities. Plasma lipid and biomarker changes were of a modest degree: there were no statistically significant differences in LDL-C levels, hsCRP and HbA1c. At week 24, mean HDL-C levels rose from a mean of 33 mg/dL to 38.0 mg/dL with apabetalone and to 36.6 mg/dL with placebo [133]. An interesting opening for apabetalone is the recently described potential activity of BRD2 and BRD4 inhibitors on SARS-CoV-2, by way of an interaction with transmembrane protein E of the virus [134].
5. Hypertension and inflammation
Recent developments in the understanding of CV risk associated to hypertension have pointed out the role of novel actors in the regulation of immune phenomena. Aside from the classical Th1 and Th2 cells, regulatory T-cells (Tregs) and Th17 have shown differential plasticities, indicating that in particular Tregs appear to attenuate hypertension target organ damage, whereas Th17 cells may exacerbate damage. Tregs are characterized by the ability to suppress inflammatory signaling of immune and non-immune cells [135] and may reduce target organ damage in hypertensive models, possibly by way of IL-10 and TGFβ mediated immunosuppression [136]. Th17 cells are instead characterized by the expression of transcription factor retinoic acid-related orphan receptor (ROR)γ together with the production of IL-17. This exacerbates tissue damage in hypertensives [137]. Tregs have been implicated in hypertension protection experienced by females, displaying, among others, a larger percentage of infiltrating T-cells in female spontaneously hypertensive rats, compared to males [138].
Inflammatory pathways and mediators are used by immune cells to drive high blood pressure (BP) and end-organ damage. Activation of immune cells and recruitment to a target organ leads to production of cytokines and chemokines that determine local inflammatory responses. Inflammatory cytokines and chemokines involved in hypertension are TNF-α, IL-17, MCP-1, and IL-6 [139]. Two TNF-α receptors have been described: TNFR1 and TNFR2 [140]. In humans, high serum TNFR1 levels strongly correlate with hypertension associated diseases, e.g. end-stage renal disease and type 2 diabetes [141]. There are, however, reports indicating that genetic deletion of TNFR1 leads to increased BP in response to angiotensin (Ang) II [142]. The epidemiological Malmo Diet and Cancer Study investigated the association of TNFR1 and TNFR2 in middle-aged individuals and reported that both were associated with an increased risk of intracerebral hemorrhage (ICH), suggesting that TNF mediated inflammation may lead to vascular changes preceding ICH [143]. Studies in hypertensive patients have shown a graded relationship of raised Intercellular adhesion molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) with blood pressure, suggestive of increased inflammatory activity in the disease [144].
5.1. Angiotensin converting enzyme (ACE)-inhibitors
ACE-inhibitors exert a significant anti-inflammatory activity at the vascular level. AngII treatment in rodents elevates BP and VCAM-1; chemokines such as monocyte chemotactic protein-1 (MCP-1) and macrophage-colony stimulating factor (M-CSF) are up-regulated in the aortas of Ang II-treated mice. In these animals, enalapril treatment reduces atherosclerosis, but in particular, vascular inflammation, by markedly reducing elevated chemokine levels [145]. A direct clinical comparison of a major ACE-inhibitor, ramipril, with the angiotensin receptor blocker telmisartan, in type 2 diabetics, showed that both exerted a similar anti-inflammatory activity, as evaluated from a fall of hsCRP [146]. The mechanisms of the proinflammatory activity of ACE activation and its inhibition by ACE inhibitors have been extensively investigated. ACE stimulation enhances the expression of inducible NO synthase and COX-2 by NF-κB activation [147], also raising the production of reactive oxygen species and of proinflammatory T cells. ACE inhibition suppresses Th17- and Th1-mediated autoimmunity, also promoting production of Treg, favorably modulating inflammation [148]. Ang II may also activate the TLR4 signaling pathway, leading to inflammatory synergism. These proinflammatory cytokines enhance abnormal serine phosphorylation of IRS-1, thus causing insulin resistance [149]. Treatment with ACE-inhibitors will rescue insulin-resistance and, in some cases even cause hypoglycemia [150].
5.2. Sartans
Among sartans with apparent additional anti-inflammatory properties, telmisartan has received the largest attention. This agent can reduce vascular SMC proliferation by a potentially additive mechanism to angiotensin receptor blockade and PPARγ activation [151]. When given to HIV+ patients with excess adiposity, a 40 mg/day treatment led to a significant 5 % visceral adipose tissue loss with raised urinary excretion of prostaglandin E2. This supports the conclusion that the anti-inflammatory activity may be linked to stimulated eicosanoid metabolism [152]. It is of interest that candesartan, another angiotensin receptor blocker, may reduce TLR2, TLR4 and NFkB activation in monocytes, thus displaying a significant anti-inflammatory effect [153]. The Val-MARC study in the US compared valsartan vs valsartan/hydrochlorothiazide in patients with stage 2 hypertension [154]. While the combination had a clearly better hypotensive activity vs valsartan monotherapy, valsartan alone reduced hsCRP to a larger extent (-8.5 %) vs the combination (+5.2 %), with modest non significantly different lowering effects on ICAM-1 and VCAM-1 vs the combination [155], possibly indicating a larger plaque stabilizing activity.
5.3. Calcium-channel antagonists
The therapeutic approach with calcium-channel antagonists [calcium channel blockers, (CCB)] is also dependent on their anti-inflammatory activity at the vascular level. Ca2+ is raised at inflammation sites and activation of the calcium-sensing receptor (CaSR) may play a pivotal role. CaSR, a cell surface coupled G protein receptor, in the vascular area can regulate the NLRP3 inflammasome, acting by way of Ca2+ and cAMP [156].
Significant differences can be found between the two classes of CCBs, although neither exerts a direct anti-inflammatory activity. The dihydropyridine CCBs, nifedipine and amlodipine, antagonize L-type calcium channels and have antioxidant effects [157]. The rate-limiting CCBs such as verapamil, may also improve endothelial function, indicating that the benefits of these agents may be related to an improved nitric oxide availability [158]. In gluteal resistance arteries in hypertensive patients, treatment with the CCB nifedipine for 1 year improved vascular relaxation responses to acetylcholine, not observed with atenolol [159]. In the coronary vasculature, nicardipine can reverse endothelial dysfunction in non-stenotic segments from hypertensive patients and in stenotic segments from both normotensives and hypertensives [160]. Nicardipine has been shown to improve endothelium-dependent vasodilation in patients with essential hypertension [161] and to raise coronary vascular responses to acetylcholine. Amlodipine increases basal NO release, whereas lacidipine enhanced the vasodilator responses to both acetylcholine and bradykinin within the forearm circulation of hypertensive patients [162].
6. Diabetes and inflammation
The pattern of pathological responses characteristic of type 2 diabetes is dependent on the sensitivity of vessels to oxidative stress, hypertension, dyslipidemia and aging, all determining the risk of atherosclerosis progression and regression. In diabetes, endothelial cell dysfunction is of frequent occurrence [163]. It is characterized by raised production of cytokines and adhesion molecules: altogether they initiate the inflammatory driven responses including leukocyte recruitment and platelet activation. Characteristic of diabetes is, in particular, the activation of the proteolytic enzymes known as matrix metalloproteinases (MMPs). MMP activity is regulated by the endogenous tissue inhibitor of metalloproteinases (TIMP). MMPs mediate the retinal neuropathy and vasculopathy that are associated with diabetes [164].
Hyperglycemia and elevated FFAs may promote inflammation by stimulating glucose utilization with altered oxidative phosphorylation. This will induce a proinflammatory trait in macrophages in the adipose tissue and other tissues, including the vasculature [165]. Glucotoxicity and lipotoxicity, by exerting oxidative and endoplasmic reticulum stress, elicit an inflammatory response by activating thioredoxin interacting proteins (TXNP and NLR families) including NLRP3. This latter stimulates the release of mature IL1-β [166] which further amplifies inflammation by recruiting immune cells including macrophages [167].
Among the molecular mechanisms underlying diabetes related inflammation is the reduction of fatty acid synthase (FAS). FAS, a large molecule producing mainly palmitate, as well as other mainly saturated FFAs, is reduced in many tissues in diabetic models. Liver specific inactivation of FAS leads to fatty liver, hypoglycemia and enhanced insulin sensitivity, a picture not different from models of the deficiency of the nuclear receptor PPARα [168]. FAS deficiency, in contrast, leads to protection from insulin resistance and with disrupted function of Cdc42, a member the RhoGTPase family, coordinating macrophage activation in target tissues [169]. Reduced activation is associated with altered phospholipid and protein composition, responsible for the inflammatory signaling coordinated by RhoGTPase. These findings have contributed to the understanding of the role of cholesterol in the inflammatory induction. FAS is required for the assembly of cholesterol and sphingomyelin in the plasma membranes of macrophages [170]. Membrane phospholipids control clustering of domains required for signaling such as in dendritic cell activation [171], Th-17 helper cell differentiation [172] and other mechanism of activation of inflammasomes in macrophages. FAS inhibition thus may lead to reduced atherosclerosis, less adiposity and reduced blood glucose in skeletal muscle. This basic knowledge allows the evaluation of potential links between inflammation and antidiabetic drugs, active not only on glucose, but also on different steps in the diabetes related inflammatory process.
6.1. Metformin
This compound is the most widely used glucose lowering drug, whose effects are believed to be mediated by activation of AMPK, a key regulator of energy homeostasis [173]. Metformin directly inhibits the production of reactive oxygen species from complex I (NADH: ubiquinone oxido-reductase) of the mitochondrial electron transport chain. Metformin inhibits the pro-form of the IL-1β in lipopolysaccharide (LPS) activated macrophages while stimulating induction of the anti-inflammatory cytokine IL-10 [174].
In the vascular endothelium, metformin inhibits monocyte-to-macrophage differentiation [175]. While these effects in rodents are well established, in particular by reducing the proinflammatory and proapoptotic protein TXNIP in β-cells and hepatocytes [176], data in humans are not well clarified. Metformin appeared to reduce hsCRP to a modest extent in patients in the US Diabetes Prevention Program [177]. In the LANCET Trial (Long acting Insulin Injections to Reduce C-Reactive Protein in Patients with Type 2 Diabetes), metformin did not modify inflammatory markers in patients with recent onset type 2 diabetes [178]. In recent years, exciting news have been provided on the potential beneficial effects of metformin in chronic inflammatory disorders and cancer, also extending lifespan independent of glucose metabolism [179].
6.2. Sulphonylureas and thiazolidinediones
While losing clinical interest because of their high potential to induce hypoglycemia, sulphonylureas have been shown to exert potentially significant anti-inflammatory effects. Glyburide appears to inhibit the NLRP3 inflammasome and the subsequent IL-1β activation in macrophages [180]. Gliclazide seems to exert a similar effect, i.e. reduced endothelial dysfunction in type 2 diabetes patients [181]. In clinical trials, however, sulphonylureas did not appear to exert a significant influence on hsCRP levels, whereas reductions were found after treatment with the thiazolidinediones (TZDs) [182].
TZDs are PPARγ agonists that increase insulin sensitivity. There is experimental evidence that PPARγ and AMPK are both targets of TZD with consequent significant anti-inflammatory effects in liver and adipose tissue [183]. Pioglitazone treatment may thus reduce infiltration of adipose tissue by proinflammatory macrophages, leading to improved hepatic and peripheral insulin sensitivity, potentially exerting an ameliorating effect on liver histology in patients with nonalcoholic steatohepatitis (NASH) [184].
Treatment with TZDs, in addition to improving endothelial function, can significant reduce hsCRP levels in people with and without diabetes irrespective of glycemic effects [185]. The apparent anti-inflammatory activity of PPARγ agonists may be possibly related to the novel observation of their interaction with NLRC5, inhibiting vascular neointima formation [22]. The reduced hsCRP and increased HDL-C after pioglitazone treatment may explain the observed consequent reduction in CV morbidity [186].
6.3. Incretins and incretin drugs
Incretin drugs are a new widely used group of glucose lowering agents which antagonize glucagon secretion and delay gastric emptying. Two groups of incretin hormones, the glucagon-like peptide-1 (GLP-1) and the glucose-dependent insulinotropic polypeptide (GIP) stimulate insulin secretion in a glucose-dependent manner and, in the case of GIP, reduce gastric acid secretion. Incretin hormone secretion can be activated by inflammatory mediators such as IL-6 and IL-1β [187]. The rapid degradation of GLP-1 and GIP by dipeptidyl peptidase-4 (DPP-IV) has led to the development of two new classes of incretin-based drugs, i.e. GLP-1R agonists and DPP-IV inhibitors [188]. GLP-1R agonists include liraglutide, exenatide, lixisenatide and albiglutide; DPP-IV inhibitors target degradation systems of incretin hormones and include sitagliptin, vildagliptin, saxagliptin, linagliptin and alogliptin [189]. These agents are listed in the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Guidelines [190].
Both drug classes have given substantial evidence of improving a variety of CV risk factors as well as inflammation. In particular DPP-IV inhibitors can suppress NLRP3, TLR4 and IL-1β expression in macrophages [191]. They can also reduce mRNA expression of CD26, TNF-α, proinflammatory kinases, and chemokine receptor CCR2, as well as plasma hsCRP, IL-6 and FFA [192]. In a clinical study evaluating endothelial dysfunction and inflammation in patients with type 2 diabetes treated with sitagliptin, there was clear evidence of reduced levels of markers including hsCRP, L-6, IL-1β phospholipase-A2, ICAM-1 and E selectin [193].
The case of GLP-1R agonists (GLP-1RAs) has provided somewhat similar findings in the lipid profile but also in statistically significant reductions of markers including hsCRP, brain natriuretic peptide and PAI-1 [194]. Balestrieri et al. tested the anti-inflammatory activity in diabetic atherosclerotic plaques, reporting that plaques from incretin-treated type 2 diabetic patients are characterized by increased collagen content, raised sirtuin-6 expression and inhibited inflammatory response with reduced numbers of macrophages, T cells and lower expression of MMP-9 and TNFα [195].
Incretin drugs, particularly GLP-1RAs, although effective on glycemia and well tolerated, have not generally provided consistent reductions in CV events. In the case of DPP-4 inhibitors outcome trials, the three-point MACE evaluation of the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial reported significant reductions of events at all ages with somewhat reduced efficacy in individuals ≥ 60y [196]. The SUSTAIN trial with semaglutide with a similar design again resulted in a significant reduction of three-point MACEs with no differences between younger and older patients [197].
6.4. Sodium glucose cotransporter 2 inhibitors (SLGT2)
In all clinical trials, SGLT2 have consistently shown a CV benefit, both in younger and older individuals. Very interestingly, in the case of empagliflozin (EMPA-REG Trial) and canagliflozin (CANVAS Trial) [198,199], CV event reduction was not only of the ischemic type but, more frequently, consequent to reduced heart failure. These impressive results on heart failure are the object of intensive investigation and the recently completed DECLARE TIMI 58 with dapagliflozin confirmed the improvement of the heart failure endpoint but no clear benefit on MACE endpoints [200].
The significant reduction in hospitalizations for heart failure and progression of renal disease have not found a clear support from changes in the inflammatory profile in earlier studies with the SGLT inhibitor phlorizin in obese gerbils. In these, a decrement in islet inflammation, possibly related to reduced glucose toxicity, was detected [201]. Similarly, in mice with type 2 diabetes, the SGLT2 inhibitor empaglifozin, in addition to positive changes in hyperglycemia and hyperlipidemia, reduced markers of inflammation (IL-6, TNF-α, MCP-1 and CRP levels) [202]. In the same experimental model, no data on inflammation were reported with canagliflozin [203].
Although data of this type have not been made available in the cardiovascular outcome trials with canagliflozin, 12 months of treatment with empagliflozin decreased hsCRP (-43 %) and lowered levels of remnant related lipoproteins probably via ameliorating insulin resistance [Hattori, 2018 #402].
The DECLARE TIMI 58 study [200] with dapagliflozin did not provide data on inflammation. While expecting clinical data from the CREDENCE Study with canagliflozin [205], attempting to establish inflammatory biomarker changes, a number of exploratory data have been provided with SLGT2 inhibitors mainly on short-term evaluations. Reduction in leptin levels and increased adiponectin are generally reported, as well as modest reductions in hsCRP TNF-α, IL-6 and IFNγ with canagliflozin, dapagliflozin and empaglifozin, mainly not reaching statistical significance [204]. In the CANTATA-SU Study [206], with canagliflozin, there was a 22 % reduction of IL-6 and a slight reduction of hsCRP (-4.4 %). Conversely, a 7 % increase in TNF-α was detected after canagliflozin vs glimepiride (Table 4 ). Overall the effects on inflammatory markers are very modest and probably not contributing to the CV outcomes.
Table 4.
Changes of inflammatory biomarkers upon drug treatments with SGLT-2 inhibitors.
| Clinical study | IL-6 | hsCRP | TNF-α | |
|---|---|---|---|---|
| Canagliflozin [262] | CANTATA-SU | −22 % | −4.4 % | +7 % |
| Empagliflozin [263] | Patients with a history of medication with SGLT2 inhibitors | – | −54 % | – |
| Dapagliflozin [264] | Patients with Nonalcoholic Steatohepatitis | – | From 0.26 (0.11-0-53) to 0.14 (0.08-0.26); p < 0.001 (after 4 weeks) | – |
SGLT-2, Sodium Glucose Cotransporter 2 Inhibitors; -, not available.
7. Microbiome and inflammation
The trillions of microbes colonizing the human intestinal tract are of major importance for human development and physiology [207]. Alterations of the microbiome, however, may be associated with multiple disorders including a raised prevalence of atherosclerotic diseases [208]. Among the best-studied mechanisms underlying this association is the increase of circulating trimethylamine oxide (TMAO) derived from the metabolism of choline and carnitine by the gut microbiome [209]. More recently the same authors indicated that the TMAO precursor trimethylysine is also associated with adverse cardiac risk and the two together provide the best risk estimate [210]. Contrasting data have been reported in an animal model: in the apoE−/− mice, Collins et al. [211] found that carnitine fed animals have raised TMAO levels but these are inversely related to aortic lesion formation. In the same model, mice fed chow or Western diet and low or no choline supplement showed raised plasma TMAO levels in the latter group in conventional (not germ free) mice [212]. Choline treatment did not affect aortic lesion size or plasma cholesterol and there was no difference in glycoprotein CD68 in macrophages. Similarly, no clear inflammatory related responses of microbiota to choline could be found, following evaluation of 10 cytokines in plasma from apo E-/- mice. There is, however, clear evidence for a wide dispersion of baseline data in the clinical studies [213]. In order to improve prediction, very recently Cassambai et al [214] suggested a choline loading test (700 mg oral choline bitartrate) followed by oral determinations for 8 h, indeed showing clear interindividual differences.
There are limited data on the direct effect of microbial changes on, particularly, endothelial function and the endothelial inflammatory phenotype. Limited information in rodent models has indicated that a major probiotic, Lactobacillus plantarum, may reduce myocardial infarct size in hypertensive rats [215], as well as suppress LPS-mediated atherosclerotic plaque inflammation [216]. The potential of Lactobacillus plantarum 299v (Ly299v) was evaluated by Malik et al [217] that directly tested in ASCVD patients the effects of oral Lp299v (20 billion-colony forming units (CFU) qd) for 6 weeks. Treatment did not change lipids, glucose or BMI, but improved flow-mediated dilation. While TMAO levels were unchanged, significant reductions of IL-8 and IL-12 were recorded. Resistance arteries isolated from patients after treatment and exposed to postbiotic plasma showed raised endothelium-dependent vasodilation.
The positive results of this last study suggested the experimental evaluation in a more frequent case of inflammation-mediated endothelial dysfunction and arterial stiffening, i.e. aging [218]. These Authors, by suppressing gut microbiota with antibiotic treatment in old mice noted reversed endothelial dysfunction and arterial stiffening, with attenuated vascular oxidative stress and inflammation. Plasma levels of TMAO were also suppressed by antibiotic treatment. The Authors noted an increase of the phylum Proteobacteria in antibiotic treated mice, almost entirely attributable to unclassified species within the Enterobacteriaceae. The issue of proinflammatory gut microbiota was examined by the use of caspase 1−/− mice with proinflammatory microbiota in LDLr−/− mice [219]. When proinflammatory casp1−/− microbiota was introduced into LDLr−/− mice a remarkable increase of systemic inflammation and atherogenesis was reported.
The aim of identifying enteral bacteria, possibly responsible for reducing inflammation and improving endothelial function, has called attention to the Akkermansia muciniphila, an enteral commensal anaerobe, postulated to exert potential anti-obesity effects, linked to reduced insulin-resistance [220]. Interestingly, beneficial properties appear to be improved by pasteurization [221]. Three-month supplementation of pasteurized A. muciniphila (1010 CFU daily) in insulin resistant volunteers reduced circulating LPS endotoxemia and white blood cells [222]. These findings support the hypothesis, now held by several Authors, that probiotic treatment with live bacteria may improve barrier function in the intestine, thus ameliorating both metabolic parameters as well inflammation [223]. It may lead to improved vascular function in aging and vascular disease.
8. Nutraceuticals
Diet, the most common variable for all individuals, may influence inflammation, in particular vascular inflammation, to a different extent according to general components, as well as to components with a drug-like activity, among these unsaturated fatty acids and commonly used nutraceuticals.
8.1. Omega- 6 fatty acids
Omega-6 fatty acids are a major component of the daily lipid intake. The most represented polyunsaturated fatty acid (PUFA) is linoleic acid (LA:18:2 n-6). A most recent assessment of omega-6 PUFA intake among adults in the UK was 10.9 ± 4.7 g/day, most of which (at least 90 %) would be LA, responsible for about 7 % of daily energy. LA is converted after a series of steps to γ-linolenic acid, dihomo-γ-linolenic acid and arachidonic acid (ARA) [224]. This last is the major precursor of eicosanoids, being converted by cyclooxygenase-1 (COX-1) to prostaglandins/thromboxanes. Concentrations of ARA-derived eicosanoids are elevated in inflammatory conditions. Pathways to ARA synthesis from LA is generally saturated in the presence of a high LA intake in humans (likely to be around 10 g/d) and thus raised LA intake will have no further effect in promoting ARA synthesis. This generally leads to unchanged levels of ARA in circulating mononuclear cells (MCs), and it is unlikely to see much of a change in inflammatory markers following raised dietary LA in individuals on a normal diet [225].
The major potential effect of LA itself on inflammation is its metabolism to lipoxygenase (LOX) derivatives such as the hydroxydecadienoic acids (HODEs) and further to oxo-HODEs and epoxy-HODEs, playing a role in inflammation and having been detected in colonic mucosal biopsies of patients with ulcerative colitis without, however, being significantly related to the degree of inflammation [226]. Dietary LA intake was not associated with the inflammatory markers CRP, IL-6, soluble (s)TNF-R and sTNF-R2 [227,228]. Despite a long-held belief, available evidence does not support the conclusion that a high dietary intake or high plasma LA concentrations raise tissue ARA or change inflammatory marker concentrations in humans.
The lack of a significant anti-inflammatory effect of LA or in general omega-6 fatty acids in spite of the beneficial activity on CV prevention is well supported by the very recent novel Consortium Evaluation of 30 prospective observational studies from 13 countries (follow up ranging between 2.5 to 31.9 years) [229]. High LA levels were associated with a lower risk of total CVD, CV mortality and ischemic stroke, with ratios between 0.93 and 0.88. ARA levels were instead not associated with a high risk of CV outcomes. Paradoxically, in extreme quintiles, higher ARA levels were linked to a lower risk of total CVD, thus supporting in general a favorable role for LA in CVD prevention.
8.2. Omega-3 fatty acids
Dietary intake of omega-3 fatty acids is essential for health, since these PUFAs cannot be synthesized to a significant extent. Omega-3 PUFAs are regarded as anti-inflammatory, exerting their effects via multiple mechanisms [230]. These include the partial inhibition of a number of steps of inflammation such as leukocyte chemotaxis, production of inflammatory cytokines, adhesion molecule expression, leukocyte-endothelial adhesive interactions, and Th1 lymphocyte reactivity [231]. From the results of a number of randomized controlled trials, the anti-inflammatory effect of omega-3 PUFA was associated to a reduction of hsCRP, IL-6 and TNF [232]. The basic mechanism is the incorporation of EPA and docosahexaenoic acid (DHA) into cell membranes at the expense of ARA, resulting in inhibited ARA metabolism and consequent decreased expression of the COX gene and, as a final consequence, reduction of ARA derived eicosanoids.
More recently, a major advance in the field of PUFA and inflammation has been the discovery of the so called pro-resolving lipid mediators. These mediators include resolvins from EPA (E-series) and DHA (D-series) and protectin and maresin from DHA. Their synthesis involves the COX and LOX pathways operating in a transcellular manner [233]. These compounds can be found in humans after EPA or DHA intake [234] and have shown anti-inflammatory benefits in vitro and in animal models of inflammation [235]. By these mechanisms, omega-3 PUFA and especially DHA decrease expression of adhesion molecules such as ICAM-1 on the surface of endothelial cells and monocyte cultures, as well as of macrophage scavenger receptor A in rats fed a high fat fish oil diet [236].
The activity on NF-κB is probably exerted by reduced translocation of the NF-κB p503 and p-65 subunits to the nucleus where they bind omega-3 response elements, upregulating inflammatory gene expression. Exposure of macrophages to DHA inhibits the TLR4 agonist (LPS) ability to promote recruitment of co-stimulatory molecules, MHC class II and to stimulate cytokine production [237].
In view of the relatively simple way of measuring circulating proinflammatory cytokines, a randomized controlled study in aging adults with chronic venous leg ulcers given EPA + DHA therapy (2.5 g/d) reported reductions in IL-6, IL-1β and TNF-α after 4 and 8 weeks of treatment [238]. A direct evaluation of the two fatty acids was carried out by Allaire et al. [239], testing in a double-blind fashion supplementation of EPA vs DHA (both 2.7 g/d) and corn oil for periods of 10 weeks. Compared to EPA, DHA led to a larger reduction of IL-1β, increased adiponectin and reduction of hsCRP (-7.9 vs -1.8 %) and TNF-α (-14.8 vs -7.6 %); effects on IL-6 (-12.0 vs -13. 4 %) were similar. Fish oil can also reduce the inflammatory effects of TMAO, as recently shown in apoE−/− mice fed a Western diet and additional TMAO [240]. Fish oil fed animals had significantly reduced atherogenesis (compared to animals fed flaxseed oil) with marked reduction of inflammatory markers.
All these findings have become of great interest after the positive outcome of the REDUCE-IT study on high dose EPA given to secondary prevention patients with elevated triglyceridemia [241]. In this study patients received 2 g bid of purified EPA vs placebo, resulting in a primary endpoint reduction of 25 % [242]. Interestingly, at the last visit, hsCRP levels were reduced by 23 %, thus probably adding to the CV benefit of EPA intake.
9. Conclusions
The clinician’s approach to arterial disease is more and more frequently calling to action on an inflammatory condition and on the potential use of agents affecting inflammation [243]. This latter is certainly consequent to the presence of cholesterol crystals in the lesion, promoting plaque instability, but newer factors have come into play, in particular from an improved knowledge of myelopoiesis related mechanisms [9]. Both raised progenitor cell recruitment after major CV events and clonal hematopoiesis related mechanisms, have provided crucial information on the inflammatory mechanisms underlying the ACS, also identifying potentially protective mechanisms such as the TET2 production [19] (Graphical abstract).
Clinical diagnostics in CVD has instead mainly relied on circulating biomarkers, in particular the hsCRP, working as an acute marker and as an event predictor. Agents affecting hsCRP levels, in particular canakinumab and, more recently, colchicine, both reduced hsCRP and inflammatory phenomena, leading to a reduced number of event relapses, independent of changes of other risk markers including lipids or hypertension [57,63,68].
Among drug classes of common use in CVD treatment, variable effects have been shown on inflammatory biomarkers, in particular hsCRP [244]. In the case of lipid lowering agents, statins have provided the first evidence indicative of the CV benefit of the reduction of hsCRP in addition to that of LDL-C [245]. Fibrates have shown hsCRP reduction in the metabolic syndrome, whereas ezetimibe has modest effects, potentially additive to those of statins in drug combinations. Finally, among newer agents, bempedoic acid appears to provide the best combination of LDL-C and hsCRP reductions. The case of PCSK9 antagonists is more complex, since these agents do not directly reduce hsCRP levels [246]. However, identification of patients with elevated hsCRP leads to the choice of individuals best responding to drug treatment [117] (Fig. 2 ).
Fig. 2.
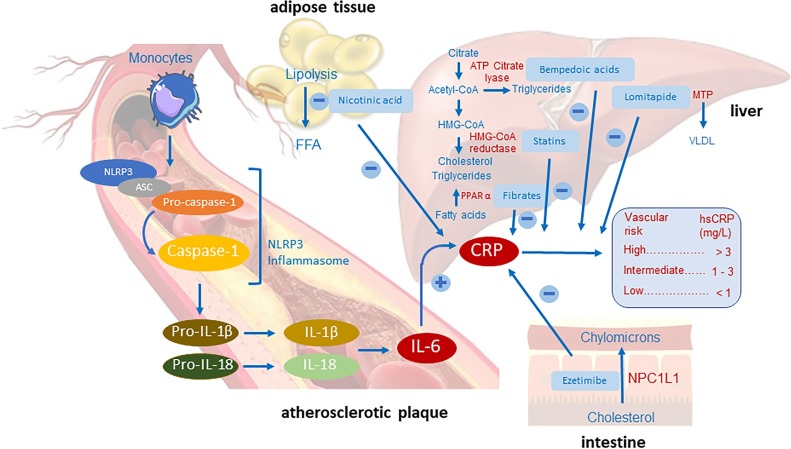
Anti-inflammatory effects of lipid lowering drugs. The NLRP3 inflammasome system induces the activation of caspase-1 which, in turn, cleaves pro-IL-1β and pro-IL-18 to their active counterparts that induce IL-6. In the liver, IL-6 induces CRP, a clinically proven biomarker of inflammatory status and cardiovascular risk. The evaluation of high sensitivity CRP (hsCRP) levels identifies patients with low (< 1 mg/L), intermediate (1–3 mg/L) and high (> 3 mg/L) risk. Several lipid lowering drugs reduce hsCRP levels, including the HMG-CoA reductase inhibitors (statins), ATP citrate lyase inhibitor (bempedoic acid), the PPARα agonists (fibrates), and the MTP inhibitor lomitapide. The NPC1L1 inhibitor, ezetimibe, reduces cholesterol absorption and hsCRP levels, similar to nicotinic acid, acting mainly in the adipose tissue by reducing lipolysis.
CRP, C-reactive protein; NLRP3, NACHT-, LRR- and pyrin domain-containing 3; ASC, apoptosis-associated speck-like protein containing a CARD; FFA, Free-fatty acids; MTP, microsomal transfer protein; NPC1L1, Niemann-Pick C1-Like 1; PPAR, Peroxisome proliferator-activated receptor gamma.
Among agents for blood pressure lowering, ACE inhibitors have an optimal anti-inflammatory activity, potentially additive to that of sartans, somewhat less effective but exerting a stimulatory activity on eicosanoid metabolism. Calcium channel blocker act by way of a CaSR, a cell surface coupled G protein receptor, regulating the NLRP3 inflammasome in the vascular area.
The diabetic condition is characterized by an increased inflammatory burden, with endothelial cell dysfunction and increased adhesion molecules. The novel antidiabetic agents, such as the incretin drugs, antagonizing glucagon secretion and delaying gastric emptying, reduce inflammatory mediators, including hsCRP, IL-6, IL1-β and ICAM-1. The SLGT-2 inhibitors, instead, have in general shown impressive CV benefit, reduction of events being not only of the ischemic type but more frequently consequent to heart failure. This impressive clinical activity has, however, been associated with modest reductions in hsCRP, TNF-α, and IL-6, thus suggesting that the anti-failure activity may not be linked to an anti-inflammatory effect (Fig. 3 ).
Fig. 3.
Anti-inflammatory effects of antidiabetic drugs. In the vascular endothelium, metformin inhibits monocyte-to-macrophage differentiation. TZDs are PPARγ agonists inhibiting vascular neointima formation. In mice with type 2 diabetes, the SGLT2 inhibitor may reduce IL-6, TNF-α, MCP-1 and CRP levels. GLP-1 agonists and DPP-IV inhibitors can reduce NLRP3, TLR4, IL-1β and PAI-1 expression in plaques and macrophages.
DDP IV: dipeptidyl peptidase-4; SGLT2: Sodium Glucose Cotransporter 2 Inhibitors; GLP1: glucagon-like peptide-1; TDZ: thiazolidinediones; PPARγ: Peroxisome proliferator-activated receptor gamma; PAI-1: Plasminogen activator inhibitor-1.
Novel findings on the microbiome have pointed to the proinflammatory activity of the metabolite TMAO, resulting from the metabolism of choline/carnitine by the gut microbiome. This circulating product leads to raised inflammatory and atherosclerotic risk in animal models, associated with an enhanced clinical risk of coronary disease. Opposite effects can have microbial changes such as those occurring with major probiotic, Lactobacillus plantarum, reducing myocardial infarction in hypertensive animals as well as suppressing LPS-mediated atherosclerotic plaque inflammation. A clinical study indicated an effective preventive activity of the oral administration of a Lactobacillus plantarum strain. This led to the investigation to the most frequent inflammation mediated endothelial dysfunction and arterial stiffening, i.e. aging. Suppression of microbiota in old mice can reverse endothelial dysfunction and the accompanying vascular oxidative stress and inflammation. Enterobacteriaceae may play a crucial role in atherogenesis induction and prevention in old age and identification of the responsible strains has become a major topic of research, the most attractive mechanism being that of improving barrier function in the intestine, thus ameliorating metabolic parameters and inflammation.
Dietary components, in particular unsaturated fatty acids and other nutraceuticals from the plant kingdom, have provided exciting results in the reduction of CV diseases and inflammation. While omega-6 fatty acids, aside from their lipid lowering properties, do not seem to exert clear anti-inflammatory activities, omega-3 fatty acids have provided very important findings in the prevention of CV disease, such as in the REDUCE-IT trial with high dose EPA. Aside from the so called pro-resolving lipid mediators, i.e. resolvins, protectins and maresin, the clear anti-inflammatory activity of both EPA and DHA has provided significant benefit, reducing proinflammatory cytokines and hsCRP.
The overall evaluation of present knowledge on inflammation and CV risk points out, to specialists and non-specialists, the major role of evaluating the pro- and antiinflammatory profile of major agents used in therapy as well as the diagnostic criteria for establishing the most appropriate guidelines for prevention and treatment.
Funding
Cariplo Foundation (2015-0552 and 2018-0511) to MR.
Declaration of Competing Interest
None.
References
- 1.Abela G.S., Kalavakunta J.K., Janoudi A., Leffler D., Dhar G., Salehi N., Cohn J., Shah I., Karve M., Kotaru V.P.K., Gupta V., David S., Narisetty K.K., Rich M., Vanderberg A., Pathak D.R., Shamoun F.E. Frequency of cholesterol crystals in culprit coronary artery aspirate during acute myocardial infarction and their relation to inflammation and myocardial injury. Am. J. Cardiol. 2017;120(10):1699–1707. doi: 10.1016/j.amjcard.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 2.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nunez G., Schnurr M., Espevik T., Lien E., Fitzgerald K.A., Rock K.L., Moore K.J., Wright S.D., Hornung V., Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17(8):588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 4.Ross R., Glomset J.A. The pathogenesis of atherosclerosis (first of two parts) N. Engl. J. Med. 1976;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 5.Ross R., Glomset J.A. The pathogenesis of atherosclerosis (second of two parts) N. Engl. J. Med. 1976;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P., Genest J., Giles J.T., Rayner K.J., Dwivedi G., Beanlands R.S., Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Back M., Yurdagul A., Jr., Tabas I., Oorni K., Kovanen P.T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg K.E., Ljungcrantz I., Andersson L., Bryngelsson C., Hedblad B., Fredrikson G.N., Nilsson J., Bjorkbacka H. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ. Cardiovasc. Genet. 2012;5(1):122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 9.Fadini G.P. Task for today: complete the puzzle of circulating stem cells and the atherosclerotic burden. Circ. Res. 2016;119(4):502–504. doi: 10.1161/CIRCRESAHA.116.309315. [DOI] [PubMed] [Google Scholar]
- 10.Massa M., Rosti V., Ferrario M., Campanelli R., Ramajoli I., Rosso R., De Ferrari G.M., Ferlini M., Goffredo L., Bertoletti A., Klersy C., Pecci A., Moratti R., Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal S., Natarajan P., Silver A.J., Gibson C.J., Bick A.G., Shvartz E., McConkey M., Gupta N., Gabriel S., Ardissino D., Baber U., Mehran R., Fuster V., Danesh J., Frossard P., Saleheen D., Melander O., Sukhova G.K., Neuberg D., Libby P., Kathiresan S., Ebert B.L. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Munoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., Croker D.E., Butler M.S., Haneklaus M., Sutton C.E., Nunez G., Latz E., Kastner D.L., Mills K.H., Masters S.L., Schroder K., Cooper M.A., O’Neill L.A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X.H., Shah P.K., Faure E., Equils O., Thomas L., Fishbein M.C., Luthringer D., Xu X.P., Rajavashisth T.B., Yano J., Kaul S., Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104(25):3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 14.Chen S., Shimada K., Crother T.R., Erbay E., Shah P.K., Arditi M. Chlamydia and lipids engage a common signaling pathway that promotes atherogenesis. J. Am. Coll. Cardiol. 2018;71(14):1553–1570. doi: 10.1016/j.jacc.2018.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L., Pagler T., Gautier E.L., Avagyan S., Siry R.L., Han S., Welch C.L., Wang N., Randolph G.J., Snoeck H.W., Tall A.R. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Q., Yang X., Lv J., Zhang J., Xia B., Kim J.D., Wang R., Xiong F., Meng S., Clements T.P., Tandon B., Wagner D.S., Diaz M.F., Wenzel P.L., Miller Y.I., Traver D., Cooke J.P., Li W., Zon L.I., Chen K., Bai Y., Fang L. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science. 2019;363(6431):1085–1088. doi: 10.1126/science.aav1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Winther M.P.J., Lutgens E. The link between Hematopoiesis and atherosclerosis. N. Engl. J. Med. 2019;380(19):1869–1871. doi: 10.1056/NEJMcibr1901397. [DOI] [PubMed] [Google Scholar]
- 18.Babaev V.R., Ding L., Zhang Y., May J.M., Ramsey S.A., Vickers K.C., Linton M.F. Loss of 2 akt (Protein kinase B) isoforms in hematopoietic cells diminished monocyte and macrophage survival and reduces atherosclerosis in ldl receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2019;39(2):156–169. doi: 10.1161/ATVBAHA.118.312206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaasinen E., Kuismin O., Rajamaki K., Ristolainen H., Aavikko M., Kondelin J., Saarinen S., Berta D.G., Katainen R., Hirvonen E.A.M., Karhu A., Taira A., Tanskanen T., Alkodsi A., Taipale M., Morgunova E., Franssila K., Lehtonen R., Makinen M., Aittomaki K., Palotie A., Kurki M.I., Pietilainen O., Hilpert M., Saarentaus E., Niinimaki J., Junttila J., Kaikkonen K., Vahteristo P., Skoda R.C., Seppanen M.R.J., Eklund K.K., Taipale J., Kilpivaara O., Aaltonen L.A. Impact of constitutional TET2 haploinsufficiency on molecular and clinical phenotype in humans. Nat. Commun. 2019;10(1):1252. doi: 10.1038/s41467-019-09198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ammirati E., Moroni F., Magnoni M., Camici P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015;179(2):173–187. doi: 10.1111/cei.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neerincx A., Castro W., Guarda G., Kufer T.A. NLRC5, at the heart of antigen presentation. Front. Immunol. 2013;4:397. doi: 10.3389/fimmu.2013.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan P., Jian W., Xu X., Kou W., Yu Q., Hu H., Li D., Wang W., Feinberg M.W., Zhuang J., Xu Y., Peng W. NLRC5 inhibits neointima formation following vascular injury and directly interacts with PPARgamma. Nat. Commun. 2019;10(1):2882. doi: 10.1038/s41467-019-10784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee G.L., Yeh C.C., Wu J.Y., Lin H.C., Wang Y.F., Kuo Y.Y., Hsieh Y.T., Hsu Y.J., Kuo C.C. TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6-Mediated RANKL induction. Arterioscler. Thromb. Vasc. Biol. 2019;39(3):432–445. doi: 10.1161/ATVBAHA.118.311874. [DOI] [PubMed] [Google Scholar]
- 24.Owsiany K.M., Alencar G.F., Owens G.K. Revealing the origins of foam cells in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2019;39(5):836–838. doi: 10.1161/ATVBAHA.119.312557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zipfel P.F., Skerka C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009;9(10):729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 26.Skerka C., Horstmann R.D., Zipfel P.F. Molecular cloning of a human serum protein structurally related to complement factor H. J. Biol. Chem. 1991;266(18):12015–12020. [PubMed] [Google Scholar]
- 27.Irmscher S., Brix S.R., Zipfel S.L.H., Halder L.D., Mutluturk S., Wulf S., Girdauskas E., Reichenspurner H., Stahl R.A.K., Jungnickel B., Wiech T., Zipfel P.F., Skerka C. Serum FHR1 binding to necrotic-type cells activates monocytic inflammasome and marks necrotic sites in vasculopathies. Nat. Commun. 2019;10(1):2961. doi: 10.1038/s41467-019-10766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruenster M., Vogl T., Roth J., Sperandio M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016;167:120–131. doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Altwegg L.A., Neidhart M., Hersberger M., Muller S., Eberli F.R., Corti R., Roffi M., Sutsch G., Gay S., von Eckardstein A., Wischnewsky M.B., Luscher T.F., Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur. Heart J. 2007;28(8):941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 30.Marinkovic G., Grauen Larsen H., Yndigegn T., Szabo I.A., Mares R.G., de Camp L., Weiland M., Tomas L., Goncalves I., Nilsson J., Jovinge S., Schiopu A. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur. Heart J. 2019;40(32):2713–2723. doi: 10.1093/eurheartj/ehz461. [DOI] [PubMed] [Google Scholar]
- 31.Calabro P., Willerson J.T., Yeh E.T. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108(16):1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 32.Biro E., Nieuwland R., Tak P.P., Pronk L.M., Schaap M.C., Sturk A., Hack C.E. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann. Rheum. Dis. 2007;66(8):1085–1092. doi: 10.1136/ard.2006.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danesh J., Wheeler J.G., Hirschfield G.M., Eda S., Eiriksdottir G., Rumley A., Lowe G.D., Pepys M.B., Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 34.Ridker P.M., Koenig W., Kastelein J.J., Mach F., Luscher T.F. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur. Heart J. 2018;39(46):4109–4111. doi: 10.1093/eurheartj/ehy723. [DOI] [PubMed] [Google Scholar]
- 35.Elliott P., Chambers J.C., Zhang W., Clarke R., Hopewell J.C., Peden J.F., Erdmann J., Braund P., Engert J.C., Bennett D., Coin L., Ashby D., Tzoulaki I., Brown I.J., Mt-Isa S., McCarthy M.I., Peltonen L., Freimer N.B., Farrall M., Ruokonen A., Hamsten A., Lim N., Froguel P., Waterworth D.M., Vollenweider P., Waeber G., Jarvelin M.R., Mooser V., Scott J., Hall A.S., Schunkert H., Anand S.S., Collins R., Samani N.J., Watkins H., Kooner J.S. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302(1):37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Interleukin-6 Receptor Mendelian Randomisation Analysis C., Swerdlow D.I., Holmes M.V., Kuchenbaecker K.B., Engmann J.E., Shah T., Sofat R., Guo Y., Chung C., Peasey A., Pfister R., Mooijaart S.P., Ireland H.A., Leusink M., Langenberg C., Li K.W., Palmen J., Howard P., Cooper J.A., Drenos F., Hardy J., Nalls M.A., Li Y.R., Lowe G., Stewart M., Bielinski S.J., Peto J., Timpson N.J., Gallacher J., Dunlop M., Houlston R., Tomlinson I., Tzoulaki I., Luan J., Boer J.M., Forouhi N.G., Onland-Moret N.C., van der Schouw Y.T., Schnabel R.B., Hubacek J.A., Kubinova R., Baceviciene M., Tamosiunas A., Pajak A., Topor-Madry R., Malyutina S., Baldassarre D., Sennblad B., Tremoli E., de Faire U., Ferrucci L., Bandenelli S., Tanaka T., Meschia J.F., Singleton A., Navis G., Mateo Leach I., Bakker S.J., Gansevoort R.T., Ford I., Epstein S.E., Burnett M.S., Devaney J.M., Jukema J.W., Westendorp R.G., Jan de Borst G., van der Graaf Y., de Jong P.A., Mailand-van der Zee A.H., Klungel O.H., de Boer A., Doevendans P.A., Stephens J.W., Eaton C.B., Robinson J.G., Manson J.E., Fowkes F.G., Frayling T.M., Price J.F., Whincup P.H., Morris R.W., Lawlor D.A., Smith G.D., Ben-Shlomo Y., Redline S., Lange L.A., Kumari M., Wareham N.J., Verschuren W.M., Benjamin E.J., Whittaker J.C., Hamsten A., Dudbridge F., Delaney J.A., Wong A., Kuh D., Hardy R., Castillo B.A., Connolly J.J., van der Harst P., Brunner E.J., Marmot M.G., Wassel C.L., Humphries S.E., Talmud P.J., Kivimaki M., Asselbergs F.W., Voevoda M., Bobak M., Pikhart H., Wilson J.G., Hakonarson H., Reiner A.P., Keating B.J., Sattar N., Hingorani A.D., Casas J.P. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsonne G., Lekander M., Akerstedt T., Axelsson J., Ingre M. Diurnal variation of circulating Interleukin-6 in humans: a meta-analysis. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke A.P., Tracy R.P., Kolodgie F., Malcom G.T., Zieske A., Kutys R., Pestaner J., Smialek J., Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105(17):2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 39.Riedel M., Lafitte M., Pucheu Y., Latry K., Couffinhal T. Prognostic value of high-sensitivity C-reactive protein in a population of post-acute coronary syndrome patients receiving optimal medical treatment. Eur. J. Prev. Cardiol. 2012;19(5):1128–1137. doi: 10.1177/1741826711420347. [DOI] [PubMed] [Google Scholar]
- 40.Kubo T., Matsuo Y., Hayashi Y., Yamano T., Tanimoto T., Ino Y., Kitabata H., Takarada S., Hirata K., Tanaka A., Nakamura N., Mizukoshi M., Imanishi T., Akasaka T. High-sensitivity C-reactive protein and plaque composition in patients with stable angina pectoris: a virtual histology intravascular ultrasound study. Coron. Artery Dis. 2009;20(8):531–535. doi: 10.1097/MCA.0b013e328332a6b0. [DOI] [PubMed] [Google Scholar]
- 41.Koskinas K.C., Zaugg S., Yamaji K., Garcia-Garcia H.M., Taniwaki M., Klingenberg R., Moschovitis A., Luscher T.F., van Tits L.J., Matter C.M., Windecker S., Raber L. Changes of coronary plaque composition correlate with C-reactive protein levels in patients with ST-elevation myocardial infarction following high-intensity statin therapy. Atherosclerosis. 2016;247:154–160. doi: 10.1016/j.atherosclerosis.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Kwon O., Kang S.J., Kang S.H., Lee P.H., Yun S.C., Ahn J.M., Park D.W., Lee S.W., Kim Y.H., Lee C.W., Han K.H., Park S.W., Park S.J. Relationship between serum inflammatory marker levels and the dynamic changes in coronary plaque characteristics after statin therapy. Circ. Cardiovasc. Imaging. 2017;10(7) doi: 10.1161/CIRCIMAGING.116.005934. [DOI] [PubMed] [Google Scholar]
- 43.Soran H., Ho J.H., Durrington P.N. Acquired low cholesterol: diagnosis and relevance to safety of low LDL therapeutic targets. Curr. Opin. Lipidol. 2018;29(4):318–326. doi: 10.1097/MOL.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 44.Ndrepepa G., Holdenrieder S., Cassese S., Xhepa E., Fusaro M., Kastrati A. Hypocholesterolaemia and mortality in patients with coronary artery disease. Eur. J. Clin. Invest. 2020;50(2) doi: 10.1111/eci.13194. [DOI] [PubMed] [Google Scholar]
- 45.Ruscica M., Zimetti F., Adorni M.P., Sirtori C.R., Lupo M.G., Ferri N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: new therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104653. [DOI] [PubMed] [Google Scholar]
- 46.Penson P.E., Long D.L., Howard G., Toth P.P., Muntner P., Howard V.J., Safford M.M., Jones S.R., Martin S.S., Mazidi M., Catapano A.L., Banach M. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the reasons for geographical and racial differences in stroke (REGARDS) study. Eur. Heart J. 2018;39(40):3641–3653. doi: 10.1093/eurheartj/ehy533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guedeney P., Claessen B.E., Kalkman D.N., Aquino M., Sorrentino S., Giustino G., Farhan S., Vogel B., Sartori S., Montalescot G., Sweeny J., Kovacic J.C., Krishnan P., Barman N., Dangas G., Kini A., Baber U., Sharma S., Mehran R. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 2019;73(19):2401–2409. doi: 10.1016/j.jacc.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 48.Group A.S.C., Bowman L., Mafham M., Wallendszus K., Stevens W., Buck G., Barton J., Murphy K., Aung T., Haynes R., Cox J., Murawska A., Young A., Lay M., Chen F., Sammons E., Waters E., Adler A., Bodansky J., Farmer A., McPherson R., Neil A., Simpson D., Peto R., Baigent C., Collins R., Parish S., Armitage J. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 2018;379(16):1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 49.McNeil J.J., Wolfe R., Woods R.L., Tonkin A.M., Donnan G.A., Nelson M.R., Reid C.M., Lockery J.E., Kirpach B., Storey E., Shah R.C., Williamson J.D., Margolis K.L., Ernst M.E., Abhayaratna W.P., Stocks N., Fitzgerald S.M., Orchard S.G., Trevaks R.E., Beilin L.J., Johnston C.I., Ryan J., Radziszewska B., Jelinek M., Malik M., Eaton C.B., Brauer D., Cloud G., Wood E.M., Mahady S.E., Satterfield S., Grimm R., Murray A.M., Group A.I. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall K.T., Kessler T., Buring J.E., Passow D., Sesso H.D., Zee R.Y.L., Ridker P.M., Chasman D.I., Schunkert H. Genetic variation at the coronary artery disease risk locus GUCY1A3 modifies cardiovascular disease prevention effects of aspirin. Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehz384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nissen S.E., Yeomans N.D., Solomon D.H., Luscher T.F., Libby P., Husni M.E., Graham D.Y., Borer J.S., Wisniewski L.M., Wolski K.E., Wang Q., Menon V., Ruschitzka F., Gaffney M., Beckerman B., Berger M.F., Bao W., Lincoff A.M., Investigators P.T. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 2016;375(26):2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 52.Reed G.W., Abdallah M.S., Shao M., Wolski K., Wisniewski L., Yeomans N., Luscher T.F., Borer J.S., Graham D.Y., Husni M.E., Solomon D.H., Libby P., Menon V., Lincoff A.M., Nissen S.E. Effect of aspirin coadministration on the safety of celecoxib, naproxen, or ibuprofen. J. Am. Coll. Cardiol. 2018;71(16):1741–1751. doi: 10.1016/j.jacc.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 53.O’Donoghue M.L., Braunwald E., White H.D., Lukas M.A., Tarka E., Steg P.G., Hochman J.S., Bode C., Maggioni A.P., Im K., Shannon J.B., Davies R.Y., Murphy S.A., Crugnale S.E., Wiviott S.D., Bonaca M.P., Watson D.F., Weaver W.D., Serruys P.W., Cannon C.P., Investigators S.-T., Steen D.L. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312(10):1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 54.Tardif J.C., Tanguay J.F., Wright S.R., Duchatelle V., Petroni T., Gregoire J.C., Ibrahim R., Heinonen T.M., Robb S., Bertrand O.F., Cournoyer D., Johnson D., Mann J., Guertin M.C., L’Allier P.L. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 2013;61(20):2048–2055. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Abbate A., Kontos M.C., Abouzaki N.A., Melchior R.D., Thomas C., Van Tassell B.W., Oddi C., Carbone S., Trankle C.R., Roberts C.S., Mueller G.H., Gambill M.L., Christopher S., Markley R., Vetrovec G.W., Dinarello C.A., Biondi-Zoccai G. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies) Am. J. Cardiol. 2015;115(3):288–292. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Gram H. The long and winding road in pharmaceutical development of canakinumab from rare genetic autoinflammatory syndromes to myocardial infarction and cancer. Pharmacol. Res. 2019 doi: 10.1016/j.phrs.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., Kastelein J.J.P., Cornel J.H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P.R.F., Troquay R.P.T., Libby P., Glynn R.J., Group C.T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 58.Ibanez B., Fuster V. CANTOS: a gigantic proof-of-concept trial. Circ. Res. 2017;121(12):1320–1322. doi: 10.1161/CIRCRESAHA.117.312200. [DOI] [PubMed] [Google Scholar]
- 59.Everett B.M., Donath M.Y., Pradhan A.D., Thuren T., Pais P., Nicolau J.C., Glynn R.J., Libby P., Ridker P.M. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 2018;71(21):2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Ridker P.M., MacFadyen J.G., Thuren T., Everett B.M., Libby P., Glynn R.J., Group C.T. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 61.Ridker P.M., MacFadyen J.G., Everett B.M., Libby P., Thuren T., Glynn R.J., Group C.T. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 62.Ridker P.M., MacFadyen J.G., Glynn R.J., Koenig W., Libby P., Everett B.M., Lefkowitz M., Thuren T., Cornel J.H. Inhibition of Interleukin-1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J. Am. Coll. Cardiol. 2018;71(21):2405–2414. doi: 10.1016/j.jacc.2018.03.490. [DOI] [PubMed] [Google Scholar]
- 63.Ridker P.M., Everett B.M., Pradhan A., MacFadyen J.G., Solomon D.H., Zaharris E., Mam V., Hasan A., Rosenberg Y., Iturriaga E., Gupta M., Tsigoulis M., Verma S., Clearfield M., Libby P., Goldhaber S.Z., Seagle R., Ofori C., Saklayen M., Butman S., Singh N., Le May M., Bertrand O., Johnston J., Paynter N.P., Glynn R.J., Investigators C. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiner Z., Sirtori C.R., Banach M., Ruscica M., Sahebkar A. Methotrexate for cardiovascular risk reduction: the right choice? Angiology. 2019 doi: 10.1177/0003319719855165. [DOI] [PubMed] [Google Scholar]
- 65.Martinez G.J., Robertson S., Barraclough J., Xia Q., Mallat Z., Bursill C., Celermajer D.S., Patel S. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J. Am. Heart Assoc. 2015;4(8) doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaidya K., Arnott C., Martinez G.J., Ng B., McCormack S., Sullivan D.R., Celermajer D.S., Patel S. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC Cardiovasc. Imaging. 2018;11(2 Pt 2):305–316. doi: 10.1016/j.jcmg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 67.Nidorf S.M., Thompson P.L. Why colchicine should Be considered for secondary prevention of atherosclerosis: an overview. Clin. Ther. 2019;41(1):41–48. doi: 10.1016/j.clinthera.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., Berry C., Lopez-Sendon J., Ostadal P., Koenig W., Angoulvant D., Gregoire J.C., Lavoie M.A., Dube M.P., Rhainds D., Provencher M., Blondeau L., Orfanos A., L’Allier P.L., Guertin M.C., Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 69.Newby L.K. Inflammation as a treatment target after acute myocardial infarction. N. Engl. J. Med. 2019;381(26):2562–2563. doi: 10.1056/NEJMe1914378. [DOI] [PubMed] [Google Scholar]
- 70.Agca R., Heslinga M., Kneepkens E.L., van Dongen C., Nurmohamed M.T. The effects of 5-year etanercept therapy on cardiovascular risk factors in patients with psoriatic arthritis. J. Rheumatol. 2017;44(9):1362–1368. doi: 10.3899/jrheum.161418. [DOI] [PubMed] [Google Scholar]
- 71.Robertson J., Porter D., Sattar N., Packard C.J., Caslake M., McInnes I., McCarey D. Interleukin-6 blockade raises LDL via reduced catabolism rather than via increased synthesis: a cytokine-specific mechanism for cholesterol changes in rheumatoid arthritis. Ann. Rheum. Dis. 2017;76(11):1949–1952. doi: 10.1136/annrheumdis-2017-211708. [DOI] [PubMed] [Google Scholar]
- 72.Barrett T.J., Distel E., Murphy A.J., Hu J., Garshick M.S., Ogando Y., Liu J., Vaisar T., Heinecke J.W., Berger J.S., Goldberg I.J., Fisher E.A. Apolipoprotein AI) promotes atherosclerosis regression in diabetic mice by suppressing myelopoiesis and plaque inflammation. Circulation. 2019;140(14):1170–1184. doi: 10.1161/CIRCULATIONAHA.119.039476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boren J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., Nordestgaard B.G., Watts G.F., Bruckert E., Fazio S., Ference B.A., Graham I., Horton J.D., Landmesser U., Laufs U., Masana L., Pasterkamp G., Raal F.J., Ray K.K., Schunkert H., Taskinen M.R., van de Sluis B., Wiklund O., Tokgozoglu L., Catapano A.L., Ginsberg H.N. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellosta S., Ferri N., Bernini F., Paoletti R., Corsini A. Non-lipid-related effects of statins. Ann. Med. 2000;32(3):164–176. doi: 10.3109/07853890008998823. [DOI] [PubMed] [Google Scholar]
- 75.Subramanian S., Emami H., Vucic E., Singh P., Vijayakumar J., Fifer K.M., Alon A., Shankar S.S., Farkouh M., Rudd J.H.F., Fayad Z.A., Van Dyke T.E., Tawakol A. High-dose atorvastatin reduces periodontal inflammation: a novel pleiotropic effect of statins. J. Am. Coll. Cardiol. 2013;62(25):2382–2391. doi: 10.1016/j.jacc.2013.08.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruscica M., Ferri N., Macchi C., Corsini A., Sirtori C.R. Lipid lowering drugs and inflammatory changes: an impact on cardiovascular outcomes? Ann. Med. 2018;50(6):461–484. doi: 10.1080/07853890.2018.1498118. [DOI] [PubMed] [Google Scholar]
- 77.Ridker P.M., Rifai N., Pfeffer M.A., Sacks F.M., Moye L.A., Goldman S., Flaker G.C., Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98(9):839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 78.Ridker P.M., Rifai N., Clearfield M., Downs J.R., Weis S.E., Miles J.S., Gotto A.M., Jr., Air Force/Texas Coronary Atherosclerosis Prevention Study I. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 2001;344(26):1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 79.Nissen S.E., Tuzcu E.M., Schoenhagen P., Crowe T., Sasiela W.J., Tsai J., Orazem J., Magorien R.D., O’Shaughnessy C., Ganz P., Reversal of Atherosclerosis with Aggressive Lipid Lowering I. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005;352(1):29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 80.Ridker P.M., Morrow D.A., Rose L.M., Rifai N., Cannon C.P., Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J. Am. Coll. Cardiol. 2005;45(10):1644–1648. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 81.Morrow D.A., de Lemos J.A., Sabatine M.S., Wiviott S.D., Blazing M.A., Shui A., Rifai N., Califf R.M., Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the aggrastat-to-zocor trial. Circulation. 2006;114(4):281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 82.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., Pfeffer M.A., Braunwald E., Pravastatin or Atorvastatin E., Infection Therapy-Thrombolysis in Myocardial Infarction I. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 83.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., Nordestgaard B.G., Shepherd J., Willerson J.T., Glynn R.J., Group J.S. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 84.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., Macfadyen J.G., Nordestgaard B.G., Shepherd J., Willerson J.T., Glynn R.J., Group J.T.S. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 85.Altmann S.W., Davis H.R., Jr., Zhu L.J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P., Maguire M., Golovko A., Zeng M., Wang L., Murgolo N., Graziano M.P. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 86.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Theroux P., Darius H., Lewis B.S., Ophuis T.O., Jukema J.W., De Ferrari G.M., Ruzyllo W., De Lucca P., Im K., Bohula E.A., Reist C., Wiviott S.D., Tershakovec A.M., Musliner T.A., Braunwald E., Califf R.M., Investigators I.-I. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Garre D., Munoz-Pacheco P., Gonzalez-Rubio M.L., Aragoncillo P., Granados R., Fernandez-Cruz A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br. J. Pharmacol. 2009;156(8):1218–1227. doi: 10.1111/j.1476-5381.2008.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearson T.A., Ballantyne C.M., Veltri E., Shah A., Bird S., Lin J., Rosenberg E., Tershakovec A.M. Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am. J. Cardiol. 2009;103(3):369–374. doi: 10.1016/j.amjcard.2008.09.090. [DOI] [PubMed] [Google Scholar]
- 89.Bohula E.A., Giugliano R.P., Cannon C.P., Zhou J., Murphy S.A., White J.A., Tershakovec A.M., Blazing M.A., Braunwald E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–1233. doi: 10.1161/CIRCULATIONAHA.115.018381. [DOI] [PubMed] [Google Scholar]
- 90.Storey B.C., Staplin N., Haynes R., Reith C., Emberson J., Herrington W.G., Wheeler D.C., Walker R., Fellstrom B., Wanner C., Landray M.J., Baigent C., Group S.C. Lowering LDL cholesterol reduces cardiovascular risk independently of presence of inflammation. Kidney Int. 2018;93(4):1000–1007. doi: 10.1016/j.kint.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinkosky S.L., Groot P.H.E., Lalwani N.D., Steinberg G.R. Targeting ATP-Citrate lyase in Hyperlipidemia and metabolic disorders. Trends Mol. Med. 2017;23(11):1047–1063. doi: 10.1016/j.molmed.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Gutierrez M.J., Rosenberg N.L., Macdougall D.E., Hanselman J.C., Margulies J.R., Strange P., Milad M.A., McBride S.J., Newton R.S. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2014;34(3):676–683. doi: 10.1161/ATVBAHA.113.302677. [DOI] [PubMed] [Google Scholar]
- 93.Ballantyne C.M., Banach M., Mancini G.B.J., Lepor N.E., Hanselman J.C., Zhao X., Leiter L.A. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Ray K.K., Bays H.E., Catapano A.L., Lalwani N.D., Bloedon L.T., Sterling L.R., Robinson P.L., Ballantyne C.M., Trial C.H. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N. Engl. J. Med. 2019;380(11):1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 95.Goldberg A.C., Leiter L.A., Stroes E.S.G., Baum S.J., Hanselman J.C., Bloedon L.T., Lalwani N.D., Patel P.M., Zhao X., Duell P.B. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 2019;322(18):1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laufs U., Banach M., Mancini G.B.J., Gaudet D., Bloedon L.T., Sterling L.R., Kelly S., Stroes E.S.G. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J. Am. Heart Assoc. 2019;8(7) doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ballantyne C.M., Laufs U., Ray K.K., Leiter L.A., Bays H.E., Goldberg A.C., Stroes E.S., MacDougall D., Zhao X., Catapano A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2019 doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan S.U., Michos E.D. Bempedoic acid and ezetimibe - better together. Eur. J. Prev. Cardiol. 2019 doi: 10.1177/2047487319864672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W., Basinger A., Neese R.A., Shane B., Myong S.A., Christiansen M., Hellerstein M.K. Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride production. Am. J. Physiol. Endocrinol. Metab. 2001;280(3):E540–7. doi: 10.1152/ajpendo.2001.280.3.E540. [DOI] [PubMed] [Google Scholar]
- 100.Watt M.J., Southgate R.J., Holmes A.G., Febbraio M.A. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J. Mol. Endocrinol. 2004;33(2):533–544. doi: 10.1677/jme.1.01499. [DOI] [PubMed] [Google Scholar]
- 101.Vega G.L., Cater N.B., Meguro S., Grundy S.M. Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am. J. Cardiol. 2005;95(11):1309–1313. doi: 10.1016/j.amjcard.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 102.Investigators A.-H., Boden W.E., Probstfield J.L., Anderson T., Chaitman B.R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 103.Group H.T.C., Landray M.J., Haynes R., Hopewell J.C., Parish S., Aung T., Tomson J., Wallendszus K., Craig M., Jiang L., Collins R., Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 104.Adiels M., Chapman M.J., Robillard P., Krempf M., Laville M., Boren J., Niacin Study G. Niacin action in the atherogenic mixed dyslipidemia of metabolic syndrome: insights from metabolic biomarker profiling and network analysis. J. Clin. Lipidol. 2018;12(3):810–821. doi: 10.1016/j.jacl.2018.03.083. e1. [DOI] [PubMed] [Google Scholar]
- 105.Wi J., Kim J.Y., Park S., Kang S.M., Jang Y., Chung N., Shim W.H., Cho S.Y., Lee S.H. Optimal pharmacologic approach to patients with hypertriglyceridemia and low high-density lipoprotein-cholesterol: randomized comparison of fenofibrate 160 mg and niacin 1500 mg. Atherosclerosis. 2010;213(1):235–240. doi: 10.1016/j.atherosclerosis.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 106.Hiukka A., Westerbacka J., Leinonen E.S., Watanabe H., Wiklund O., Hulten L.M., Salonen J.T., Tuomainen T.P., Yki-Jarvinen H., Keech A.C., Taskinen M.R. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008;52(25):2190–2197. doi: 10.1016/j.jacc.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 107.Keech A., Simes R.J., Barter P., Best J., Scott R., Taskinen M.R., Forder P., Pillai A., Davis T., Glasziou P., Drury P., Kesaniemi Y.A., Sullivan D., Hunt D., Colman P., d’Emden M., Whiting M., Ehnholm C., Laakso M. F.s. investigators, Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 108.Agouridis A.P., Tsimihodimos V., Filippatos T.D., Dimitriou A.A., Tellis C.C., Elisaf M.S., Mikhailidis D.P., Tselepis A.D. The effects of rosuvastatin alone or in combination with fenofibrate or omega 3 fatty acids on inflammation and oxidative stress in patients with mixed dyslipidemia. Expert Opin. Pharmacother. 2011;12(17):2605–2611. doi: 10.1517/14656566.2011.591383. [DOI] [PubMed] [Google Scholar]
- 109.Belfort R., Berria R., Cornell J., Cusi K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 2010;95(2):829–836. doi: 10.1210/jc.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferri N., Corsini A., Macchi C., Magni P., Ruscica M. Proprotein convertase subtilisin kexin type 9 and high-density lipoprotein metabolism: experimental animal models and clinical evidence. Transl. Res. 2016;173:19–29. doi: 10.1016/j.trsl.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Macchi C., Banach M., Corsini A., Sirtori C.R., Ferri N., Ruscica M. Changes in circulating pro-protein convertase subtilisin/kexin type 9 levels - experimental and clinical approaches with lipid-lowering agents. Eur. J. Prev. Cardiol. 2019;26(9):930–949. doi: 10.1177/2047487319831500. [DOI] [PubMed] [Google Scholar]
- 112.Casula M., Olmastroni E., Boccalari M.T., Tragni E., Pirillo A., Catapano A.L. Cardiovascular events with PCSK9 inhibitors: an updated meta-analysis of randomised controlled trials. Pharmacol. Res. 2019;143:143–150. doi: 10.1016/j.phrs.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 113.Macchi C., Sirtori C.R., Corsini A., Santos R.D., Watts G.F., Ruscica M. A new dawn for managing dyslipidemias: the era of rna-based therapies. Pharmacol. Res. 2019;150 doi: 10.1016/j.phrs.2019.104413. [DOI] [PubMed] [Google Scholar]
- 114.Seidah N.G., Prat A., Pirillo A., Catapano A.L., Norata G.D. Novel strategies to target proprotein convertase subtilisin kexin 9: beyond monoclonal antibodies. Cardiovasc. Res. 2019;115(3):510–518. doi: 10.1093/cvr/cvz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ricci C., Ruscica M., Camera M., Rossetti L., Macchi C., Colciago A., Zanotti I., Lupo M.G., Adorni M.P., Cicero A.F.G., Fogacci F., Corsini A., Ferri N. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018;8(1):2267. doi: 10.1038/s41598-018-20425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macchi C., Ferri N., Favero C., Cantone L., Vigna L., Pesatori A.C., Lupo M.G., Sirtori C.R., Corsini A., Bollati V., Ruscica M. Long-term exposure to air pollution raises circulating levels of proprotein convertase subtilisin/kexin type 9 in obese individuals. Eur. J. Prev. Cardiol. 2019;26(6):578–588. doi: 10.1177/2047487318815320. [DOI] [PubMed] [Google Scholar]
- 117.Ruscica M., Tokgozoglu L., Corsini A., Sirtori C.R. PCSK9 inhibition and inflammation: a narrative review. Atherosclerosis. 2019;288:146–155. doi: 10.1016/j.atherosclerosis.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Bohula E.A., Giugliano R.P., Leiter L.A., Verma S., Park J.G., Sever P.S., Lira Pineda A., Honarpour N., Wang H., Murphy S.A., Keech A., Pedersen T.R., Sabatine M.S. Inflammatory and cholesterol risk in the FOURIER trial. Circulation. 2018;138(2):131–140. doi: 10.1161/CIRCULATIONAHA.118.034032. [DOI] [PubMed] [Google Scholar]
- 119.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., Harrington R.A., Jukema J.W., Lecorps G., Mahaffey K.W., Moryusef A., Pordy R., Quintero K., Roe M.T., Sasiela W.J., Tamby J.F., Tricoci P., White H.D., Zeiher A.M., Committees O.O. Investigators, alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 120.Pradhan A.D., Aday A.W., Rose L.M., Ridker P.M. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation. 2018;138(2):141–149. doi: 10.1161/CIRCULATIONAHA.118.034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Landmesser U., Haghikia A., Leiter L.A., Wright R.S., Kallend D., Wijngaard P., Stoekenbroek R., Kastelein J.J.P., Ray K.K. Effect of inclisiran, the siRNA against PCSK9, on platelets, immune cells and immunological biomarkers - a pre-specified analysis from ORION-1. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa077. [DOI] [PubMed] [Google Scholar]
- 122.Blom D.J., Averna M.R., Meagher E.A., du Toit Theron H., Sirtori C.R., Hegele R.A., Shah P.K., Gaudet D., Stefanutti C., Vigna G.B., Larrey D., Bloedon L.T., Foulds P., Rader D.J., Cuchel M. Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation. 2017;136(3):332–335. doi: 10.1161/CIRCULATIONAHA.117.028208. [DOI] [PubMed] [Google Scholar]
- 123.Pharmaceuticals A. 2014. Investigator’s Brochure Lomitapide (AEGR-733, Formerly BMS-201038-04) [Google Scholar]
- 124.Filippakopoulos P., Picaud S., Mangos M., Keates T., Lambert J.P., Barsyte-Lovejoy D., Felletar I., Volkmer R., Muller S., Pawson T., Gingras A.C., Arrowsmith C.H., Knapp S. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kempen H.J., Bellus D., Fedorov O., Nicklisch S., Filippakopoulos P., Picaud S., Knapp S. Stimulation of hepatic apolipoprotein A-I production by novel thieno-triazolodiazepines: roles of the classical benzodiazepine receptor, PAF receptor, and bromodomain binding. Lipid Insights. 2013;6:47–54. doi: 10.4137/Lpi.s13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Picaud S., Wells C., Felletar I., Brotherton D., Martin S., Savitsky P., Diez-Dacal B., Philpott M., Bountra C., Lingard H., Fedorov O., Muller S., Brennan P.E., Knapp S., Filippakopoulos P. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc. Natl. Acad. Sci. U. S. A. 2013;110(49):19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nicholls S.J., Gordon A., Johansson J., Wolski K., Ballantyne C.M., Kastelein J.J., Taylor A., Borgman M., Nissen S.E. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J. Am. Coll. Cardiol. 2011;57(9):1111–1119. doi: 10.1016/j.jacc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 128.Gilham D., Tsujikawa L.M., Sarsons C.D., Halliday C., Wasiak S., Stotz S.C., Jahagirdar R., Sweeney M., Johansson J.O., Wong N.C.W., Kalantar-Zadeh K., Kulikowski E. Apabetalone downregulates factors and pathways associated with vascular calcification. Atherosclerosis. 2019;280:75–84. doi: 10.1016/j.atherosclerosis.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 129.Haarhaus M., Ray K.K., Nicholls S.J., Schwartz G.G., Kulikowski E., Johansson J.O., Sweeney M., Halliday C., Lebioda K., Wong N., Brandenburg V., Beddhu S., Tonelli M., Zoccali C., Kalantar-Zadeh K. Apabetalone lowers serum alkaline phosphatase and improves cardiovascular risk in patients with cardiovascular disease. Atherosclerosis. 2019;290:59–65. doi: 10.1016/j.atherosclerosis.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 130.Nicholls S.J., Puri R., Wolski K., Ballantyne C.M., Barter P.J., Brewer H.B., Kastelein J.J., Hu B., Uno K., Kataoka Y., Herrman J.P., Merkely B., Borgman M., Nissen S.E. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE trial. Am. J. Cardiovasc. Drugs. 2016;16(1):55–65. doi: 10.1007/s40256-015-0146-z. [DOI] [PubMed] [Google Scholar]
- 131.Nicholls S.J., Ray K.K., Johansson J.O., Gordon A., Sweeney M., Halliday C., Kulikowski E., Wong N., Kim S.W., Schwartz G.G. Selective BET protein inhibition with apabetalone and cardiovascular events: a pooled analysis of trials in patients with coronary artery disease. Am. J. Cardiovasc. Drugs. 2018;18(2):109–115. doi: 10.1007/s40256-017-0250-3. [DOI] [PubMed] [Google Scholar]
- 132.Ray K.K., Nicholls S.J., Ginsberg H.D., Johansson J.O., Kalantar-Zadeh K., Kulikowski E., Toth P.P., Wong N., Cummings J.L., Sweeney M., Schwartz G.G. Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: rationale, design, and baseline characteristics of the BETonMACE trial. Am. Heart J. 2019;217:72–83. doi: 10.1016/j.ahj.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 133.Ray K.K., Nicholls S.J., Buhr K.A., Ginsberg H.N., Johansson J.O., Kalantar-Zadeh K., Kulikowski E., Toth P.P., Wong N., Sweeney M., Schwartz G.G., Investigators B.E., Committees Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and Type 2 diabetes: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huettenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O’Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 136.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int. Immunol. 2009;21(10):1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 137.Madhur M.S., Lob H.E., McCann L.A., Iwakura Y., Blinder Y., Guzik T.J., Harrison D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tipton A.J., Baban B., Sullivan J.C. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303(4):R359–67. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramseyer V.D., Garvin J.L. Tumor necrosis factor-alpha: regulation of renal function and blood pressure. Am. J. Physiol. Renal Physiol. 2013;304(10):F1231–42. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruscica M., Baragetti A., Catapano A.L., Norata G.D. Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: gaps and open questions. Nutr. Metab. Cardiovasc. Dis. 2017;27(5):379–395. doi: 10.1016/j.numecd.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Saulnier P.J., Gand E., Ragot S., Ducrocq G., Halimi J.M., Hulin-Delmotte C., Llaty P., Montaigne D., Rigalleau V., Roussel R., Velho G., Sosner P., Zaoui P., Hadjadj S., Group S.S. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. Diabetes Care. 2014;37(5):1425–1431. doi: 10.2337/dc13-2580. [DOI] [PubMed] [Google Scholar]
- 142.Chen C.C., Pedraza P.L., Hao S., Stier C.T., Ferreri N.R. TNFR1-deficient mice display altered blood pressure and renal responses to ANG II infusion. Am. J. Physiol. Renal Physiol. 2010;299(5):F1141–50. doi: 10.1152/ajprenal.00344.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Svensson E.H., Soderholm M., Abul-Kasim K., Engstrom G. Tumor necrosis factor receptor 1 and 2 are associated with risk of intracerebral hemorrhage. Stroke. 2017;48(10):2710–2715. doi: 10.1161/STROKEAHA.117.017849. [DOI] [PubMed] [Google Scholar]
- 144.Chae C.U., Lee R.T., Rifai N., Ridker P.M. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38(3):399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 145.da Cunha V., Tham D.M., Martin-McNulty B., Deng G., Ho J.J., Wilson D.W., Rutledge J.C., Vergona R., Sullivan M.E., Wang Y.X. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis. 2005;178(1):9–17. doi: 10.1016/j.atherosclerosis.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 146.Koulouris S., Symeonides P., Triantafyllou K., Ioannidis G., Karabinos I., Katostaras T., El-Ali M., Theodoridis T., Vratsista E., Thalassinos N., Kokkinou V., Nanas I., Stamatelopoulos S., Toutouzas P. Comparison of the effects of ramipril versus telmisartan in reducing serum levels of high-sensitivity C-reactive protein and oxidized low-density lipoprotein cholesterol in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2005;95(11):1386–1388. doi: 10.1016/j.amjcard.2005.01.092. [DOI] [PubMed] [Google Scholar]
- 147.Ruiz-Ortega M., Lorenzo O., Ruperez M., Konig S., Wittig B., Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ. Res. 2000;86(12):1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 148.Platten M., Youssef S., Hur E.M., Ho P.P., Han M.H., Lanz T.V., Phillips L.K., Goldstein M.J., Bhat R., Raine C.S., Sobel R.A., Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(35):14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou M.S., Schulman I.H., Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc. Med. 2012;17(5):330–341. doi: 10.1177/1358863X12450094. [DOI] [PubMed] [Google Scholar]
- 150.Herings R.M., de Boer A., Stricker B.H., Leufkens H.G., Porsius A. Hypoglycaemia associated with use of inhibitors of angiotensin converting enzyme. Lancet. 1995;345(8959):1195–1198. doi: 10.1016/s0140-6736(95)91988-0. [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto K., Ohishi M., Ho C., Kurtz T.W., Rakugi H. Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54(6):1353–1359. doi: 10.1161/HYPERTENSIONAHA.109.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Le C.N., Hulgan T., Tseng C.H., Milne G.L., Lake J.E. Urine eicosanoids in the metabolic abnormalities, Telmisartan, and HIV infection (MATH) trial. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dasu M.R., Riosvelasco A.C., Jialal I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis. 2009;202(1):76–83. doi: 10.1016/j.atherosclerosis.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ridker P.M., Danielson E., Rifai N., Glynn R.J., Val M.I. Valsartan, blood pressure reduction, and C-reactive protein: primary report of the Val-MARC trial. Hypertension. 2006;48(1):73–79. doi: 10.1161/01.HYP.0000226046.58883.32. [DOI] [PubMed] [Google Scholar]
- 155.Conen D., Everett B.M., Glynn R.J., Ridker P.M. Effect of valsartan compared with valsartan/hydrochlorothiazide on plasma levels of cellular adhesion molecules: the Val-MARC trial. Heart. 2008;94(3):e13. doi: 10.1136/hrt.2007.126169. [DOI] [PubMed] [Google Scholar]
- 156.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dudenbostel T., Glasser S.P. Effects of antihypertensive drugs on arterial stiffness. Cardiol. Rev. 2012;20(5):259–263. doi: 10.1097/CRD.0b013e31825d0a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Touyz R.M., Dominiczak A.F. Hypertension guidelines: is it time to reappraise blood pressure thresholds and targets? Hypertension. 2016;67(4):688–689. doi: 10.1161/HYPERTENSIONAHA.116.07090. [DOI] [PubMed] [Google Scholar]
- 159.Schiffrin E.L., Deng L.Y. Structure and function of resistance arteries of hypertensive patients treated with a beta-blocker or a calcium channel antagonist. J. Hypertens. 1996;14(10):1247–1255. doi: 10.1097/00004872-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 160.Frielingsdorf J., Seiler C., Kaufmann P., Vassalli G., Suter T., Hess O.M. Normalization of abnormal coronary vasomotion by calcium antagonists in patients with hypertension. Circulation. 1996;93(7):1380–1387. doi: 10.1161/01.cir.93.7.1380. [DOI] [PubMed] [Google Scholar]
- 161.Sudano I., Virdis A., Taddei S., Spieker L., Corti R., Noll G., Salvetti A., Luscher T.F. Chronic treatment with long-acting nifedipine reduces vasoconstriction to endothelin-1 in essential hypertension. Hypertension. 2007;49(2):285–290. doi: 10.1161/01.HYP.0000254645.33321.a3. [DOI] [PubMed] [Google Scholar]
- 162.Lyons D., Webster J., Benjamin N. The effect of antihypertensive therapy on responsiveness to local intra-arterial NG-monomethyl-L-arginine in patients with essential hypertension. J. Hypertens. 1994;12(9):1047–1052. [PubMed] [Google Scholar]
- 163.Semenkovich C.F. We know more than we can tell about diabetes and vascular disease: the 2016 Edwin Bierman award lecture. Diabetes. 2017;66(7):1735–1741. doi: 10.2337/db17-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Opdenakker G., Abu El-Asrar A. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell. Mol. Life Sci. 2019;76(16):3157–3166. doi: 10.1007/s00018-019-03177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Richardson S.J., Willcox A., Bone A.J., Foulis A.K., Morgan N.G. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- 166.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L.A., Harris J., Coll R.C., Mills K.H., Mok K.H., Newsholme P., Nunez G., Yodoi J., Kahn S.E., Lavelle E.C., O’Neill L.A. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 2010;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J.A., Kerr-Conte J., Pattou F., Halban P.A., Weir G.C., Donath M.Y. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 2008;93(10):4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guerre-Millo M., Rouault C., Poulain P., Andre J., Poitout V., Peters J.M., Gonzalez F.J., Fruchart J.C., Reach G., Staels B. PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50(12):2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- 169.Huang Q.Y., Lai X.N., Qian X.L., Lv L.C., Li J., Duan J., Xiao X.H., Xiong L.X. Cdc42: a novel regulator of insulin secretion and diabetes-associated diseases. Int. J. Mol. Sci. 2019;20(1) doi: 10.3390/ijms20010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Slotte J.P., Bierman E.L. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem. J. 1988;250(3):653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Everts B., Amiel E., Huang S.C., Smith A.M., Chang C.H., Lam W.Y., Redmann V., Freitas T.C., Blagih J., van der Windt G.J., Artyomov M.N., Jones R.G., Pearce E.L., Pearce E.J. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014;15(4):323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bahre H., Tschirner S.K., Gorinski N., Gohmert M., Mayer C.T., Huehn J., Ponimaskin E., Abraham W.R., Muller R., Lochner M., Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20(11):1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 173.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 174.Kelly B., Tannahill G.M., Murphy M.P., O’Neill L.A. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of Interleukin-1beta (IL-1beta) and boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 2015;290(33):20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vasamsetti S.B., Karnewar S., Kanugula A.K., Thatipalli A.R., Kumar J.M., Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64(6):2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 176.Shaked M., Ketzinel-Gilad M., Cerasi E., Kaiser N., Leibowitz G. AMP-activated protein kinase (AMPK) mediates nutrient regulation of thioredoxin-interacting protein (TXNIP) in pancreatic beta-cells. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Haffner S., Temprosa M., Crandall J., Fowler S., Goldberg R., Horton E., Marcovina S., Mather K., Orchard T., Ratner R., Barrett-Connor E., Diabetes Prevention Program Research G. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54(5):1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pradhan A.D., Everett B.M., Cook N.R., Rifai N., Ridker P.M. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302(11):1186–1194. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 179.Zi F., Zi H., Li Y., He J., Shi Q., Cai Z. Metformin and cancer: an existing drug for cancer prevention and therapy. Oncol. Lett. 2018;15(1):683–690. doi: 10.3892/ol.2017.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lamkanfi M., Mueller J.L., Vitari A.C., Misaghi S., Fedorova A., Deshayes K., Lee W.P., Hoffman H.M., Dixit V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rakel A., Renier G., Roussin A., Buithieu J., Mamputu J.C., Serri O. Beneficial effects of gliclazide modified release compared with glibenclamide on endothelial activation and low-grade inflammation in patients with type 2 diabetes. Diabetes Obes. Metab. 2007;9(1):127–129. doi: 10.1111/j.1463-1326.2006.00571.x. [DOI] [PubMed] [Google Scholar]
- 182.Pfutzner A., Marx N., Lubben G., Langenfeld M., Walcher D., Konrad T., Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J. Am. Coll. Cardiol. 2005;45(12):1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 183.LeBrasseur N.K., Kelly M., Tsao T.S., Farmer S.R., Saha A.K., Ruderman N.B., Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am. J. Physiol. Endocrinol. Metab. 2006;291(1):E175–81. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 184.Boettcher E., Csako G., Pucino F., Wesley R., Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Esposito K., Ciotola M., Carleo D., Schisano B., Saccomanno F., Sasso F.C., Cozzolino D., Assaloni R., Merante D., Ceriello A., Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29(5):1071–1076. doi: 10.2337/diacare.2951071. [DOI] [PubMed] [Google Scholar]
- 186.Lincoff A.M., Wolski K., Nicholls S.J., Nissen S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 187.Kahles F., Meyer C., Mollmann J., Diebold S., Findeisen H.M., Lebherz C., Trautwein C., Koch A., Tacke F., Marx N., Lehrke M. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014;63(10):3221–3229. doi: 10.2337/db14-0100. [DOI] [PubMed] [Google Scholar]
- 188.Tahrani A.A., Barnett A.H., Bailey C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016;12(10):566–592. doi: 10.1038/nrendo.2016.86. [DOI] [PubMed] [Google Scholar]
- 189.Gallego-Colon E., Wojakowski W., Francuz T. Incretin drugs as modulators of atherosclerosis. Atherosclerosis. 2018;278:29–38. doi: 10.1016/j.atherosclerosis.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 190.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C., D’Alessio D.A., Davies M.J. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63(2):221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 191.Dai Y., Wang X., Ding Z., Dai D., Mehta J.L. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol. 2014;51(3):471–478. doi: 10.1007/s00592-013-0541-3. [DOI] [PubMed] [Google Scholar]
- 192.Makdissi A., Ghanim H., Vora M., Green K., Abuaysheh S., Chaudhuri A., Dhindsa S., Dandona P. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 2012;97(9):3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tremblay A.J., Lamarche B., Deacon C.F., Weisnagel S.J., Couture P. Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism. 2014;63(9):1141–1148. doi: 10.1016/j.metabol.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 194.Song X., Jia H., Jiang Y., Wang L., Zhang Y., Mu Y., Liu Y. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 diabetes mellitus: a meta-analysis. Sci. Rep. 2015;5:10202. doi: 10.1038/srep10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Balestrieri M.L., Rizzo M.R., Barbieri M., Paolisso P., D’Onofrio N., Giovane A., Siniscalchi M., Minicucci F., Sardu C., D’Andrea D., Mauro C., Ferraraccio F., Servillo L., Chirico F., Caiazzo P., Paolisso G., Marfella R. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64(4):1395–1406. doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 196.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., Steinberg W.M., Stockner M., Zinman B., Bergenstal R.M., Buse J.B., Committee L.S., Investigators L.T. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., Woo V., Hansen O., Holst A.G., Pettersson J., Vilsboll T., Investigators S.- Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 198.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E., Investigators E.-R.O. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 199.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N., Shaw W., Law G., Desai M., Matthews D.R., Group C.P.C. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 200.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., Silverman M.G., Zelniker T.A., Kuder J.F., Murphy S.A., Bhatt D.L., Leiter L.A., McGuire D.K., Wilding J.P.H., Ruff C.T., Gause-Nilsson I.A.M., Fredriksson M., Johansson P.A., Langkilde A.M., Sabatine M.S., Investigators D.-T. Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 201.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A., Kaiser N., Halban P.A., Donath M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 2002;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., Takasu T., Imamura M., Li Q., Tomiyama H., Kobayashi Y., Noda A., Sasamata M., Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur. J. Pharmacol. 2013;715(1–3):246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 203.Osataphan S., Macchi C., Singhal G., Chimene-Weiss J., Sales V., Kozuka C., Dreyfuss J.M., Pan H., Tangcharoenpaisan Y., Morningstar J., Gerszten R., Patti M.E. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019;4(5) doi: 10.1172/jci.insight.123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bonnet F., Scheen A.J. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44(6):457–464. doi: 10.1016/j.diabet.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 205.Jardine M.J., Mahaffey K.W., Neal B., Agarwal R., Bakris G.L., Brenner B.M., Bull S., Cannon C.P., Charytan D.M., de Zeeuw D., Edwards R., Greene T., Heerspink H.J.L., Levin A., Pollock C., Wheeler D.C., Xie J., Zhang H., Zinman B., Desai M., Perkovic V., investigators C.s. the canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am. J. Nephrol. 2017;46(6):462–472. doi: 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cefalu W.T., Leiter L.A., Yoon K.H., Arias P., Niskanen L., Xie J., Balis D.A., Canovatchel W., Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 207.Sircana A., De Michieli F., Parente R., Framarin L., Leone N., Berrutti M., Paschetta E., Bongiovanni D., Musso G. Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol. Res. 2019;144:390–408. doi: 10.1016/j.phrs.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 208.Griffin J.L., Wang X., Stanley E. Does our gut microbiome predict cardiovascular risk? A review of the evidence from metabolomics. Circ. Cardiovasc. Genet. 2015;8(1):187–191. doi: 10.1161/CIRCGENETICS.114.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li X.S., Obeid S., Wang Z., Hazen B.J., Li L., Wu Y., Hurd A.G., Gu X., Pratt A., Levison B.S., Chung Y.M., Nissen S.E., Tang W.H.W., Mach F., Raber L., Nanchen D., Matter C.M., Luscher T.F., Hazen S.L. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur. Heart J. 2019;40(32):2700–2709. doi: 10.1093/eurheartj/ehz259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Collins H.L., Drazul-Schrader D., Sulpizio A.C., Koster P.D., Williamson Y., Adelman S.J., Owen K., Sanli T., Bellamine A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37. doi: 10.1016/j.atherosclerosis.2015.10.108. [DOI] [PubMed] [Google Scholar]
- 212.Lindskog Jonsson A., Caesar R., Akrami R., Reinhardt C., Fak Hallenius F., Boren J., Backhed F. Impact of gut microbiota and diet on the development of atherosclerosis in apoe(-/-) mice. Arterioscler. Thromb. Vasc. Biol. 2018;38(10):2318–2326. doi: 10.1161/ATVBAHA.118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li X.S., Obeid S., Klingenberg R., Gencer B., Mach F., Raber L., Windecker S., Rodondi N., Nanchen D., Muller O., Miranda M.X., Matter C.M., Wu Y., Li L., Wang Z., Alamri H.S., Gogonea V., Chung Y.M., Tang W.H., Hazen S.L., Luscher T.F. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017;38(11):814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cassambai S., Salzano A., Yazaki Y., Bernieh D., Wong M., Israr M.Z., Heaney L.M., Suzuki T. Impact of acute choline loading on circulating trimethylamine N-oxide levels. Eur. J. Prev. Cardiol. 2019;26(17):1899–1902. doi: 10.1177/2047487319831372. [DOI] [PubMed] [Google Scholar]
- 215.Lam V., Su J., Koprowski S., Hsu A., Tweddell J.S., Rafiee P., Gross G.J., Salzman N.H., Baker J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26(4):1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim J.Y., Kim H., Jung B.J., Kim N.R., Park J.E., Chung D.K. Lipoteichoic acid isolated from Lactobacillus plantarum suppresses LPS-mediated atherosclerotic plaque inflammation. Mol. Cells. 2013;35(2):115–124. doi: 10.1007/s10059-013-2190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Malik M., Suboc T.M., Tyagi S., Salzman N., Wang J., Ying R., Tanner M.J., Kakarla M., Baker J.E., Widlansky M.E. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ. Res. 2018;123(9):1091–1102. doi: 10.1161/CIRCRESAHA.118.313565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brunt V.E., Gioscia-Ryan R.A., Richey J.J., Zigler M.C., Cuevas L.M., Gonzalez A., Vazquez-Baeza Y., Battson M.L., Smithson A.T., Gilley A.D., Ackermann G., Neilson A.P., Weir T., Davy K.P., Knight R., Seals D.R. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019;597(9):2361–2378. doi: 10.1113/JP277336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brandsma E., Kloosterhuis N.J., Koster M., Dekker D.C., Gijbels M.J.J., van der Velden S., Rios-Morales M., van Faassen M.J.R., Loreti M.G., de Bruin A., Fu J., Kuipers F., Bakker B.M., Westerterp M., de Winther M.P.J., Hofker M.H., van de Sluis B., Koonen D.P.Y. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 2019;124(1):94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., de Vos W.M., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., Myridakis A., Delzenne N.M., Klievink J., Bhattacharjee A., van der Ark K.C., Aalvink S., Martinez L.O., Dumas M.E., Maiter D., Loumaye A., Hermans M.P., Thissen J.P., Belzer C., de Vos W.M., Cani P.D. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 222.Depommier C., Everard A., Druart C., Plovier H., Van Hul M., Vieira-Silva S., Falony G., Raes J., Maiter D., Delzenne N.M., de Barsy M., Loumaye A., Hermans M.P., Thissen J.P., de Vos W.M., Cani P.D. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li J., Lin S., Vanhoutte P.M., Woo C.W., Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe-/- mice. Circulation. 2016;133(24):2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 224.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 225.Rett B.S., Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr. Metab. (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Masoodi M., Pearl D.S., Eiden M., Shute J.K., Brown J.F., Calder P.C., Trebble T.M. Altered colonic mucosal Polyunsaturated Fatty Acid (PUFA) derived lipid mediators in ulcerative colitis: new insight into relationship with disease activity and pathophysiology. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pischon T., Hankinson S.E., Hotamisligil G.S., Rifai N., Willett W.C., Rimm E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108(2):155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 228.Ferrucci L., Cherubini A., Bandinelli S., Bartali B., Corsi A., Lauretani F., Martin A., Andres-Lacueva C., Senin U., Guralnik J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006;91(2):439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 229.Marklund M., Wu J.H.Y., Imamura F., Del Gobbo L.C., Fretts A., de Goede J., Shi P., Tintle N., Wennberg M., Aslibekyan S., Chen T.A., de Oliveira Otto M.C., Hirakawa Y., Eriksen H.H., Kroger J., Laguzzi F., Lankinen M., Murphy R.A., Prem K., Samieri C., Virtanen J., Wood A.C., Wong K., Yang W.S., Zhou X., Baylin A., Boer J.M.A., Brouwer I.A., Campos H., Chaves P.H.M., Chien K.L., de Faire U., Djousse L., Eiriksdottir G., El-Abbadi N., Forouhi N.G., Michael Gaziano J., Geleijnse J.M., Gigante B., Giles G., Guallar E., Gudnason V., Harris T., Harris W.S., Helmer C., Hellenius M.L., Hodge A., Hu F.B., Jacques P.F., Jansson J.H., Kalsbeek A., Khaw K.T., Koh W.P., Laakso M., Leander K., Lin H.J., Lind L., Luben R., Luo J., McKnight B., Mursu J., Ninomiya T., Overvad K., Psaty B.M., Rimm E., Schulze M.B., Siscovick D., Skjelbo Nielsen M., Smith A.V., Steffen B.T., Steffen L., Sun Q., Sundstrom J., Tsai M.Y., Tunstall-Pedoe H., Uusitupa M.I.J., van Dam R.M., Veenstra J., Monique Verschuren W.M., Wareham N., Willett W., Woodward M., Yuan J.M., Micha R., Lemaitre R.N., Mozaffarian D., Riserus U., Cohorts for H., Aging Research in Genomic Epidemiology Fatty A., Outcomes Research C. Biomarkers of dietary Omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139(21):2422–2436. doi: 10.1161/CIRCULATIONAHA.118.038908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Heshmati J., Morvaridzadeh M., Maroufizadeh S., Akbari A., Yavari M., Amirinejad A., Maleki-Hajiagha A., Sepidarkish M. Omega-3 fatty acids supplementation and oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019;149 doi: 10.1016/j.phrs.2019.104462. [DOI] [PubMed] [Google Scholar]
- 231.Calder P.C. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 232.Wan X., Gao X., Bi J., Tian F., Wang X. Use of n-3 PUFAs can decrease the mortality in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. Lipids Health Dis. 2015;14:23. doi: 10.1186/s12944-015-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bannenberg G., Serhan C.N. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim. Biophys. Acta. 2010;1801(12):1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Weylandt K.H. Docosapentaenoic acid derived metabolites and mediators - the new world of lipid mediator medicine in a nutshell. Eur. J. Pharmacol. 2016;785:108–115. doi: 10.1016/j.ejphar.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 235.Calder P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 2017;45(5):1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 236.Miles E.A., Wallace F.A., Calder P.C. Dietary fish oil reduces intercellular adhesion molecule 1 and scavenger receptor expression on murine macrophages. Atherosclerosis. 2000;152(1):43–50. doi: 10.1016/s0021-9150(99)00446-3. [DOI] [PubMed] [Google Scholar]
- 237.Weatherill A.R., Lee J.Y., Zhao L., Lemay D.G., Youn H.S., Hwang D.H. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 2005;174(9):5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 238.Tan A., Sullenbarger B., Prakash R., McDaniel J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot. Essent. Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Allaire J., Couture P., Leclerc M., Charest A., Marin J., Lepine M.C., Talbot D., Tchernof A., Lamarche B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016;104(2):280–287. doi: 10.3945/ajcn.116.131896. [DOI] [PubMed] [Google Scholar]
- 240.He Z., Hao W., Kwek E., Lei L., Liu J., Zhu H., Ma K.Y., Zhao Y., Ho H.M., He W.S., Chen Z.Y. Fish oil is more potent than flaxseed oil in modulating gut microbiota and reducing Trimethylamine-N-oxide-Exacerbated atherogenesis. J. Agric. Food Chem. 2019;67(49):13635–13647. doi: 10.1021/acs.jafc.9b06753. [DOI] [PubMed] [Google Scholar]
- 241.Mason R.P., Libby P., Bhatt D.L. Emerging mechanisms of cardiovascular protection for the Omega-3 fatty acid eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 2020;40(5):1135–1147. doi: 10.1161/ATVBAHA.119.313286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M., Investigators R.-I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 243.Ridker P.M., MacFadyen J.G., Thuren T., Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehz542. [DOI] [PubMed] [Google Scholar]
- 244.Quispe R., Michos E.D., Martin S.S., Puri R., Toth P.P., Al Suwaidi J., Banach M., Virani S.S., Blumenthal R.S., Jones S.R., Elshazly M.B. High-sensitivity C-Reactive protein discordance with atherogenic lipid measures and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. J. Am. Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Diamantis E., Kyriakos G., Quiles-Sanchez L.V., Farmaki P., Troupis T. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr. Cardiol. Rev. 2017;13(3):209–216. doi: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Banach M., Penson P.E. What have we learned about lipids and cardiovascular risk from PCSK9 inhibitor outcome trials: ODYSSEY and FOURIER? Cardiovasc. Res. 2019;115(3):e26–e31. doi: 10.1093/cvr/cvy301. [DOI] [PubMed] [Google Scholar]
- 247.Sacks F.M., Pfeffer M.A., Moye L.A., Rouleau J.L., Rutherford J.D., Cole T.G., Brown L., Warnica J.W., Arnold J.M., Wun C.C., Davis B.R., Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 248.Ridker P.M., Rifai N., Pfeffer M.A., Sacks F., Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The cholesterol and recurrent events (CARE) investigators. Circulation. 1999;100(3):230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 249.Albert M.A., Danielson E., Rifai N., Ridker P.M., Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 250.Downs J.R., Clearfield M., Weis S., Whitney E., Shapiro D.R., Beere P.A., Langendorfer A., Stein E.A., Kruyer W., Gotto A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 251.Kinlay S., Schwartz G.G., Olsson A.G., Rifai N., Leslie S.J., Sasiela W.J., Szarek M., Libby P., Ganz P., Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108(13):1560–1566. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- 252.Schwartz G.G., Olsson A.G., Ezekowitz M.D., Ganz P., Oliver M.F., Waters D., Zeiher A., Chaitman B.R., Leslie S., Stern T., Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 253.Nissen S.E., Tuzcu E.M., Schoenhagen P., Brown B.G., Ganz P., Vogel R.A., Crowe T., Howard G., Cooper C.J., Brodie B., Grines C.L., DeMaria A.N., Investigators R. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291(9):1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 254.Cannon C.P., Braunwald E., McCabe C.H., Rader D.J., Rouleau J.L., Belder R., Joyal S.V., Hill K.A., Pfeffer M.A., Skene A.M., Pravastatin or Atorvastatin E., Infection Therapy-Thrombolysis in Myocardial Infarction I. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 255.de Lemos J.A., Blazing M.A., Wiviott S.D., Lewis E.F., Fox K.A., White H.D., Rouleau J.L., Pedersen T.R., Gardner L.H., Mukherjee R., Ramsey K.E., Palmisano J., Bilheimer D.W., Pfeffer M.A., Califf R.M., Braunwald E. Investigators, Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 256.Heart Protection Study Collaborative G., Jonathan E., Derrick B., Emma L., Sarah P., John D., Jane A., Rory C. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20,536 patients in the heart protection study. Lancet. 2011;377(9764):469–476. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sever P.S., Poulter N.R., Chang C.L., Thom S.A., Hughes A.D., Welsh P., Sattar N., Investigators A. Evaluation of C-reactive protein before and on-treatment as a predictor of benefit of atorvastatin: a cohort analysis from the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm. J. Am. Coll. Cardiol. 2013;62(8):717–729. doi: 10.1016/j.jacc.2013.02.098. [DOI] [PubMed] [Google Scholar]
- 258.Soedamah-Muthu S.S., Livingstone S.J., Charlton-Menys V., Betteridge D.J., Hitman G.A., Neil H.A., Bao W., DeMicco D.A., Preston G.M., Fuller J.H., Stehouwer C.D., Schalkwijk C.G., Durrington P.N., Colhoun H.M., Investigators C. Effect of atorvastatin on C-reactive protein and benefits for cardiovascular disease in patients with type 2 diabetes: analyses from the Collaborative Atorvastatin Diabetes Trial. Diabetologia. 2015;58(7):1494–1502. doi: 10.1007/s00125-015-3586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ballantyne C.M., Houri J., Notarbartolo A., Melani L., Lipka L.J., Suresh R., Sun S., LeBeaut A.P., Sager P.T., Veltri E.P., Ezetimibe Study G. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 260.Ballantyne C.M., Weiss R., Moccetti T., Vogt A., Eber B., Sosef F., Duffield E., Investigators E.S. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study) Am. J. Cardiol. 2007;99(5):673–680. doi: 10.1016/j.amjcard.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 261.Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Bregeault M.F., Dalby A.J., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., Harrington R.A., Jukema J.W., Lecorps G., Mahaffey K.W., Moryusef A., Ostadal P., Parkhomenko A., Pordy R., Roe M.T., Tricoci P., Vogel R., White H.D., Zeiher A.M., Schwartz G.G. Effect of Alirocumab on mortality after acute coronary syndromes. Circulation. 2019;140(2):103–112. doi: 10.1161/CIRCULATIONAHA.118.038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Garvey W.T., Van Gaal L., Leiter L.A., Vijapurkar U., List J., Cuddihy R., Ren J., Davies M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 263.Hattori S. Anti-inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol. Metab. Syndr. 2018;10:93. doi: 10.1186/s13098-018-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tobita H., Sato S., Miyake T., Ishihara S., Kinoshita Y. Effects of Dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr. Ther. Res. Clin. Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]