Graphical abstract
Keywords: Rheumatoid arthritis, Rheumatic disease, Biologic, Small molecule, Tumor necrosis factor, Coronavirus, COVID-19
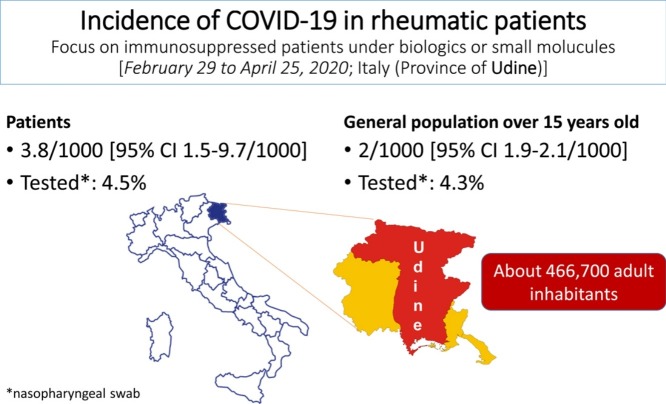
Abstract
Objective
The aim of this study is to determine the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease 2019 (COVID-19) among adult patients treated with biologic agents or small molecules for chronic inflammatory rheumatic diseases, in particular for chronic inflammatory arthritides.
Methods
To this end, a population-based study, in the province of Udine (466,700 inhabitants, with age > 15 years old, Friuli Venezia Giulia region, Italy) was planned. The primary outcome was the prevalence of COVID-19 in the first two months of the outbreak. All the rheumatic patients treated with biologic agents or small molecules in the last 6 months in our province were included (N = 1051).
Results
From February 29 to April 25, 2020, 4 adult patients (4/1051, i.e. 3.8/1000, 95% Confidence Interval 1.5–9.7/1000) were registered as swab test positive by PCR for COVID-19. Overall, a total of 47/1051 (4.5%) cases were tested for COVID-19 by PCR in the same period, and 15 of them due to symptoms compatible with COVID-19. In the general population, the prevalence was 937 cases/466700 (2/1000, 95% Confidence Interval 1.9–2.1/1000, P-value = 0.33, chi square test), and 20,179/466,700 (4.3%) swab tests for COVID-19 were performed.
Conclusion
The risk of COVID-19 in rheumatic patients under biologic agents or small molecules does not appear different from that observed in the general population. Patients should be informed to safely proceed with their treatment and follow the rules for self-protection to COVID-19.
1. Introduction
The ongoing outbreak by novel coronavirus (COVID-19) has been defined as a global public health emergency by World Health Organization (WHO) [1]. COVID-19 is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with droplets and contact as the main way of transmission. Currently, the research on COVID-19 is growing at great speed. Italy is one of the country showing the highest rate of mortality in the world, mainly in the northern regions [2]. While pre-existing pulmonary and cardiovascular disease as well as diabetes mellitus are known risk factors for the worst outcome for COVID-19 [3], the impact of chronic rheumatic diseases, and, in particular, if the risk of COVID-19 while using a biologic agent (b-DMARD) or a small molecule (ts-DMARD) for chronic inflammatory arthritis is higher than in the general population, is still unknown [1]. Biologic agents increase the risk for infections, although the advantages largely overcome that risk [4]. Moreover, the most severe complication of COVID-19 pneumonia seem to be caused by a cytokine storm syndrome [5] as an exaggerated response of the immune system to the virus [4], [6] for whom many rheumatic drugs, including biologic agents, are currently under investigations [7], [8].
Prevalence data in patients with chronic inflammatory rheumatic diseases treated with b-DMARDs or ts-DMARDs and their risk of COVID-19 are still scarce, and limited to case series [9], [10], [11]. Those preliminary data may be reassuring for clinicians, but, importantly, they lack of comparison to the reference population. Therefore, the aim of this study is to compare the prevalence of COVID-19 in this population of patients with the general population in order to provide a stronger evidence supporting the management of patients with inflammatory rheumatic diseases during COVID-19 outbreak [12].
2. Methods
2.1. Objective
The primary objective of the present study is to define the prevalence and the severity of COVID-19 in a population of patients suffering from a chronic inflammatory rheumatic disease under treatment with a biologic agent or a small molecule during the first two months of COVID-19 outbreak.
2.2. Study population and reference population
The cases were all the adult patients with a rheumatic disease and who were under treatment with a b- or a ts-DMARD from September 2019 to April 2020 in the province of Udine, Italy. A computer database from the Pharmacy service of the province of Udine recording past and present treatments with b/ts-DMARDs in the same cases was used. All the clinical charts of these cases were revised to verify they were proceeding with treatment at the last contact. The prevalence of COVID-19 during the month of March 2020 and April 2020 was compared to that of the general population in the province of Udine after excluding subjects ≤ 15 years old (a total of 466,700 inhabitants), the first COVID-19 case in this province being reported on February 29, 2020. Importantly, in our region, all the patients who undergo a biologic or small molecule treatment must be evaluated by a public consultant rheumatologist every six months for renewing and proceeding with their own therapeutic plan and then they need to be registered by the Pharmacy service that supplies the drug about every two months until to the treatment plan expiration.
2.3. Procedure to make diagnosis of COVID-19
Diagnostic tests for COVID-19 were conducted in accordance with the indication provided by the Italian Ministry of Health, following the rules suggested by the WHO, in symptomatic subjects. For initial diagnostic testing for COVID-19, the Italian Ministry of Health recommends collecting and testing an upper respiratory specimen. A nasopharyngeal specimen is the preferred choice for swab-based COVID-19 testing. All the people of the province of Udine, and in particular, all the rheumatic patients treated with b/ts-DMARDS, as well as other patients treated with immunosuppressive or cytotoxic agents for other diseases, were informed by national and local media to contact their own general practitioner (GP) in case of symptoms such as body temperature over 37.4 °C, cough, or dyspnoea. In turn, the GP sent the patients to undergo testing for COVID-19, whenever this infection was suspected.
Routine identification of cases of COVID-19 is based on the detection of unique sequences of virus RNA by nucleic acid amplification tests (NAAT) such as RT-PCR with confirmation by nucleic acid sequencing. The following genes are investigated: E gene for screening and then RdRp and N genes of SARS-CoV-2 for confirmation. WHO provided an interim guidance to laboratories showing the strategic use of diagnostic testing in different transmission scenarios of the COVID-19 outbreak, including how to justify testing when having to prioritize patients due to lack of proper facilities. The WHO document specifies the conditions necessary to consider a case laboratory-confirmed by NAAT for areas with no known or established COVID-19 circulation [13].
2.4. Statistical methods
Variables were expressed as mean ± standard deviation or frequency, as appropriate. Comparison between study population and general reference population was performed by Chi2 test. P-value < 0.05 was considered significant. The study was conducted in accordance with the ethical principles of the Helsinki Declaration, and approved by the local Ethics Committee (CEUR-2020-Os-129).
3. Results
3.1. Epidemiology data
Within the province of Udine, 1053 patients taking biologic drugs or small molecules were identified. Of them, two patients were excluded since they suspended the drug after a diagnosis of cancer. They were 703 (66.9%) women and 348 (33.1%) men, with a mean age of 58.4 ± 14.6 years.
From February 29 (first case of COVID-19 infection reported in the province of Udine) to April 25, 2020, a total of 47/1051 cases were tested for COVID-19 (4.5%): 15/47 (31.9%) because of symptoms, and 20/47 (42.5%) since they were healthcare providers with a previous contact with a proven COVID-19 positive subject, and 11/47 (23.4%) for ward triage rules. Thus, 15/1051 (1.4%) patients underwent COVID-19 swab test because of symptoms.
Four cases (4/1051, i.e. 3.8/1000, 95% Confidence Interval 1.5-9.7/1000) were proved to be test positive to COVID-19, none of them among the healthcare providers. Detailed description of them is reported in tables from 1 to 3. In the reference general population, on April 25, 2020, the prevalence was 937 cases/466,700 (2/1000, 95% Confidence Interval 1.9-2.1/1000, P-value = 0.33, chi square test). Screening swab tests were performed in 20,179 subjects in a general population of about 466,700 inhabitants (4.3%).
In order to provide background information on our study population, complete data on the underlying rheumatic disease were available for 925/1051 (88%). The diagnoses were: rheumatoid arthritis (362, 39.1%), psoriatic arthritis (275, 26.7%), ankylosing spondylitis or non-radiographic spondyloarthritis (176, 19.0%), systemic vasculitides (74, 8.0%), systemic lupus erythematosus (38, 4.1%), other chronic inflammatory diseases (19, 2.1%). Overall, 549 (59.3%) patients were taking anti-TNF agent (in particular adalimumab, 202, 21.8%, or etanercept, 210, 22.7%), 90 (9.7%) patients were taking an anti-IL6 receptor antibody, 67 (9.6%) patients were taking an anti-IL17 or anti-IL12/23 agent, 73 (6.9%) were taking a JAK inhibitor. The other treatments were abatacept (48, 4.8%), rituximab (38, 4.1%), belimumab (29, 3.1%), anakinra (13, 1.4%), canakinumab (1, 0.1%), apremilast (2, 0.2%). Comorbidities were hypertension in 277 (29.9%), type 2 diabetes in 65 (7.0%), heart disease in 109 (11.8%). Seven hundred and fifty-eight of these patients (81.9%) had been under biologic treatment for more than one year, and 393 patients (42.5%) for more than 5 years. Concomitant therapy with glucocorticoids (mean dosage at the last follow-up visit 4.7 ± 3.9 mg/day of prednisone equivalent) was used by 146 patients (15.8%), and a traditional synthetic DMARD, was used by 355 patients (38.4%), mainly methotrexate (257/355, i.e. 72.4% of them). Antimalarials were employed by 72 patients (7.8%).
3.2. Case series description
They were two females and two males, aged from 42 to 69 years, three affected by seronegative spondyloarthritis and one by rheumatoid arthritis (Table 1 ). The disease duration is longer than one year in all of them and the exposure to biologic drugs or small molecules along the clinical history of those patients ranged from 12 to 72 months. Other concomitant treatments for the rheumatic disease including methotrexate and low-dose of prednisone in two of them. Three out of four presented pre-existing comorbidities including active pulmonary or cardiovascular diseases.
Table 1.
Demographic and clinical characteristics of the four patients.
| Feature | Pt. 1 | Pt.2 | Pt.3 | Pt.4 |
|---|---|---|---|---|
| Sex | Female | Male | Female | Male |
| Age | 42 | 69 | 68 | 62 |
| Rheumatic disease as indication for the biologic agent | Non-radiographic SpA and suppurative hydradenitis | AS | RA | AS |
| Ongoing b/ts-DMARD | ADA 40/weekly | GOL 50 mg monthly | TOF 5 mg twice daily | GOL 50 mg monthly |
| Exposure to the last b/ts-DMARD (months) | 12 | 72 | 4 | 38 |
| Exposure to b/ts-DMARDs | From 2019 | From 2006 | From 2012 | From 2017 |
| Previous treatments | None | MTX, LEF ETA, ADA, | LEF, CYC, ETA, ADA, ABA, TCZ, CZP, BARI | None |
| Other concomitant DMARD | None | MTX 15 mg/weekly | MTX 10 mg/weekly | None |
| Glucocorticoids (prednisone equivalent/day) | No | Yes (5 mg/day) | Yes (5 mg/day) | No |
| Other ongoing treatments | Isoniazide, budesonide/formoterol fumarate dihydrate | Bisoprolol, furosemide, kanrenol, digoxin, apixaban, duloxetine, oxycodone/naloxone, lansoprazole, folic acid | Valsartan/hydrochlorothiazide, fentanyl, folic acid | None |
| Smoking | Yes, currently | No | No | Yes, currently |
| Obesity | No | Yes | No | No |
| Comorbidity | Renal angiomyolipoma, latent TB (still under isoniazid), asthma | Chronic heart failure; chronic atrial fibrillation, aortic valve insufficiency, mild dilatation of the ascending aorta; chronic bronchitis; fibromyalgia | Systemic arterial hypertension, complicated osteoporosis with vertebral fractures | Latent TB (previously treated with isoniazid for 9 months) |
| Influenza vaccination | Yes | Yes | Yes | Yes |
SpA: spondyloarthritis; AS: ankylosing spondylitis; RA: rheumatoid arthritis; b/ts-DMARD, biologic/targeted synthetic disease modifying drug; ADA, adalimumab; GOL: golimumab; ETA: etanercept; CZP, certolizumab pegol; ABA, abatacept; TCZ, tocilizumab; BARI, baricitinib; TOF: tafacitinib; MTX, methotrexate, LEF, leflunomide; CYC, cyclosporine; TB, tuberculosis.
3.3. Viral infection
Characteristics of the COVID-19 infection are reported in Table 2 . Briefly, three patients required hospital admission and two of them needed oxygen support, but none was subsequently transferred to ICU. All but one of the patients received antiviral drugs and one also hydroxychloroquine. All patients showed a favorable outcome. Laboratory features of the three hospitalized patients were reported in Table 3 .
Table 2.
Characteristics of Covid-19 infection in the four patients.
| Pt.1 | Pt.2 | Pt.3 | Pt.4 | |
|---|---|---|---|---|
| Swab test positive, date | March, 03 | March, 26 | March, 13 | March, 21 |
| Risk factors for COVID-19 | Yes, recent holiday in North western Italy | Not known | Not known | Not known |
| COVID-19 manifestations | Fever | Fatigue, dyspnoea, intense oral dryness | Fever, dyspnoea, fatigue, nausea | Fever |
| COVID-19 severity | Mild | High | Moderate | Mild |
| Hospitalization | Yes | Yes | Yes | No |
| Oxygen support | No | Yes | Yes | No |
| Mechanical ventilation | No | No | No | No |
| Admission to ICU | No | No | No | No |
| Treatment for COVID-19 | Lopinavir/Ritonavir, ribavirin | Lopinavir/Ritonavir | Lopinavir/Ritonavir, hydroxychloroquine | None |
| Lung imaging | RX negative | RX and CT scan positive for pneumonia | RX positive for pneumonia | No |
| Recovery | Yes (discharge after one day) | Yes (discharge after 10 days) | Yes (discharge after 11 days) | Yes |
| Swab re-test | Negative after 10 days | Negative after 7 days | Negative after 27 days | Negative after 25 days |
Table 3.
Laboratory data at hospital admission.
| Pt.1 | Pt.2 | Pt.3 | |
|---|---|---|---|
| Hemoglobin (g/dl) | 13.7 | 12.5 | 8.4 |
| White Blood cell count (cell/mcl) | 11040 | 4870 | 6390 |
| Neutrophils (cell/mcl) | 6100 | 3720 | 5600 |
| Lymphocyte (cell/mcl) | 2140 | 470 | 370 |
| Platelets (cell/mcl) | 437000 | 230000 | 345000 |
| C-Reactive Protein (mg/l) | 4.17 | 88.5 | 92.1 |
| Procalcitonin (ng/ml) | < 0.01 | 0.14 | 0.03 |
| Creatinine (mg/dl) | 0.56 | 1.00 | 0.89 |
| Electrolytes | Normal | Hyponatriemia, 128 mMol/l | Hyponatriemia, 129 mMol/l |
| AST/ALT (IU/l) | Normal | Normal | Normal |
| CK (IU/l) | 74 | 193 | 449 |
| LDH (IU/l) | 272 | 470 | 278 |
| BNP (pg/ml) | Not available | 213 | Not available |
| Troponin I (ng/ml) | Not available | Negative | Negative |
| D-dimer (ng/ml) | Not available | 488 | Not available |
| Ferritin (ng/ml) | Not available | Not available | 40 |
AST: aspartate transaminase; ALT: alanine transaminase; CK: creatine kinase; LDH: lactate dehydrogenase; BNP: b-type natriuretic peptide.
4. Discussion
To our knowledge, this is the first report comparing the COVID-19 incidence among patients suffering from chronic rheumatic inflammatory diseases under ongoing treatment with a biologic agent or a small molecule with the reference general population of adults. In fact, by matching the data from our Institute of Epidemiology, Pharmacy service and electronic clinical charts of our Hospital, the study design allow us to capture all the patients taking a biologic agent or a small molecule for a chronic inflammatory muscoloskeletal disease, who were tested for COVID-19, and to compare the prevalence of COVID-19 with that observed in the reference general population of adults in the same province. TheCOVID-19 prevalence of 3.8/1000 (95% Confidence Interval 1.5–9.7/1000) reported by us is not different from that estimated in the reference general population. On the contrary, it is known that b-DMARDs and ts-DMARDs put patients at significantly higher risk of infections [6], especially in the respiratory tract, than the general population. Therefore, our result is reassuring for clinicians, and, thus, b- or ts-DMARDs in rheumatic diseases could neither substantially increase the risk of COVID-19, nor worsen the course of COVID-19, when it occurs. This observation is consistent with previous findings during the previous outbreaks, such as SARS and Middle East respiratory syndrome, regarding favorable outcome in immunosuppressed patients for organ transplantation, cancer or autoimmune diseases [14], [15], [16].
Even if the true rate of COVID-19 is not known either in the general population or among rheumatic patients, rheumatic patients under immunosuppressors are usually more prone to alert clinicians in the case of symptoms of infection, and to protect themselves earlier from possible contagion. The number of patients, who were tested for COVID-19 (4.5%) was similar to the reference population (4.3%). However, among rheumatic patients tested for COVID-19, only one third (15/47, 31.9%) referred to healthcare providers for symptoms compatible with COVID-19, thus underlying a possible social behavior of patients with rheumatic disease (i.e., being more careful in social contacts because of their immunosuppression) that likely played an important role in infection rate and control. This feature needs appropriate studies. Overall, it is an important observation, though it needs to be confirmed by other groups, since this is a large population of chronic immunosuppressed patients, and, among the different subgroups of rheumatic patients, they probably represent those at highest risk for infectious complications both for their own clinical characteristics (age, comorbidity, moderate to severe inflammatory chronic rheumatic disease) and related treatments (chronic glucocorticosteroids, b- or ts-DMARD, long disease duration, combination of two immunosuppressors), as already known [17]. We limited our analysis to rheumatic diseases that required biologic treatments or small molecules, thus the majority of patients with chronic inflammatory arthritis not requiring those treatments, as well as the majority of patients with rarer autoimmune systemic diseases, like systemic lupus erythematosus or systemic vasculitides, were not included. Anyway, among patients suffering from chronic inflammatory arthritides, those taking biologics are at greater infectious risk than the others. Previous epidemiologic data also in our region of Italy supported this notion [18]. The possible mitigation of symptoms by biologic agents or small molecules cannot be excluded, thus causing an underestimation of COVID-19 in our study population. Finally, the weight of antimalarials in possibly decreasing the prevalence of COVID-19 in our study population was negligible (< 10%). However, antimalarials failed to reduce mortality in critically ill COVID-19 patients in a recent randomized controlled trial [19], and further studies are warranted [20].
Yet, more than 50% of our patients have been in treatment with an anti-TNF agent (adalimumab and etanercept, in particular), therefore, our conclusions are more properly applicable in patients with chronic inflammatory arthritides who are taking an anti-TNF agent.
Finally, regarding our four patients positive for COVID-19, the infectious disease was generally characterized by a favorable outcome, in line with the recently reported case series [9], [10], [11].
To conclude, the risk of COVID-19 in chronically immunosuppressed rheumatic patients under biologics or small molecules does not appear higher than the general population, in particular for those patients with chronic inflammatory arthritides taking an anti-TNF agent. Overall, the outcome of the infection might be favorable. Thus, patients should be informed to safely proceed with their treatments during the outbreak, and to strictly adhere to the principles for self-protection to COVID-19.
Disclosure of interest
The authors declare that they have no competing interest.
Contributions
LQ designed data collection tools, monitored data collection for the whole study, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. He is guarantor. FV and SDV implemented the statistical analysis, analysed the data, drafted and revised the paper. EP, CT analysed the data, drafted and revised the paper.
Funding
This research received no external funding.
Ethical approval information
The study was conducted in accordance with the ethical principles of the Helsinki Declaration. Written patients’ consents for using data for research purpose were obtained at the time of hospital admission, and the local Ethics Committee approved the study (CEUR-2020-Os-129).
Data sharing statement
The data that support the findings of this study are available on request from the corresponding author, [LQ].
Acknowledgements
Ginevra De Marchi, MD, Stefania Sacco, MD, Alen Zabotti, MD, Elena Treppo, MD, Germana Modesti, MPH, Roberto Agarinis, MD, Giulia Del Frate MD contributed to this work.
Local patients’ associations for rheumatic diseases and general practitioners were involved in the identifying this topic as an urgent need for the best management of immunosuppressive treatments, and in particular biologic agents or small molecules, in this outbreak.
References
- 1.Wang L., Wang Y., Ye D., et al. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents. 2020:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuite A.R., Ng V., Rees E. Estimation of COVID-19 outbreak size in Italy. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Sepriano A., Kerschbaumer A., Smolen J.S., et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;6736:19–20. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 7.Benucci M., Damiani A., Infantino M., et al. Old and new antirheumatic drugs for the treatment of COVID-19. Joint Bone Spine. 2020;87:195–197. doi: 10.1016/j.jbspin.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quartuccio L., Semerano L., Benucci M., et al. Urgent avenues in the treatment of COVID-19: Targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87:191–193. doi: 10.1016/j.jbspin.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberman R., Axelrad J., Chen A., et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med. 2020 doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti S., Balduzzi S., Delvino P., et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217424. annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favalli E.G., Agape E., Caporali R. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol. 2020 doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 12.Richez C., Lazaro E., Lemoine M., et al. Implications of COVID-19 for the management of patients with inflammatory rheumatic diseases. Joint Bone Spine. 2020;87:187–189. doi: 10.1016/j.jbspin.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . World Health Organization; 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance.https://apps.who.int/iris/handle/10665/331329 [Google Scholar]
- 14.D’Antiga L. Coronaviruses immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020 doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020:382. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui D.S., Azhar E.I., Kim Y.-J., et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favalli E.G., Ingegnoli F., De Lucia O., et al. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020 doi: 10.3899/jrheum.200507. 102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quartuccio L., Zabotti A., Del Zotto S., et al. Risk of serious infection among patients receiving biologics for chronic inflammatory diseases: usefulness of administrative data. J Adv Res. 2018;15:87–93. doi: 10.1016/j.jare.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borba M.G.S., Val F.F.A., Sampaio V.S., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastick K.A., Okafor E.C., Wang F., et al. Review: Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19) Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa130. ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]