In December 2019, a novel coronavirus, currently defined as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), was identified as the aetiological agent of a cluster of pneumonia in Wuhan, China [1]. Since this outbreak, the novel coronavirus disease (COVID-19) has spread worldwide. By 30 March 2020, COVID-19 had reached pandemic proportions, involving >110 countries and >600 000 cases [2]. Most cases of COVID-19 are self-limiting, but up to 20% of infected patients show a severe or critical disease, including severe pneumonia and multi-organ failure [3]. Systemic immune abnormalities feature in severe COVID-19. Despite peripheral blood showing a reduced lymphocyte number, there is a hyperactivation state of T cells, with an increase of Th17 and a high cytotoxic activity of CD8 [4]. Moreover, patients with severe COVID-19 show increased serum IL-6 levels and reduced number of circulating NK cells. Globally, these clinical and serological abnormalities characterize a cytokine release syndrome (CRS) [5]. During CRS, the systemic activation of immune cells causes the release of a large quantity of cytokines with the aim of limiting viral diffusion and clearing the infection. However, uncontrolled immune system activation can cause terminal organ damage, evolving towards multi-organ failure [6].
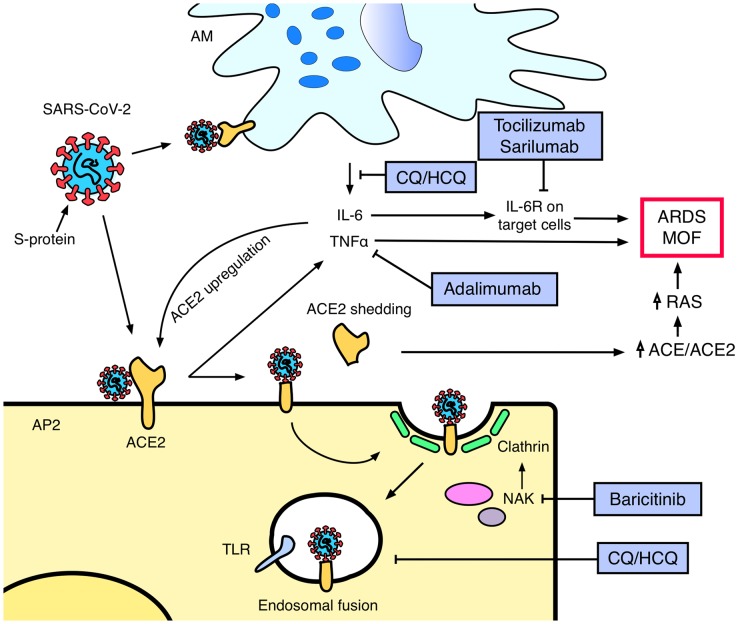
So far, there is no available specific antiviral treatment for COVID-19, and management is largely supportive. However, in light of the increasing understanding of SARS-CoV-2 biology and COVID-19 pathophysiology, several drugs commonly used in rheumatology have been proposed as potential COVID-19 treatments (Fig. 1).
Fig. 1.
Antiviral mechanisms of action of anti-rheumatic drugs in COVID-19
ACE: angiotensin-converting enzyme; AM: alveolar macrophage; AP2: alveolar pneumocyte type 2; ARDS: acute respiratory distress syndrome; CQ/HCQ: chloroquine/hydroxychloroquine; IL-6R: interleukin 6 receptor; MOF: multi-organ failure; NAK: numb-associated kinases; RAS: renin–angiotensin system; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; TLR: toll-like receptor.
Chloroquine (CQ) and hydroxychloroquine (HCQ) are antimalarial agents with immune-modulatory activities largely used in rheumatology. These agents present also a well-known antiviral activity, involving a broad spectrum of viral species [7]. The drugs act by increasing endosomal pH and inhibiting toll-like receptors, interfering with virus–cell fusion, as well as interfering with the glycosylation of angiotensin-converting enzyme 2 (ACE2), which represents the cellular receptor of the virus [8]. In vitro studies demonstrated an antiviral activity against SARS-COV-2 at concentrations achievable at the usual therapeutic doses. Moreover, the immune-modulatory activity of these agents, limiting the systemic immune activation associated to COVID-19, could act synergistically to the antiviral properties [9]. Several clinical trials conducted in China demonstrated superiority of CQ treatment with respect to placebo in improving the evolution of COVID-19 pneumonia and promoting viral clearance [10]. Accordingly, several medical agencies, including Chinese and Italian ones, included CQ and HCQ in the recommendations for treatment of COVID-19 [11, 12]. Recently, a small non-randomized trial evaluating the combination of HCQ and azithromycin in 36 SARS-CoV-2 positive subjects showed a significant efficacy of the combination in clearing the viral nasopharyngeal carriage compared with the control treatment [13]. Azithromycin activates antiviral interferon pathways in bronchial epithelial cells, suggesting an additive effect to its antimalarial action and a potential utility against viral spread [14]. Moreover, HCQ shows a higher antiviral activity compared with CQ on in vitro SARS-CoV-2 infected cells [15]. However, the small size and the non-randomized design limit the strength of the studies. Larger randomized clinical trials (RCT) investigating HCQ efficacy, with or without azithromycin, in COVID-19 patients as well as prophylactic treatment in healthcare providers have been announced in several countries, including Australia, Brazil (NCT04321278), Denmark (NCT04322396) and Spain (NCT04304053).
The development of a CRS has a pivotal role in severe COVID-19. The persistent viral stimulation leads to a significant increase of circulating cytokines such as IL-6 and TNFα, which are negatively related to the absolute lymphocyte count and can trigger inflammatory organ damage [16]. IL-6 is central in the pathogenesis of CRS associated to SARS-CoV-2 and consequently tocilizumab, a humanized anti-IL-6 receptor (IL-6R) monoclonal antibody, gained interest as a potential treatment of COVID-19. A retrospective study on 21 patients affected by severe COVID-19 showed that tocilizumab treatment improved the clinical manifestations in most of the patients [17]. Despite the fact that RCTs investigating the safety and the efficacy of tocilizumab in COVID-19 are still ongoing (ChiCTR2000029765; NCT04317092), both Chinese and Italian recommendations led to tocilizumab being introduced as an option for patients with extensive and bilateral lung disease or severely ill patients with elevated IL-6 levels [11, 12]. Similarly, sarilumab, a fully human anti-IL6R antibody, is currently under investigation in severe COVID-19 (NCT04315298).
SARS-CoV-2 shares several similarities with SARS-CoV, the coronavirus strain responsible for the 2002 SARS pandemic. Both viruses use the spike (S)-proteins to engage their cellular receptor, ACE2, for cell invasion [18]. ACE2 expression is upregulated by both SARS-CoV-2 infection and inflammatory cytokine stimulation [19]. In SARS-CoV infection, S-proteins can induce shedding of the ectodomain of ACE2, a process strictly coupled to TNFα production [20]. This loss of ACE2 activity caused by shedding has been associated to lung injury as a consequence of an increased activity of the renin–angiotensin system [21]. Although mainly demonstrated for SARS-CoV, the homology between the structures of S-proteins suggests that also SARS-CoV-2 S-proteins may show a similar mechanism [22]. The increased TNFα production could consequently both facilitate viral infection and cause organ damage. Indeed, anti-TNFα treatment has been suggested as a possible treatment option in COVID-19 [23], and a RCT investigating adalimumab in COVID-19 has recently been registered (ChiCTR2000030089).
Clathrin-dependent endocytosis is crucial for viral invasion of pneumocytes [24]. This process is promoted by members of the numb-associated kinase (NAK) family, which have been proposed as targets to limit intracellular viral traffic. Tyrosine kinase inhibitors, targeting NAK family members, showed good antiviral activity in vitro [25]. JAK inhibitors, including baricitinib, ruxolitinib and fedratinib, show the ability to inhibit NAK, limiting also systemic inflammatory response and cytokine production through the inhibition of the canonical JAK–STAT pathway [26]. Among these, baricitinib is the only JAK inhibitor to reach, at therapeutic and well-tolerated doses, plasmatic concentrations sufficient to inhibit NAK members [27]. A RCT investigating baricitinib efficacy in COVID-19 is currently ongoing (NCT04320277).
Severe COVID-19 represents the first example of an infectious disease successfully treatable with immune-modulating therapies. While the ongoing outbreak of COVID-19 requires the urgent development of a vaccine, this unexpected indication for anti-rheumatic therapies underlines the need to better understand how infectious agents trigger the immune system to produce severe clinical manifestations, especially in the case of pandemics.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Gorbalenya AE. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv 2020, doi: 10.1101/2020.02.07.937862.
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 69. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200329-sitrep-69-covid-19.pdf? sfvrsn=8d6620fa_4(29 March 2020, date last accessed).
- 3. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. (in press), doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L. et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020, doi: 10.1101/2020.02.10.20021832. [Google Scholar]
- 6. Shimabukuro-Vornhagen A, Gödel P, Subklewe M. et al. Cytokine release syndrome. J Immunother Cancer 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rolain J-M, Colson P, Raoult D.. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 2007;30:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent MJ, Bergeron E, Benjannet S. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao J, Tian Z, Yang X.. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72–3. [DOI] [PubMed] [Google Scholar]
- 11.SIMIT – Società Italiana di Malattie Infettive e Tropicali – Sezione Regione Lombardia. Vademecum per la cura delle persone con malattia da COVI-19. Edizione 2.0, 13 marzo. 2020. http://www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf.
- 12.National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (7th interim edition). Beijing, China, 2020. http://www.kankyokansen.org/uploads/uploads/files/jsipc/protocol_V7.pdf (9 April 2020, date last accessed).
- 13. Gautret P, Lagier J-C, Parola P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020. (in press), doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Schögler A, Kopf BS, Edwards MR. et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J 2015;45:428–39. [DOI] [PubMed] [Google Scholar]
- 15. Yao X, Ye F, Zhang M. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020. (in press), doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L. et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). medRxiv 2020, doi: 10.1101/2020.02.18.20024364. [DOI] [PMC free article] [PubMed]
- 17. Xu X, Han M, Li T, Sun W, Wang D, Fu B. et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv 2020, 202003.00026. [DOI] [PMC free article] [PubMed]
- 18. Hoffmann M, Kleine-Weber H, Schroeder S. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020. (in press), doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang P-H. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv 2020, doi: 10.1101/2020.02.24.963348. [DOI] [PMC free article] [PubMed]
- 20. Haga S, Yamamoto N, Nakai-Murakami C. et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci USA 2008;105:7809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai Y, Kuba K, Rao S. et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu Y, Cheng Y, Wu Y.. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin 2020, doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng X, Yu X, Pei J.. Regulation of interferon production as a potential strategy for COVID-19 treatment. arXiv 2020:200300751. [Google Scholar]
- 24. Li X, Geng M, Peng Y, Meng L, Lu S.. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020. (in press), doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pu S-Y, Xiao F, Schor S. et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res 2018;155:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richardson P, Griffin I, Tucker C. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020;395:e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stebbing J, Phelan A, Griffin I. et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020;20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]