Abstract
We prepared a novel amphiphile with a penta-phenylene lipophilic group and a branched trimaltoside head group. This new agent, designated penta-phenylene maltoside (PPM), showed a high tendency to self-assembly into micelles via strong aromatic-aromatic interactions in aqueous media, as evidenced by 1H NMR and fluorescence studies. When applied for membrane protein studies, this new agent was superior to DDM, a gold standard conventional detergent, in stabilizing multiple proteins for a long term. The ability of this agent to form aromatic-aromatic interactions is likely responsible for enhanced protein stabilization when associated with a target membrane protein.
Keywords: amphiphile, molecular design, micelles, membrane proteins, protein stability
Graphical Abstract
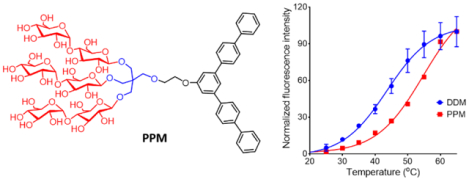
A novel amphiphilic molecule with a penta-phenylene group as a lipophilic group (PPM) was designed. This detergent formed small and stable micelles via strong aromatic-aromatic interactions, and displayed notable protein stabilization efficacy with a few membrane proteins including β2 adrenergic receptor (β2AR).
Self-assembly, a general phenomenon in nature, plays a pivotal role in various biological functions.[1] Inspired by the abundance of self-assembly processes in biological systems, considerable efforts have been devoted to the design and synthesis of self-assembly-based nanostructures for a wide range of applications in chemistry, biology, and material sciences.[2] Amphipathic agents are known to self-assemble into nano-sized aggregates, for example micelles, liposomes, nanotubes, when above a critical concentration in an aqueous environment. Of these nanostructures, micelles are particularly interesting as they are widely used in biochemical studies for solubilization and stabilization of integral membrane proteins as well as providing a medium for crystallisation and ultimately structure determination.[3] These micellar assemblies typically have a spherical or elliptical shape and are traditionally depicted as having a core of loosely packed hydrophobic tails surrounded by hydrophilic head groups.[4] For structural studies of membrane proteins, conventional detergents (e.g., n-dodecyl-β-D-maltoside (DDM) and n-octyl-β-D-glucoside (OG)) are widely used.[5] Over the last three decades, several alternative micellar systems comprising small synthetic amphiphiles have demonstrated significant utility for membrane protein study. Representatives include tripod amphiphiles (TPAs),[6] fluorinated surfactants (FSs),[7] facial amphiphiles (FAs),[8] glyco-diosgenin (GDN),[9] neopentyl glycol (NG) amphiphiles (GNGs and MNGs),[10] penta-saccharide-bearing amphiphiles (PSEs) and norbornane-based maltosides (NBMs).[11] Large molecules/assemblies have also been invented for the same purpose, as exemplified by lipopeptide detergents (LPDs),[12] amphipol (APols) and nanodics (NDs).[13] Most of these amphiphiles (both the small synthetic amphiphiles and large molecules/assemblies) contain linear and flexible alkyl chains as the hydrophobic groups and thus hydrophobic interaction between these alkyl chains is likely to be the main driving force for spontaneous formation of aggregates in water. We hypothesised that an amphiphile with an extended aromatic scaffold, would form stable assemblies due to the formation of additional aromatic-aromatic associations. To date, there has been no report describing amphiphile micelles stabilized by strong aromatic-aromatic interactions for application to membrane proteins. This study introduces a new aromatic ring-bearing amphiphile that proved useful for stabilizing multiple membrane proteins. The new amphiphilic molecule consists of penta-phenylene unit as a hydrophobic part and a branched trimaltoside as a hydrophilic group, designated penta-phenylene maltoside (PPM; Figure 1). The current study showed that PPM self-assembled into a micellar architecture via strong aromatic-aromatic interactions in aqueous solution, which directly correlated to enhanced membrane protein stability conferred by this amphiphile.
Figure 1.
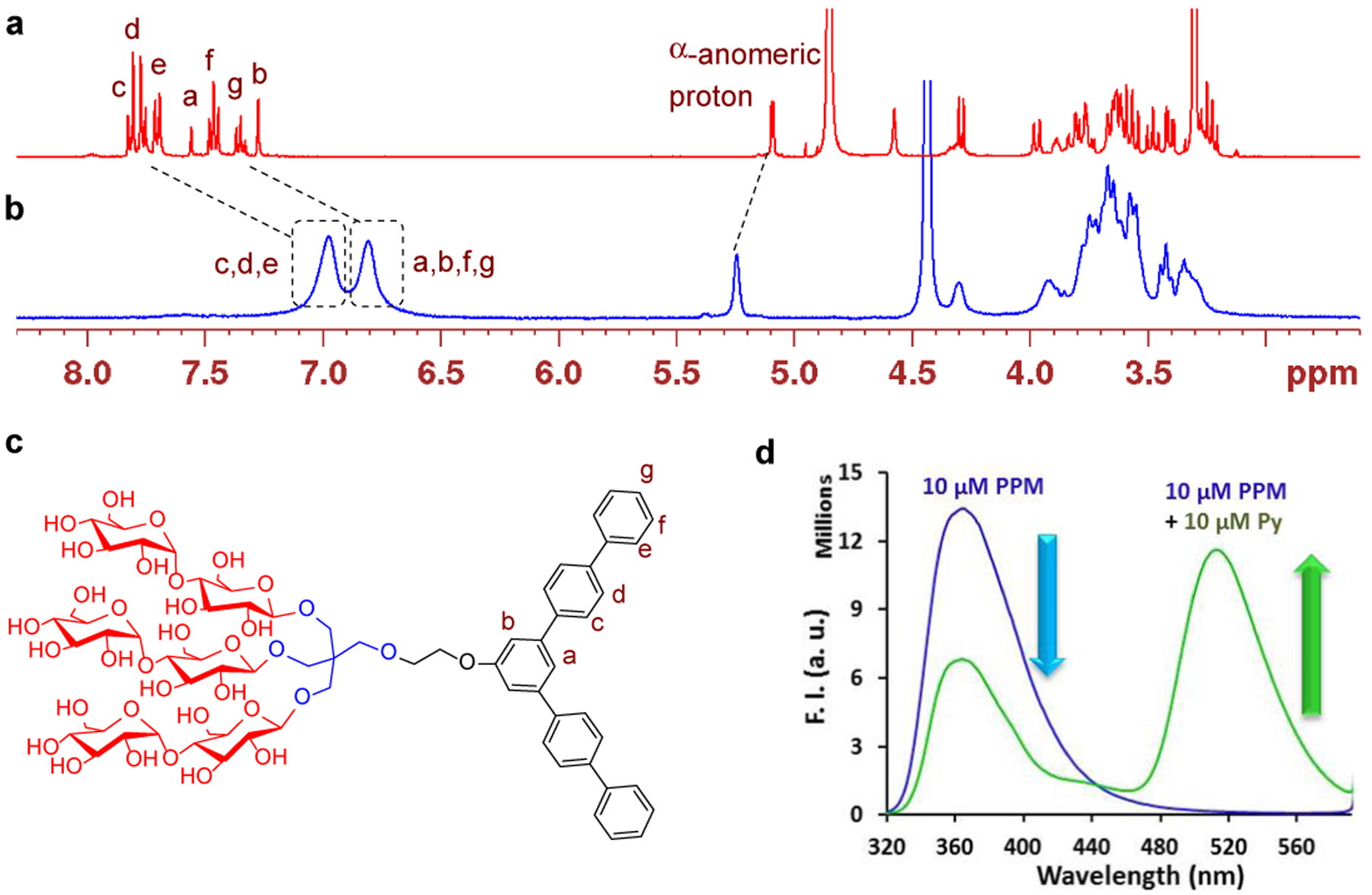
(a) 1H NMR spectrum (400 MHz, room temperature, 1.0 mM) of a solution of PPM in CD3OD. (b) 1H NMR spectrum (400 MHz, 1.0 mM) of a solution of PPM in D2O at 60 °C. Tetramethylsilane (TMS) was used as an internal standard. (c) Chemical structure of PPM showing proton assignment in the aromatic region. (d) Emission spectra change of aqueous solutions of PPM (10 mM) and PPM combined with one equivalent pyranine dye (Py; 10 mM) at an excitation wavelength of 300 nm.
For membrane protein study, a highly conjugated aromatic group (e.g., penta-phenylene) is rarely used as the amphiphile hydrophobic group. Detergents with a single aromatic ring (e.g., benzene) have been reported previously, as exemplified by styrene-maleic acid (SMA) copolymers,[14] TPAs[6] and trans-4-(4’-propylphenyl)cyclohexyl-β-D-maltoside (PPC-b-M).[15] However, these amphiphiles exhibit no extensive conjugation in their aromatic group. Due to a rigid conformation and relatively high polarity compared to an alkyl chain, a detergent with a highly conjugated aromatic group is likely to be less effective at forming strong interactions with the hydrophobic surfaces of proteins. Thus, without a clever design, it would be challenging to develop novel detergents effective at membrane protein stabilization. In addition, the light-absorbing aromatic group could potentially interfere with optical spectroscopic measurements. However, it also needs to be considered that aromatic group-bearing detergents can be synthetically more accessible than non-aromatic analogues because of high reactivity and selectivity. In addition, the availability of diverse reactions for structural modifications of an aromatic group can introduce a wide variety of detergent structures, facilitating the optimization of detergent properties. More importantly, as aromatic-aromatic interactions are additive to micellar stability we can develop highly stable micelles for membrane protein study by utilizing an aromatic scaffold.
A six-step synthetic protocol including Suzuki coupling and glycosylation produced PPM with an overall good yield (~50%). The structure of the PPM was characterized by NMR and ESI-MS analyses (Figure S1 & S2 ESI†). Exclusive β-stereochemistry in the newly formed glycosidic bonds was confirmed by the 1H NMR spectrum of PPM where a peak corresponding to the β-anomeric proton appeared at 4.30 ppm as a doublet with a vicinal coupling constant of J = 8.0 Hz (Figure S1). The 2D NMR spectrum (1H-1H NOSEY) was also consistent with the chemical structure of the penta-phenylene group in this agent (Figure S2). Good water-solubility is important for biological applications of a detergent. Stirring PPM (1.0 mmol) in D2O (1.0 mL) at 60 °C for 1 min resulted in a clear solution.
Aggregation behaviour of PPM was assessed in terms of critical micelle concentration (CMC) and the hydrodynamic diameter (Dh) of the micelles. The summarized results of PPM along with a conventional detergent (DDM) are presented in Table 1. The CMC of this agent (~8 μM), estimated using a hydrophobic fluorophore (i.e., diphenylhexatriene (DPH)).[16] To exclude the potential effect of DPH on detergent CMC determination, we also used fluorescence intensity ratio (I375/I363) of the penta-phenylene unit for measure of the CMC of PPM. This probe-free method gave ~6 μM as a CMC of PPM (Figure S3), comparable to that obtained from DPH use. Thus, the CMC of PPM (~6 or 8 μM) was much smaller than that of DDM (170 μM). The standard Gibbs free energies (ΔG°) corresponding to the transfer of DDM and PPM (~8 μM) from a water to a micellar phase were −31.5 and −39.1 kJ/mol, respectively. This water-to-micellar free energy of PPM was significantly smaller than triton X-100, an aromatic group-bearing conventional detergent (−39.1 vs −30.6 kJ/mol), indicating a strong tendency to self-assembly.[17] The size of aggregates (Dh) formed by PPM, measured by dynamic light scattering (DLS) experiments at 25 °C, was approximately 6.2 nm (Figure S4a,b), indicating that this agent forms small micelles like DDM (6.8 nm). The micelle sizes of both PPM and DDM were similar over a detergent concentration range of 0.3 to 2.0 wt% (Figure S4c). When micelle size was measured with increasing temperature, size variation was less for PPM than DDM, indicating enhanced thermal stability of the PPM micelles. Over the course of the temperature increase from 15 °C to 65 °C, PPM micelles only decreased in micelle size by ~10% while DDM micelles decreased by ~21% (Figure S4d).
Table 1.
Molecular weights (MWs), critical micelle concentrations (CMCs) of PPM and DDM, and hydrodynamic diameters (Dh) (mean ± S.D., n = 5) of their micelles in water
| Detergent | MW[a] | CMC (μM) | CMC (wt%) | Dh (nm)[b] |
|---|---|---|---|---|
| PPM | 1533.5 | ~8 (~6)[c] | ~0.0012 (~0.009)[c] | 6.2±0.1 |
| DDM | 510.6 | ~170 | ~0.0087 | 6.8±0.1 |
Molecular weight of detergents.
Hydrodynamic diameter of micelles determined at 1.0 wt % by dynamic light scattering.
CMC obtained from using fluorescence intensity ratio (I375/I363) of the penta-phenylene unit.
The 1H NMR spectrum of PPM in CD3OD at room temperature showed the well-dispersed aromatic signals (Ha-g) in the range of δ = 7.82–7.27 ppm (Figure 1a,c). When D2O instead of CD3OD was used as an NMR solvent, serious broadenings and collapses of all NMR peaks were observed, further indicating aggregate formation of this agent in water (Figure S5). The peak resolutions were significantly improved when the sample temperature was increased from room temperature to 50 °C, probably due to the increased rotational speed of the aggregates (Figure S5). Despite this improvement in peak resolution, the aromatic peaks collapsed into two broad peaks centred at 6.81 and 6.97 ppm, respectively, at a high temperature of 60 °C. However, it was evident that these aromatic signals significantly shifted upfield when the NMR solvent was changed from CD3OD to D2O (Figure 1a,b). This contrasted with a relatively a small downfield shift of the α-anomeric proton peak (Δδ = +0.14 ppm) or no noticeable change in the chemical shifts of the other aliphatic peaks. The large upfield shifts of the aromatic signals observed here can be categorized into two proton groups with Δδ = −0.46 ~ −0.74 (Ha, Hb, Hf, and Hg,) and −0.74 ~ −0.85 ppm (Hc, Hd, and He), respectively (Table S1). This is likely due to efficient aromatic shielding, indicating the presence of strong intermolecular aromatic-aromatic interactions.[18] DDM micelles showed minor peak shifts for alkyl chain protons under the same conditions (Figure S6), indicating that the large peak shifts observed for the aromatic protons of PPM are likely caused neither by a change in solvent polarity nor by an environmental change from a hydrophilic (solvent) to hydrophobic medium (micelle interior). When the NMR spectrum of this agent was measured with increasing D2O content (0, 50, 80, 100%), a main upfield shift of the aromatic peaks occurred with water concentration variation from 50% to 80% (Figure S7), suggesting that there is a critical water concentration in this solvent system necessary for aromatic-aromatic interactions between PPM molecules. Based on these self-assembly behaviours, PPM would form small micelles with the strong aromatic-aromatic interactions of the penta-phenylene units in the interior, along with the hydrophobic interactions applicable to all hydrophobic groups. Of note, the presence of an aromatic ring in the lipophilic region does not necessarily mean the formation of aromatic-aromatic interactions between detergent hydrophobic groups. In order to support this statement, we carried out an NMR study with TPA-6 which contains a phenyl ring in the lipophilic region.[19] Few aromatic-aromatic interactions between the aromatic rings were observed for this aromatic ring-bearing amphiphile (Figure S8). Other amphiphiles such as SMA copolymers and PPC-b-M have a phenyl ring as a hydrophobic group and for these molecules there is no evidence for formation of such strong aromatic interactions. This suggests that the presence of a rigid and/or highly conjugated aromatic ring with a large surface area is essential for strong aromatic-aromatic interactions in the micellar environment.
The aromatic-aromatic interaction was further supported by intercalation of a hydrophobic aromatic dye (pyranine) into the penta-phenylene groups in PPM micelles. The aromatic dye intercalation limits the aromatic interactions among PPM molecules and results in a loosening of the penta-phenylene packing within the micelle interior (Figure 1d). Upon excitation at 300 nm, the solution containing PPM at 10 μM showed an intense emission at 365 nm. The intensity of emission was significantly reduced following pyranine dye addition (10 μM), along with the appearance of a large peak at 510 nm. This new peak corresponds to pyranine emission. Because of intercalation of the dye molecules into the penta-phenylene packing, these two aromatic units (pyranine and penta-phenylene) come in close proximity, leading to efficient Förster resonance energy transfer (FRET) from the penta-phenylene unit to pyranine.[20] With increasing dye concentration from 1 to 10 μM, the peak at 365 or 510 nm showed a steady decrease or increase in the intensity, indicating that the intercalation of the dye into the penta-phenylene packing occurs gradually rather than critically (Figure S9).
While PPM is of great interest in terms of its self-assembly architecture/behaviour, it also has potential for use in manipulation of membrane proteins. Accordingly, we evaluated this agent with a few membrane proteins. PPM was first tested with Rhodobacter (R.) capsulatus super-assembly, comprising light harvesting complex I and the reaction centre complex (LHI-RC).[21] Long-term protein stability was assessed by monitoring complex integrity via absorbance value at 875 nm (A875) over time. Due to the presence of multiple cofactors (e.g., chlorophylls and carotenoids), the intact LHI-RC complex gives rise to an intense absorption peak at this wavelength. DDM-purified LHI-RC complex was diluted into buffer solutions containing PPM or DDM to give final concentrations of CMCs+0.05 wt%. To investigate the effect of temperature on protein stability, the protein samples were incubated at 25 °C for the first 10 days and then at 35 °C for the next 10 days. The DDM-solubilized LHI-RC showed a gradual loss in its integrity over the incubation at 25 °C. The integrity loss accelerated with the elevated temperature of 35 °C, ending with an only ~5 % intact protein at day 20 (Figure 2a). In contrast, PPM was fully effective at maintaining complex integrity at 25 °C. Integrity of the complex was also maintained reasonably well even at the elevated temperature (35 °C), with ~75% protein integrity retained at the end of the test period (Figure 2a). This result clearly demonstrates that PPM is superior to DDM in stabilizing a complex known to be sensitive to denaturation.[22]
Figure 2.
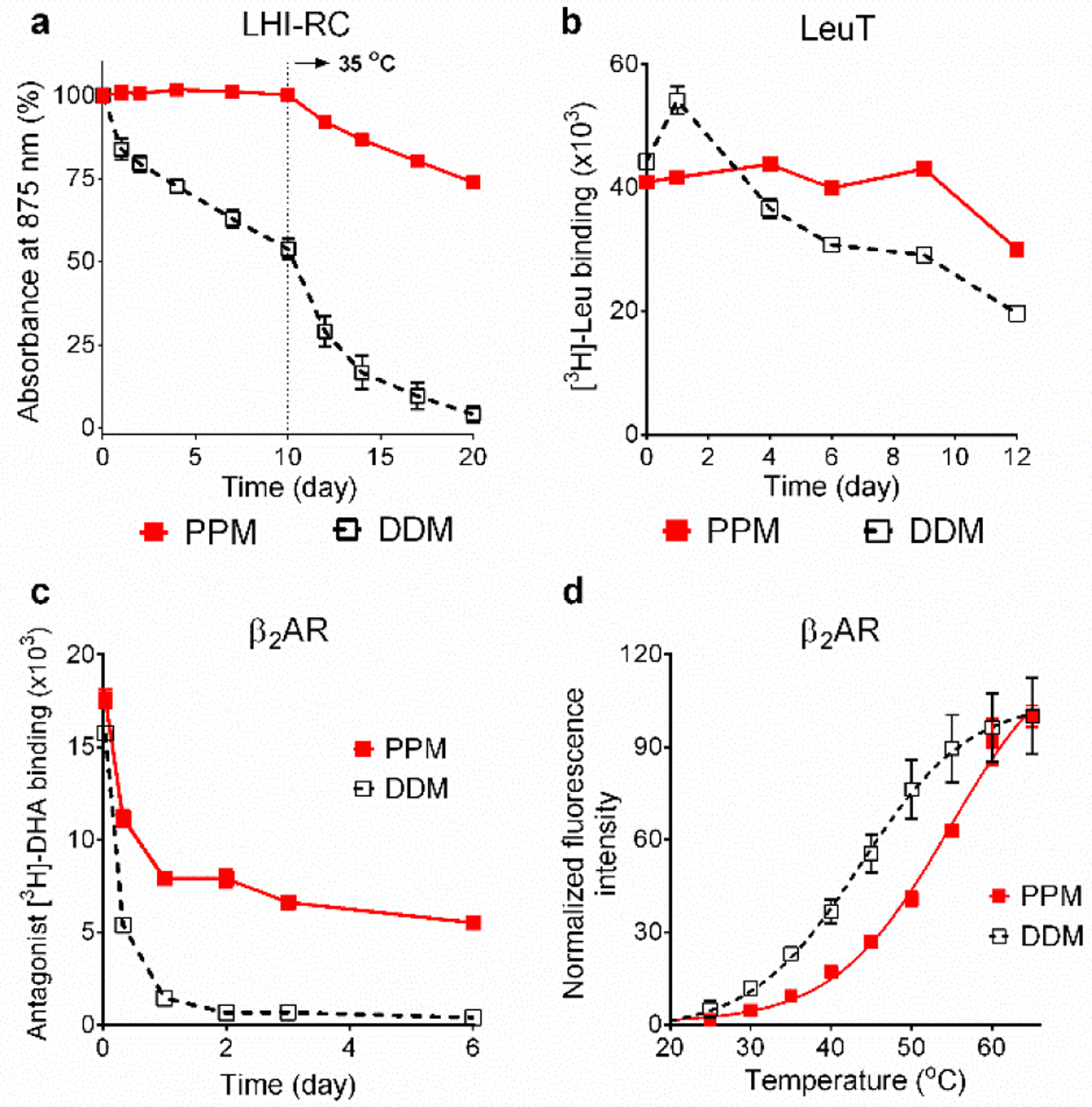
Stability of LHI-RC complex (a), LeuT (b) and β2AR (c,d) solubilized in PPM or DDM. LHI-RC stability was assessed by monitoring the absorbance of the complexes at 875 nm (A875) at regular intervals during a 20-day incubation. LeuT stability was assessed by measuring the ability of the transporter to bind the radio-labeled substrate (3[H]-leucine (Leu)) at regular intervals during a 12-day incubation at room temperature via scintillation proximity assay (SPA). β2AR stability was assessed by measuring the receptor ability to bind the radio-labelled antagonist ([3H]-dihydroalprenolol (DHA)) during a 6-day incubation at room temperature (c) or by measuring the melting temperature (Tm) of the receptor using CPM assay (d). The detergents were tested at CMC+0.05 wt% for LHI-RC, CMC+0.04 wt% for LeuT, 0.2 wt% (ligand binding assay; c) or 0.1 wt% (CPM assay; d) for β2AR. As for the LHI-RC complex study, the incubation temperature was increased to 35 °C after the first 10-day incubation at room temperature. Error bars: SEM, n = 2 (LHI-RC); n = 2–3 (LeuT); n = 3 (β2AR).
Enhanced efficacy for protein stabilization was also found when PPM was evaluated with the bacterial leucine transporter (LeuT) from Aquifex aeolicus.[23] Protein stability was assessed by monitoring the substrate binding ability of the transporter using a radiolabelled substrate ([3H]-Leucine (Leu)) via scintillation proximity assay (SPA).[24] LeuT solubilized in PPM or DDM was prepared from DDM-purified LeuT via a dilution method. The final detergent concentration was CMC+0.04 wt%. LeuT stability was measured at regular intervals during a 12-day incubation at room temperature. Following detergent dilution, PPM yielded initial activity comparable to that of DDM, and this initial activity was fully preserved through to day 9, while DDM exhibited a small increase (day 1) before a gradual loss of activity (Figure 2b), indicating that the new agent was better than DDM at long-term stabilisation of LeuT. Both detergents displayed a limited decline from day 9 to 12.
The long-term protein-stabilizing efficacy of PPM was further evaluated with a G protein-coupled receptor (GPCR), the human β2 adrenergic receptor (β2AR).[25] DDM-purified receptor was diluted into each detergent-containing buffer to give final detergent concentration of CMC+0.2 wt%. Protein stability was assessed by measuring receptor ability to bind the radio-labelled antagonist ([3H]-dihydroalprenolol (DHA))[26] at regular intervals during a 6-day incubation at room temperature. The DDM-solubilized receptor rapidly lost activity over time, resulting in less than 10% retention of the initial activity at day 2 (Figure 2c). In contrast, PPM was substantially more effective than DDM at retaining receptor activity long term, along with high initial receptor activity. To further support this result, we utilized an alternative methodology widely used for portein stability assessment, N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl] maleimide (CPM) assay.[27] This assay gives the melting temperature (Tm) of a target protein purified in detergent micelles. For this experiment, detergent exchange was first carried out using an affinity chromatography column to exclude the effect of residual DDM on receptor stability. As consistent with the result of ligand binding assay, the PPM-purified receptor gave a Tm of 11.0 °C higher than the receptor in DDM (54.7 vs 43.7 °C) (Figure 2d), revealing that PPM is clearly superior to DDM for receptor stability. This results also indicates that PPM could be useful for protein stability in the absence of the conventional detergent. Of note, as mixed detergent micelles are often used for membrane protein structural study, PPM-DDM mixed micelles used here for detergent efficacy evaluation should not be problematic. The use of a single detergent often gives protein degradation, particularly when working with challenging membrane proteins. Collectively, our results showed that PPM was effective at stabilizing the receptor in both the presence and absence of DDM.
Amphiphile efficiency for protein extraction was evaluated with the melibiose permease of Salmonella typhimurium (MelBSt).[28] Escherichia coli membranes expressing MelBSt at 10 mg/mL were treated with DDM or PPM at 1.5 wt%, incubated at three different temperatures (0, 45, and 55 °C; pH 7.4) for 90 min. The high temperatures were employed for protein extraction to investigate thermostability of detergent-extracted MelBSt. Thus, the amount of soluble MelBSt at the low temperature (0 °C) gives information of detergent efficiency for protein extraction, while MelBSt result obtained for the use of a high temperature (45 or 55 °C) provides information of detergent efficacy for protein thermo-stabilization. The amount of MelBSt extracted by PPM or DDM was estimated by SDS-PAGE and Western blot analysis. At 0 °C, PPM extracted MelBSt at much lower efficiency than DDM (Figure S10), indicating the limitation of this agent in protein extraction. When incubation temperature was increased to 45 °C, PPM efficiency was substantially enhanced (60%), but still inferior to DDM. With a further increase to 55°C, however, a reverse trend was observed; DDM yielded only ~10% soluble transporter while the new agent yielded ~30% soluble protein, suggesting that this agent is more effective than DDM in maintaining the extracted transporter in a soluble state. In order to investigate the effect of solution pH and detergent concentration on protein solubilization efficiency, the same experiment was carried out with solution pH of 8.2 and/or detergent concentration of 3.0 wt%. With the increase of PPM concentration (3.0 wt%) and/or the solution pH (8.2), we detected an increased amount of soluble MelBSt at both 45 °C (~80%) and 55°C (~40%), whereas DDM gave little change under the varied conditions. Taken together, this result suggests that PPM is clearly less efficient than DDM at extracting MelBSt but is a little more effective at maintaining the extracted transporter in a soluble state.
In summary, we have prepared penta-phenylene-bearing amphiphile (PPM) with a strong tendency to self-assemble into micelles in aqueous solution. Thanks to the incorporation of strong aromatic-aromatic interactions in amphiphile micelles, the current aromatic group-bearing amphiphile (PPM) with an extended conjugation system formed micelles with enhanced stability compared to DDM only utilizing relatively weak hydrophobic interactions. The presence of the two rigid branches at both sides of the central benzene ring is likely responsible for strong aromatic-aromatic interactions. Enhanced micellar stability of PPM was supported by the very low CMC compared to that of DDM (~ 8 vs 170 μM) and little variation in micelle size with increasing temperature. The effective aromatic-aromatic interactions between the penta-phenylene groups of PPM were demonstrated by the large upfield shifts of the aromatic peaks in the solvent-varied 1H NMR study and by efficient energy transfer from the penta-phenylene unit to the aromatic dye in the fluorescence study. Introducing such a rigid and conjugated scaffold into a self-assembly system has been reported to enhance aggregate stability,[29] but with no associated application for protein research. As expected from the presence of the rigid and polar hydrophobic group (i.e., penta-phenylene), PPM showed the rather limited solubilisation efficiency for MelBSt. However, PPM conferred enhanced stability to the multiple membrane proteins (LHI-RC, LeuT, β2AR, and MelBSt) compared to DDM, a gold standard conventional detergent. It is challenging to develop a novel detergent compatible with several membrane proteins as individual membrane proteins have different characteristics. This study indicates that PPM is markedly effective at stabilizing denaturation-sensitive membrane protein complexes (e.g., LHI-RC) as well as transporters (e.g., LeuT and MelB) and GPCRs (e.g., β2AR). The remarkable effect of PPM on long-term protein stability implies the importance of aromatic-aromatic interactions in stabilizing micelles surrounding a target membrane protein and thus achieving enhanced protein stability. Thus, the current study not only introduces a new detergent tool effective for membrane protein study, but also provided a novel design concept, related to aromatic hydrophobic interactions, that will help rational design of novel amphiphiles in the future.
Supplementary Material
Scheme 1.
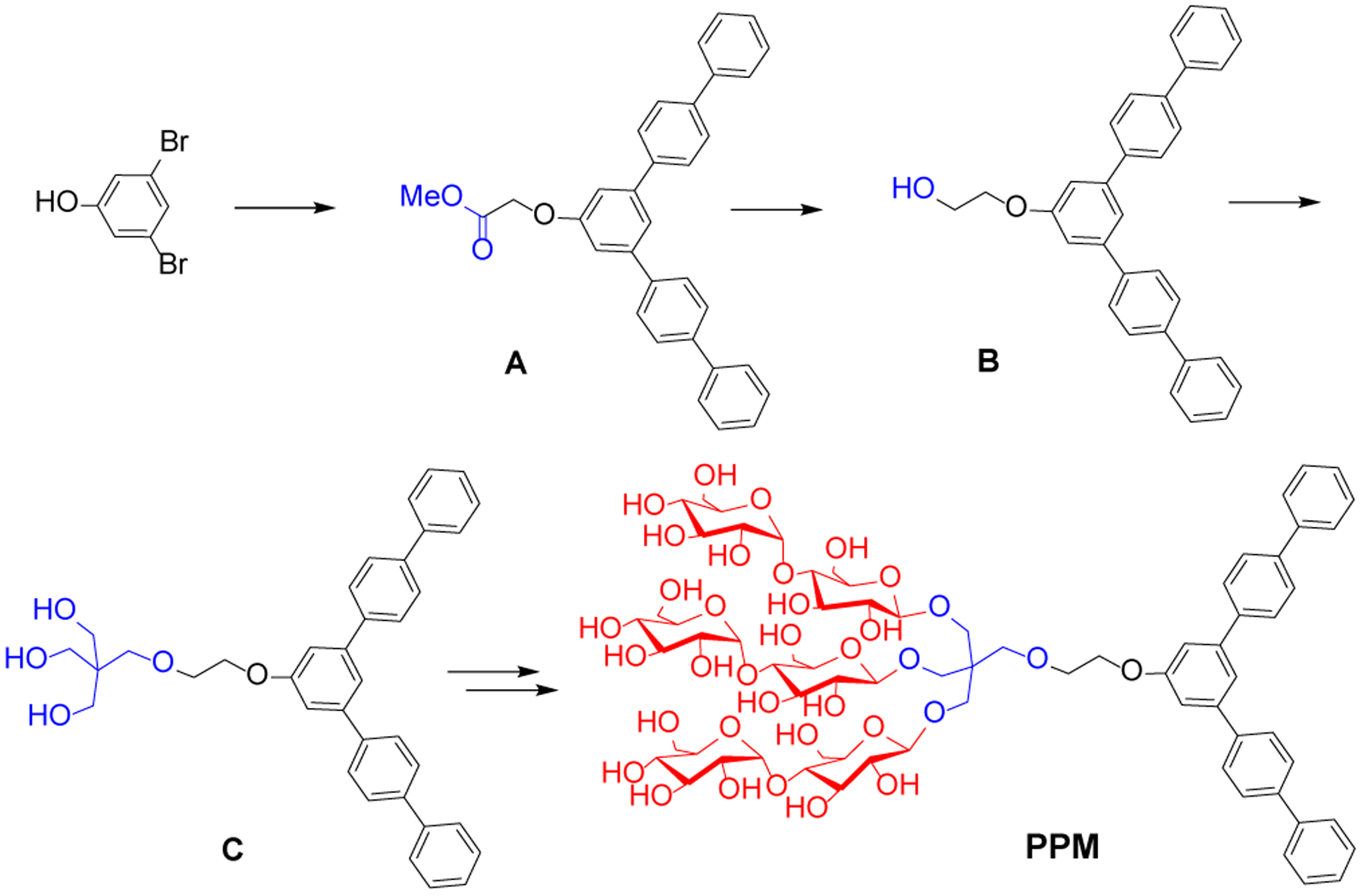
Synthetic scheme of PPM. Methyl ester-functionalized penta-phenylene derivative (A) was prepared from 1,3-dibromophenol using a boronic acid-based Suzuki coupling reaction. The resulting compound was further modified by reduction and pentaerythritol conjugation to give mono-ol and tri-ol compounds (B and C), respectively. The triol-functionalized aromatic compound (C) was used for β-selective glycosylation and global deprotection, providing penta-phenylene maltoside (PPM).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) (2016R1A2B2011257 and 2018R1A6A1A03024231 to P.S.C.) and by the National Institutes of Health (R01 GM122759 and R21 NS105863 to L.G.).
Footnotes
Experimental Section
Synthesis and characterization of novel amphiphiles, and membrane protein stability assays: Experimental details can be found in the Supporting Information.
References
- [1] a).Lehn JM, Science 2002, 295, 2400–2403; [DOI] [PubMed] [Google Scholar]; b) Antonietti M, Förster S, Adv. Mater 2003, 15, 1323–1333. [Google Scholar]
- [2] a).Palmer LC, Stupp SI, Acc. Chem. Res 2008, 41, 1674–1684; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seeman NC, Nature 2003, 421, 427–431; [DOI] [PubMed] [Google Scholar]; c) Rudick JG, Percec V, Acc. Chem. Res 2008, 41, 1641–1652; [DOI] [PubMed] [Google Scholar]; c) Azevedo HS, Velichko YS, Stupp SI, Science 2008, 319, 1812–1816. [DOI] [PubMed] [Google Scholar]
- [3] a).Columbus L, Lipfert J, Klock H, Millett I, Doniach S, Lesley SA, Protein Sci. 2006, 15, 961–975; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Eshaghi S, Hedren M, Nasser MI, Hammarberg T, Thornell A, Nordlund P, Protein Sci. 2005, 14, 676–683; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sanders CR, Sonnichsen F, Magn. Reson. Chem 2006, 44, 24–40. [DOI] [PubMed] [Google Scholar]
- [4] a).Israelachvili JN, Intermolecular and Surface Forces, 2nd ed.; Academic Press: New York, 1992; [Google Scholar]; b) Evans DF, Wennerström H, The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet, 2nd ed.; Wiley-VCH: New York, 1999. [Google Scholar]
- [5].Newstead S, Hobbs J, Jordan D, Carpenter EP, Iwata S, Mol. Membr. Biol 2008, 25, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6] a).McQuade DT, Quinn MA, Yu SM, Polans AS, Krebs MP, Gellman SH, Angew. Chem 2000, 112, 774–777; Angew. Chem. Int. Ed. 2000, 39, 758–761; [PubMed] [Google Scholar]; b) Chae PS, Wander MJ, Bowling AP, Laible PD, Gellman SH, ChemBioChem 2008, 9, 1706–1709; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chae PS, Cho KH, Wander MJ, Bae HE, Gellman SH, Labile PD, Biochim. Biophys. Acta 2014, 1838, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] a).Frotscher E, Danielczak B, Vargas C, Meister A, Durand G, Keller S, Angew. Chem. Int. Ed 2015, 54, 5069–5073; Angew. Chem. 2015, 127, 5158–5162; [DOI] [PubMed] [Google Scholar]; b) Abla M, Unger S, Keller S, Bonnete F, Ebel C, Pucci B, Breyton C, Durand G, J. Colloid Interface Sci 2015, 445, 127–136. [DOI] [PubMed] [Google Scholar]
- [8] a).Chae PS, Gotfryd K, Pacyna J, Miercke LJW, Rasmussen SGF, Robbins RA, Rana RR, Loland CJ, Kobilka B, Stroud R, Byrne B, Gether U, Gellman SH, J. Am. Chem. Soc 2010, 132, 16750–16752; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee SC, et al. , Proc. Natl. Acad. Sci. U. S. A 2013, 110, E1203–E1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, Gellman SH, Chem. Eur. J 2012, 18, 9485–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10] a).Chae PS, Rana RR, Gotfryd K, Rasmussen SGF, Kruse AC, Cho KH, Capaldi S, Carlsson E, Kobilka BK, Loland CJ, Gether U, Banerjee S, Byrne B, Lee JK, Gellman SH, Chem. Commun 2013, 49, 2287–2289; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka BK, Gellman SH, Nat. Methods 2010, 7, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11] a).Ehsan M, Du Y, Scull NJ, Tikhonova E, Tarrasch J, Mortensen JS, Loland CJ, Skiniotis G, Guan L, Byrne B, Kobilka BK, Chae PS, J. Am. Chem. Soc 2016, 138, 3789–3796; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Das M, Du Y, Ribeiro O, Hariharan P, Mortensen JS, Patra D, Skiniotis G, Loland CJ, Guan L, Kobilka BK, Byrne B, Chae PS, J. Am. Chem. Soc 2017, 139, 3072–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McGregor CL, Chen L, Pomroy NC, Hwang P, Go S, Chakrabartty A, Prive GG, Nat. Biotechnol 2003, 21, 171–176. [DOI] [PubMed] [Google Scholar]
- [13] a).Popot JL, et al. , Annu. Rev. Biophys 2011, 40, 379–408; [DOI] [PubMed] [Google Scholar]; b) Tao HC, Lee SC, Moeller A, Roy RS, Siu FY, Zimmermann J, Stevens RC, Potter CS, Carragher B, Zhang Q, Nat. Methods 2013, 10, 759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Swainsbury DJK, Scheidelaar S, van Grondelle R, Killian JA, Jones MR, Angew. Chem. Int. Ed 2014, 53, 11803–11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hovers J, Potschies M, Polidori A, Pucci B, Raynal S, Bonneté F, Serrano-Vega MJ, Tate CG, Picot D, Pierre Y, Popot J-L, Nehmé R, Bidet M, Mus-Veteau I, Bußkamp H, Jung K-H, Marx A, Timmins PA, Welte W, Mol. Membr. Biol 2011, 28, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chattopadhyay A, London E, Anal. Biochem 1984, 139, 408–412. [DOI] [PubMed] [Google Scholar]
- [17].Carnero Ruiz C, Molina-Bolivar JA, Aguiar J, Langmuir 2001, 17, 6831–6840. [Google Scholar]
- [18] a).Okazawa Y, Kondo K, Akita M, Yoshizawa M, J. Am. Chem. Soc 2015, 137, 98–101; [DOI] [PubMed] [Google Scholar]; b) Kondo K, Suzuki A, Akita M, Yoshizawa M, Angew. Chem 2013, 125, 2364–2368; Angew. Chem., Int. Ed. 2013, 52, 2308–2312. [Google Scholar]
- [19].Chae PS, Cho KH, Wander MJ, Bae HE, Gellman SH, Laible PD, Biochem. Biophys. Acta 2014,1838, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ryu J-H, Lee E, Lim Y.-b., Lee M, J. Am. Chem. Soc 2007, 129, 4808–4814. [DOI] [PubMed] [Google Scholar]
- [21].Laible PD, Kirmaier C, Udawatte SMC, Hofman SJ, Holten D, Hanson DK, Biochemistry 2003, 42, 1718–1730. [DOI] [PubMed] [Google Scholar]
- [22].Cho KH, Husri M, Amin A, Gotfryd K, Lee HJ, Go J, Kim JW, Loland CJ, Guan L, Byrne B, Chae PS, Analyst 2015, 140, 3157–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ, Swanson RV, Nature 1998, 392, 353–358. [DOI] [PubMed] [Google Scholar]
- [24].Quick M, Javitch JA, Proc. Natl. Acad. Sci. U. S. A 2007, 104, 3603–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK, Science 2007, 318, 1266–1273. [DOI] [PubMed] [Google Scholar]
- [26] a).Yao X, Parnot C, Deupi X, Ratnala VRP, Swaminath G, Farrens D, Kobilka B, Nat. Chem. Biol 2006, 2, 417–422; [DOI] [PubMed] [Google Scholar]; b) Swaminath G, Steenhuis J, Kobilka B, Lee TW, Mol. Pharmacol 2002, 61, 65–72. [DOI] [PubMed] [Google Scholar]
- [27] a).Alexandrov A, Mileni M, Chien EY, Hanson MA, Stevens RC, Structure 2008, 16, 351–359; [DOI] [PubMed] [Google Scholar]; b) Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola V-P, Chien EYT, Velasquez J, Kuhn P, Stevens RC, Structure 2008, 16, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28] a).Guan L, Nurva S, Ankeshwarapu SP, J. Biol. Chem 2011, 286, 6367–6374; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR, Guan L, Nat. Commun 2014, 5, 3009–3020; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hariharan P, Tikhonova E, Medeiros-Silva L, Jeucken A, Bogdanov MV, Dowhan W, Brouwers JF, Weingarth M, Guan L, BMC Biol 2018, 16, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoo YS, Choi JH, Song JH, Oh NK, Zin WC, Park S, Chang T, Lee M, J. Am. Chem. Soc 2004, 126, 6294–6300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


