Sir,
Cao and co-workers1 reported no added benefit of lopinavir/ritonavir in hospitalized adult patients with severe COVID-19 treated with standard of care. Beyond the challenging efficacy, the use of lopinavir/ritonavir has also raised some safety concerns. Indeed, frequent episodes of severe diarrhoea have been reported in patients with COVID-19 treated with lopinavir/ritonavir at 400/100 mg twice daily, the standard dose safely used for years in people living with HIV.2,3
Recent studies have shown that liver damage is common in patients infected by SARS-CoV-2 and is associated with the severity of disease.4,5 Accordingly, it can be hypothesized that acute liver injury may impair the activity of metabolic enzymes involved in lopinavir/ritonavir metabolism, ultimately resulting in drug overexposure and poorer drug tolerability.
To address this issue, we measured lopinavir and ritonavir trough concentrations in COVID-19 inpatients from the Department of Infectious Diseases of our hospital, one of the two Italian reference hospitals for the treatment of SARS-CoV-2 infections, and compared data with those routinely collected from HIV-infected patients on lopinavir-based maintenance ART. Blood samples for therapeutic drug monitoring (TDM) were collected just before the morning dose (corresponding to trough levels) at steady-state (from Day 3 after starting therapy); drug concentrations were measured by a validated chromatography method coupled with tandem MS, originally developed by Crommentuyn et al.6
Twenty-one patients with COVID-19 were included (7 female, 14 male; mean ± SD age of 59 ± 19 years); all were concomitantly treated with hydroxychloroquine. The majority of patients showed mild elevated levels of liver enzymes (mean ± SD aspartate aminotransferase 49 ± 38 U/L, reference values 11–34; mean ± SD ALT 55 ± 54 U/L, reference values <33 for female patients and <49 for male patients; mean ± SD lactate dehydrogenase 317 ± 82 U/L, reference values 125–220) and a trend for low serum albumin concentrations (29 ± 5 g/L, reference values 35–50). Lopinavir and ritonavir trough concentrations ranged from 5185 to 30 149 ng/mL and from 183 to 2245 ng/mL, respectively. For comparison, we looked at the TDM data collected from 22 HIV-infected patients (4 female, 18 male; mean ± SD age of 53 ± 10 years) who were on maintenance lopinavir-based ART for at least 5 years.
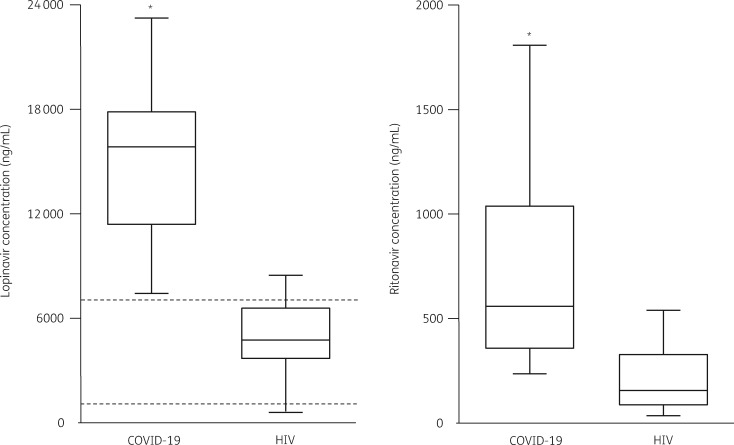
As shown in Figure 1 (left panel), the trough concentrations of lopinavir were 3-fold higher in COVID-19 patients compared with HIV patients (15 235 ± 5905 versus 4882 ± 2347 ng/mL, P < 0.0001). The same trend was also observed for ritonavir trough concentrations (Figure 1, right panel: 772 ± 563 versus 214 ± 165 ng/mL, P < 0.0001). Remarkably, all the lopinavir TDM performed in COVID-19 patients resulted in values above the therapeutic range (set in our laboratory at 1000–7000 ng/mL).7,8 Six out of the 21 COVID-19 patients reported gastrointestinal side effects.
Figure 1.
Box plot (showing 5th, 25th, 50th, 75th and 95th percentiles) of lopinavir (left side) and ritonavir (right side) trough concentrations measured in COVID-19 versus HIV-infected patients. Dashed lines represent the therapeutic window of lopinavir concentrations described in HIV-infected patients. *P < 0.0001 versus HIV-infected patients.
Our real-life TDM data analysis documents that COVID-19 patients had high lopinavir (and ritonavir) concentrations, with virtually all samples exceeding the threshold concentration of 7000 ng/mL that has been associated with the worst drug tolerability, at least in HIV-infected patients.7,8 It is worthy of note that the distribution of lopinavir and ritonavir trough concentrations between COVID-19 and HIV patients showed only a minimal overlap, clearly segregating two completely different populations.
The observed high antiviral concentrations might be explained by the well-described damaging effects of coronaviruses on liver function, including on the expression of drug-metabolizing enzymes.4 Also a potential role of drug–drug interaction between lopinavir/ritonavir and hydroxychloroquine cannot be ruled out. Indeed, it has been recently demonstrated that chloroquine and hydroxychloroquine are inhibitors of the human organic anion-transporting polypeptide 1A2 (OATP1A2), an influx transport protein involved in the entry of many substrates, including lopinavir, into liver cells.9,10 Accordingly, concomitant administration of hydroxychloroquine, by inhibiting OATP1A2, may completely block the metabolism of lopinavir, magnifying its systemic concentrations.
We believe that our findings could be interpreted in two main ways. Firstly, the failure of lopinavir/ritonavir to significantly accelerate clinical improvement, reduce mortality or diminish throat SARS-CoV-2 RNA detectability reported by Cao et al.1 cannot be ascribed to poor/inadequate drug exposure. Therefore, despite the higher drug exposure compared with HIV-infected patients, the response of COVID-19 patients to antiviral treatment resulted in limited clinical value, arguing against the use of lopinavir/ritonavir in this clinical setting. Instead, if lopinavir/ritonavir is still believed to be a valid therapeutic option for the treatment of COVID-19, an investigation is warranted for a reduced dose (i.e. reduced to 400/100 mg daily perhaps) to improve drug tolerability. This is relevant not only for the patients but also for the safety of healthcare providers: SARS-CoV-2 can be transmitted through faeces, with a theoretical increased risk of transmission during the handling of diarrhoea stools.11,12 The issue of the optimal lopinavir/ritonavir dosage may be of particular relevance now, considering that large-scale, randomized controlled clinical trials including lopinavir/ritonavir as one of the key treatment arms are actually in the planning/ongoing phases.13
Funding
This study was carried out as part of our routine work.
Transparency declarations
C.G. has received personal fees from MSD, ViiV, Gilead and Jansen Cilag, outside the submitted work. D.C. has received personal fees from MSD, ViiV and Jansen Cilag, outside the submitted work. All other authors: none to declare.
References
- 1. Cao B, Wang Y, Wen D. et al. A trial of lopinavir/ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartiromo M, Borchi B, Botta A. et al. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19). Transpl Infect Dis 2020; doi: 10.1111/tid.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young BE, Ong SWX, Kalimuddin S. et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu L, Liu J, Lu M. et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020; 40: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang C, Shi L, Wang FS.. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020; 5: 428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crommentuyn KM, Rosing H, Nan-Offeringa LG. et al. Rapid quantification of HIV protease inhibitors in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Mass Spectrom 2003; 38: 157–66. [DOI] [PubMed] [Google Scholar]
- 7. Cattaneo D, Baldelli S, Castoldi S. et al. Is it time to revise antiretrovirals dosing? A pharmacokinetic viewpoint. AIDS 2014; 28: 2477–80. [DOI] [PubMed] [Google Scholar]
- 8. Solas C, Poizot-Martin I, Drogoul MP. et al. Therapeutic drug monitoring of lopinavir/ritonavir given alone or with a non-nucleoside reverse transcriptase inhibitor. Br J Clin Pharmacol 2004; 57: 436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu C, Zhu L, Chan T. et al. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide 1A2. J Pharm Sci 2016; 105: 884–90. [DOI] [PubMed] [Google Scholar]
- 10. Hartkoorn RC, Kwan WS, Shallcross V. et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics 2010; 20: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang A, Tong ZD, Wang HL. et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis 2020; doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Y, Wang Z, Li F. et al. Public health might be endangered by possible prolonged discharge of SARS-CoV-2 in stool. J Infect 2020; 80: e18–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kupferschmidt K, Cohen J.. WHO Launches Global Megatrial of the Four Most Promising Coronavirus Treatments. Science Magazine. https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments. [Google Scholar]