Abstract
The outbreak of the coronavirus disease (COVID-19) occurred in Wuhan, China, in December 2019. As of 21 March 2020, this epidemic has spread to 179 countries with more than 200 000 confirmed cases and 8578 deaths. The outbreak has put enormous pressure on the medical establishment and even led to exhaustion of medical resources in the most affected areas. Other medical work has been significantly affected in the context of COVID-19 epidemic. In order to reduce or avoid cross-infection with COVID-19, many hospitals have taken measures to limit the number of outpatient visits and inpatients. For example, emergency surgery can only be guaranteed, and most other surgeries can be postponed. Patients with cancer are one of the groups most affected by the epidemic because of their systematic immunosuppressive state and requirement of frequent admission to hospital. Consequently, specific adjustments for their treatment need to be made to cope with this situation. Therefore, it is of significance to summarize the relevant experience of China in the prevention and control of COVID-19 infection and treatment of patients with cancer during the epidemic.
Keywords: COVID-19, SARS-CoV-2, cancer, treatment, diagnosis
We share the experience of the medical management of cancer patients from China in the situation of exhaustion of medical resources during the epidemic.
Introduction
On December 2019, several cases of pneumonia with unknown etiology occurred in Wuhan, Hubei, China (1), and the disease quickly spread to the whole country and all over the world. As of 20 March 2020, the Chinese government officially announced a total of 81 307 cases had been confirmed of COVID-19 in China, killing 3253 people. Moreover 164 556 cases had also been cumulatively reported in 173 countries outside China. The pathogen was identified as a novel coronavirus (2019 novel coronavirus); full-length genome sequencing showed that the sequence of this virus was 79.6% consistent with that of SARS (severe acute respiratory syndrome) coronavirus (2, 3). The World Health Organization (WHO) named the virus SARS-COV-2 and declared the disease (coronavirus disease 2019, COVID-19) a public health emergency of international concern (4).
As the effective reproductive number (R0) is estimated to be higher than the reported effective reproductive number of SARS at the early stage, SARS-COV-2 has higher levels of transmissibility and pandemic risk than SARS-COV (5). The latest guidelines from the National Health and Health Commission of China stated an average incubation duration of 7 days, ranging from 2 to 14 days (6). The primary patients with COVID-19 remain the main source of infection. However, asymptomatic carriers and those in the incubation period may also be infectious (7). Clusters of infected family members and medical workers confirmed the presence of human-to-human transmission by respiratory droplets, contact and fomite (3, 7). Besides, it can also transmit through aerosols when exposing to high concentrations of aerosols for a long time in a relatively closed environment (6).
In the early stage of the epidemic, the number of infected people doubles every 7.4 days (7). In addition to confirmed infections, there were hundreds of thousands of suspected patients in Wuhan, Hubei. Admission to hospital of so many patients at the same period put a lot of pressure on hospitals and caused potential cross-infection among individuals including patients and medical workers. Wang et al. showed 41.3% of confirmed cases were presumed to have been infected by hospital-associated transmission at Zhongnan Hospital affiliated to Wuhan University, including 40 health professionals (8). In addition, on 3 February 2020, the cluster epidemic at the cardiology department of Fuxing Hospital of Xicheng District of Beijing was reported to confirm a total of 36 cases infected, including 17 medical workers. Statistic data have shown a total of 3387 medical workers were infected with COVID-19, 90% of them was from Hubei (9). Hospital-associated transmission and the followed quarantine may paralyze an entire medical institution.
Therefore, the medical services were obviously affected because of the lack of doctors and enforced quarantine during the outbreak of the epidemic. Among them, patients with malignant tumors have high risk in COVID-19 epidemic due to their immunosuppression status and requirement of frequent access to hospital. The outbreak of COVID-19 makes the medical treatment of cancers difficult. For example, maintenance chemotherapy and/or immunotherapy treatment for patients with advanced cancer is often suspended. Here we share the experience of the medical management of cancer patients from China. However, clinicians should make individual clinical decisions based on the specific state of each patient and the local epidemic situation.
Threat of COVID-19 for patients with cancer
In general, the cases with cancer are usually old people with systemic immunosuppressive state caused by malignancy and anticancer therapies, who are more susceptible to viral infection compared with those without cancer. It was reported that the mortality of viral pneumonia in patients with tumors was significantly higher than that of patients without tumors, with the mortality of coronavirus pneumonia being 24.4 and 3.0% in the two groups, respectively (P < 0.01) (10). Of the 138 hospitalized patients with COVID-19 in Zhongnan Hospital, there are 10 (7.2%) patients with cancer (8). Among the 72 314 COVID-19 cases reported by the Chinese Center for Disease Control and Prevention (CDC) in mainland China as of 11 February 2020, 107 (0.5%) patients have the co-exist disease of tumor, among which 6 person died; the crude mortality rate in patients with cancer (5.6%) is higher than that of the general population (2.3%) (11). Moreover, in the study by Liang and his colleagues, the incidence of cancer in COVID-19 cases was higher than that in the overall Chinese population (1.3 vs. 0.29%). In addition, compared with cases without cancer, cases with cancer had a higher risk of severe events (39 vs. 8%) and deteriorated more rapidly (median time to severe events 13 vs. 43 days). Moreover, cases who received chemotherapy or surgery in the past month had a higher risk of severe events compared with those who did not (75 vs. 43%) (12). These data show that COVID-19 infection may pose a greater threat to patients with cancer than those without cancer.
Although the percentage of cases with cancer in the COVID-19 cohort was higher than that of the overall population, it is not sufficient to conclude that case with cancer had a higher risk of COVID-19 (13). And according to Wang et al., all the people are susceptible to COVID-19, and the existing data could not demonstrate that tumor is the susceptibility factor of COVID-19. Meanwhile, since the average age of tumor patients (63.1 years old) was significantly greater than that of non-tumor patients (48.7 years old), old age may be the factor influencing the poor prognosis of COVID-19. In addition, Wang et al. believe that the greatest risk for patients with cancer during the COVID-19 epidemic is that they cannot receive the necessary treatment due to the impact of the epidemic (14).
In conclusion, although it is unclear whether cancer directly increases the incidence and risk of COVID-19 infection, it is certain that the prevalence of COVID-19 will have a significant impact on cancer patients both in terms of infection and receiving treatment for cancer. Therefore, the admission and treatment of patients with cancer need to be adjusted according to the local epidemic situation.
Suggestions for treatment of cancers in COVID-19 epidemic (what we learn)
Lung cancer
Bronchoscopy
Lung cancer is the leading cause of worldwide cancer mortality. COVID-19 epidemic seems to make the effective diagnostic and therapeutic management for lung cancer more difficult because of the high risk of interference of COVID-19, which needs to be highly concerned by clinicians (15). Bronchoscopy remains an important diagnostic tool in patients with lung cancer. However, as an aerosol-generating procedure, it could pose a high risk of infection to proximate healthcare staff. Thus, careful preparation and precautions are necessary, such as elective bronchoscopy should be postponed, measure the temperature and ask the history of travel and COVID-19 patient contact before the procedure, and perform the RT-PCR for SARS-CoV-2 before the procedure as much as possible (16). Lung cancer is associated with high COVID-19 prevalence, morbidity and mortality due to specific conditions that represent cumulative risk factors for COVID-19 complications, including old age, severe cardiovascular and respiratory complications, smoking and related lung injury (17).
Non-small cell lung cancer
Early stage
Surgery is a definitive treatment for early stage non-small cell lung cancer (NSCLC). The delay of surgery because of COVID-19 epidemic may significantly affect the prognosis of patients with early stage lung cancer. Therefore, experts of thoracic surgery department suggest that surgery for patients with early lung cancer should be carried out as early as possible once they are completely ruled out of infection of COVID-19. If there are any suspected symptoms of COVID-19 of the patients, SARS-COV-2 nucleic acid detection should be done to rule out infection before surgery (18). For patients requiring adjuvant therapy after lung cancer surgery, studies have shown that the interval between adjuvant therapy and surgery is not related to the therapeutic effect, and even starting adjuvant chemotherapy 4 months after the surgery can still benefit from it (19). Therefore, it is recommended to appropriately extend the interval between adjuvant therapy and surgery during the outbreak of COVID-19 (19, 20). For patients with postoperative N2 lymph node metastasis of EGFR-mutated lung cancer, targeted therapy may also be one of the adjuvant treatment options. According to ADJUVANT/CTONG1104 trial, adjuvant gefitinib led to longer disease-free survival with reduced myelosuppression risk compared with that for vinorelbine plus cisplatin in patients with completely resected stage II–IIIA EGFR-mutant NSCLC. Meanwhile, the orally administered EGFR-TKI drugs could be taken by patients at home, thereby reducing the risk of cross-infection caused by repeated hospital visits (20, 21).
Advanced stage
A questionnaire survey was conducted among lung cancer experts from all over the country by the Lung Cancer Committee, Respiratory Society, Chinese Medical Association and Chinese Respiratory Tumor Cooperative Group to establish the guidelines for patients with advanced NSCLC during the epidemic (22). The guidelines suggested that (i) the infection should be treated first for patients who are infected with COVID-19 and all anticancer therapy should be discontinued for severe patients. For mild and moderate patients, continuing or suspending targeted therapy could both be considered; chemotherapy and immunotherapy is not recommended. (ii) For patients cured from COVID-19 treatment, targeted therapy is recommended to be started 2 weeks after pneumonia cure; chemotherapy, immunotherapy and intravenous anti-angiogenesis therapy are recommended to be started more than 4 weeks after pneumonia cure. (iii) For advanced NSCLC patients without COVID-19 infection, routine antitumor therapy is recommended.
It should be noted that the symptoms and imaging features of interstitial pneumonia caused by immunotherapy are quite similar to those of COVID-19 pneumonia and it’s rather important to distinguish immunotherapy-induced interstitial pneumonia from COVID-19. As COVID-19 has been widespread in the world, it is difficult to distinguish them accurately just with the epidemiological history and etiology any longer. Then, RT-PCR (real-time polymerase chain reaction) detection for SARS-COV-2 should be performed actively in these patients. Meanwhile, immunotherapy should be undertaken with caution in the special epidemic period to avoid this problem (20, 22). Instead, if the pneumonia is mild, simultaneously EGFR-TKI therapy and antiviral therapy may be safe for patients infected with COVID-19. It’s reported that a patient with NSCLC was treated with Kaletra (lopinavir/ritonavir) and osimertinib at the same time after the diagnosis of mild COVID-19 infection; 2 weeks after Kaletra treatment, pneumonia was cured and the tumor remained stable (23). However, in the randomized, controlled, open-label trial evaluating the efficacy of lopinavir/ritonavir in patients with confirmed SARS-CoV-2 infection, there is no difference in the time to clinical improvement between lopinavir/ritonavir and standard treatment [hazard ratio for clinical improvement, 1.24; 95% confidence interval (CI), 0.90–1.72] (24). The case report had suggested that the combination of lopinavir/ritonavir with osimertinib resulted in virologic clearance and stable tumor. However, because convincing data about the efficacy of this approach in patients with confirmed SARS-CoV-2 infection and NSCLC is lacking, future trials are needed to further identify the safety of targeted therapy combined with antiviral therapy.
Small cell lung cancer
Small cell lung cancer (SCLC) progresses rapidly, and the median survival time of patients with extensive stage SCLC is only 9–11 months. Delayed treatment will lead tumor progression and worse prognosis in patients with extensive stage SCLC. Therefore, for patients with extensive stage SCLC, regular chemotherapy should be carried out in local hospitals on the premise of excluding COVID-19. If the disease progresses, chemotherapy regimens can be adjusted according to the condition, and oral anlotinib is also an option in China, which has been approved by the National Medical Products Administration only in China as a third-line or beyond therapy for SCLC based on the results of the phase II clinical trial (ALTER 1202) (20, 25).
Digestive tumors
Endoscopy
During the outbreak of COVID-19, it should be careful to conduct endoscopy for medical institutions because there is a relatively high risk of cross-infection (26). This brings difficulties in the diagnosis and treatment of digestive tract tumors. Yun Zhang (27) suggested that noninvasive inspections should be preferred in the diagnosis of digestisve system cancers during the outbreak, such as by MRI and PET-CT imaging examinations and tumor markers in the laboratory and needle biopsy of metastatic lesions when necessary. For patients who must undergo endoscopic examination, COVID-19 should be completely excluded first.
Early and local advanced stages
For endoscopically resectable malignant tumors of the digestive system, due to the slow growth of early tumors, appropriate delayed resection during the epidemic period has no significant impact on the prognosis of tumors. For operable esophageal, gastric and colorectal cancers, 2–4 cycles of neoadjuvant therapy could be selected, followed by surgery after the epidemic is over. However, surgery should be performed as early as possible after assessing the risk of COVID-19 in patients with bleeding, perforation, or obstruction. It is recommended to choose a long cycle chemotherapy regimen of 3–4 weeks in neoadjuvant therapy during the epidemic period to shorten hospital exposure time and reduce the risk of infection. In the period of neoadjuvant chemoradiotherapy for esophageal cancer and rectal cancer, it is recommended to choose long-term radiotherapy to reduce the waiting time for surgery after chemoradiotherapy. For patients with early tumors of the hepatobiliary and pancreatic systems, surgery as early as possible is recommended on the premise of assessing the risk of COVID-19 due to their relatively rapid progression and poor outcome of chemotherapy (27). For colorectal cancer, laparoscopic surgery should be the first choice to reduce the risk of virus exposure. Meanwhile, in order to reduce the infection fever caused by anastomotic leakage after digestive tract reconstruction, it is recommended to perform preventive stoma for patients with risk factors of anastomotic leakage, but the risk of virus infection during the postoperative care of enterostomy should be considered during the epidemic (28).
Advanced stage
Advanced tumors of the digestive system progress relatively quickly, and systemic treatment is the main option. During the outbreak, it is recommended to choose the treatment with long interval and short stay in hospital; oral chemotherapy or targeted therapy should be preferred. In the AXEPT research for metastatic colorectal cancer, the efficacy of irinotecan combined with capecitabine was not inferior to FOLFIRI. Therefore, CapeOX and XELIRI should be preferred for patients with metastatic colorectal cancer in the context of COVID-19 epidemic (27, 29).
Breast cancer
As most breast cancer is a chronic disease, professor Xu suggested that appropriate countermeasures should be selected according to the regional epidemic severity and status of tumor disease. In the severe epidemic areas of COVID-19, stable breast cancer patients may appropriately postpone the admission for review or treatment. For breast cancer patients undergoing postoperative adjuvant chemotherapy, there was no difference between starting adjuvant chemotherapy 90 and 30 days after surgery in efficacy. Deferment of medication for 4–6 weeks will not significantly affect the efficacy for patients receiving oral endocrine drugs or maintenance therapy with trastuzumab, while, for patients requiring pharmacologic ovarian function suppression, a long-acting preparation once every 3 months can be used to reduce the number of patients’ visits. For patients with advanced breast cancer, individual treatment plan should be performed according to the patient’s situation. Endocrine therapy or oral chemotherapy drugs can be considered to replace the intravenous treatment, so as to ensure the continuity of treatment during the epidemic (30).
Hematological malignancies
As COVID-19 spreads widely in the community, the unique impact on hematology patients and the practical measures should be urgently taken into consideration to reduce their risk during ongoing treatment. Patients who are conducted outpatient hematopoietic cell transplantations (HCTs) should remain near the transplant center. While community transmission of COVID-19 has been confirmed, patients with HCT who are actively undergoing conditioning or treatment at the pre-implantation stage can be admitted early. The Worldwide Network for Blood and Marrow Transplantation (WBMT) recommends postponing non-emergency HCT in endemic or high-frequency COVID-19 infected areas (31). In the absence of vaccines or effective antiviral drugs at this stage, personal protection should be emphasized to the greatest extent, and good hygiene should be maintained for patients at home but still receiving immunosuppressive treatment. For patients who had a requirement for chemotherapy, regular chemotherapy should be carried out in local hospitals on the premise of excluding COVID-19.
Recommendations for screening COVID-19 for cancer patients upon admission
In the early stage of the epidemic, cross-infection in hospital is one of the most risk for patients and medical workers, so it is very important to screen COVID-19 patients as soon as admission of patients for cancer hospitals. Because the most common symptom of COVID-19 infection is fever, a study with 1099 COVID-19 patients showed that 88.7% had fever (32). As a consequence, our hospital has developed an admission screening process according to this symptom (Fig. 1). First, temperature tests are performed at the entrances of the hospital, the outpatient clinic and the wards. And the contact and travel histories regarding the epidemic area of all individuals are recorded. Second, for patients preparing to be admitted, mandatory routine blood tests and high-resolution computed tomography scans of the lungs are performed. COVID-19 virus nucleic acid tests and IgM/G ELISA will be carried out for patients with suspected pneumonia on computed tomography imaging. Third, the confirmed cases will be transferred to the designated hospital. Patients excluded from COVID-19 will receive antitumor treatment.
Figure 1.
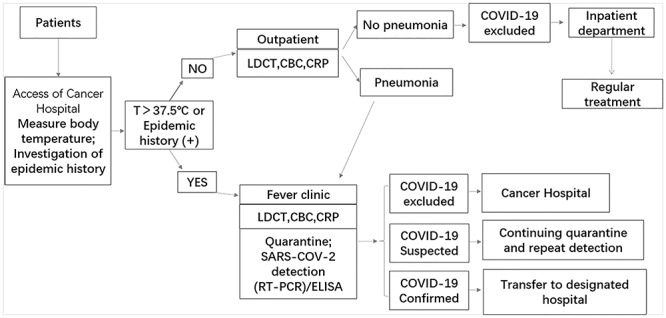
Admission screening process of COVID-19 for cancer hospitals.
The patients that met screening criteria for COVID-19 include those with influenza-like symptoms (such as fever, sore throat or cough), significant respiratory symptoms (such as, shortness of breath) and/or exposure risk to COVID-19 (33). As the asymptomatic COVID-19 infection had been widely documented in the literature (34, 35). We suggested currently that the RT-PCR test should be carried out for all patients with potential exposure history even without any respiratory symptoms.
In the screening of COVID-19 infections, it should be noticed that the detection sensitivity of SARS-COV-2 by RT-PCR was not high enough. The sensitivity for a single respiratory swab was 70% and can increase to 94% with a second test, and 98% with a third test (36). Thus, repeating testing may be important and necessary to make a diagnosis of COVID-19 (37). In the case of poor detection sensitivity of RT-PCR, the pulmonary CT findings are of great value for the early diagnosis of COVID-19. Multiple reports have revealed the significance of CT chest with a higher sensitivity in early detection of COVID-19 cases. In most cases, concordant CT scan and PCR test results were used to diagnose COVID-19 (38). In the study involved 1014 SARS-CoV-2 patients, 59% of which had positive RT-PCR, while 88% had positive CT scan. The sensitivity of chest CT imaging was 97%, which is higher than that of RT-PCR (39). Therefore, in the admission screening of COVID-19 for cancer patients, attention should be paid to the chest CT performance. However, the dose delivered to patients through CT scans is much greater than that produced by modalities including plain radiograph, which may cause serious problems in the future, including carcinogenesis and heritable issues (40).
The National Lung Screening Trial (NLST) from the USA showed low-dose computed tomography (LDCT) can reduce the mortality of lung cancer, which is a safe tool for screening (41). And international academic organizations and many medical institutions have proposed low-dose spiral CT screening and developed corresponding screening guidelines (42). The median duration of IgM antibody produced against SARS-CoV-2 was 5 days. IgM detection resulted in a higher efficiency compared with qPCR detection after 5.5 days of symptom set. The positive rate of IgM ELISA assay accompanied with qPCR is significantly increased compared with a single qPCR test, including in subclinical cases (43).
For those patients whose imaging cannot exclude COVID-19, further judgment should be made based on the patients’ epidemiological history, clinical symptoms and complete blood count (CBC) results. Suspected cases should be isolated and treated as soon as possible.
Conclusion
COVID-19 is a major global human threat that has turned into a pandemic and has specifically high morbidity in the elderly and in comorbid populations. Cancer patients may be more susceptible to infection, and tumor history is defined as an important factor of poor prognosis, which challenges both doctors and patients. However, the countermeasures of integrative cancer therapy in the epidemic of COVID-19 are not fully understood and more clinical studies are needed. During the epidemic, in addition to assessing the stage of the tumor and the status of the patient, treatment of the tumor needs to make necessary adjustments according to the local epidemic status to reduce the risk of tumor progression and COVID 19 infection (44).
Under the epidemic of COVID-19 worldwide, it is very important to balance the effective prevention of cross infection of COVID-19 and the rational arrangement of anticancer treatment. As the spread of COVID-19 in China is effectively controlled after taking strict prevention and control measures, medical work gradually returned to normal, including anticancer treatment. However, because the cases imported from outside mainland China and asymptomatic infected persons may also be infectious, the contact and travel histories regarding the epidemic area of all individual record is always needed in controlled phase. And COVID-19 virus nucleic acid tests should be carried out for patients preparing to be admitted if available. Overall, to ensure the normal treatment of patients with cancer and simultaneously avoid cross infection of COVID-19, more attention should be paid by clinicians to meet the challenges of different COVID-19 epidemic phases.
Funding
This work was supported by a project cosponsored by the Henan Province and Ministry of Health Medical Science and Technology Program (No. 201601026) and in part by the National Natural Science Foundation of China (No. 81272600), Zhongyuan 1000 Talents Plan, Natural Science Foundation of Henan Province (No. 162300410300) and the 51282 project Leading Talent of Henan Provincial Health Science and Technology Innovation Talents (No. [2016]32). It was also supported by the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 18HASTIT044). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest statement
None declared.
References
- 1. Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133(9):1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organization WH Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV), 2020. https://wwwwhoint/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)s.
- 5. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. General Office of National Health Committee OosaotCm Notice on the Issuance of a Program for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infected Pneumonia (Trial Seven Edition) [EB/OL], 2020. http://bgssatcmgovcn/zhengcewenjian/2020-03-04/13594html (3 March 2020, date last accessed).
- 7. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7]. JAMA 2020;323(11):1061–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Group W-CJIE Press Conference of WHO-China Joint Mission on COVID-19, 2020. https://newqqcom/rain/a/20200224A0QTPR00.
- 10. Kim YJ, Lee ES, Lee YS. High mortality from viral pneumonia in patients with cancer. Infect Dis 2019;51:502–9. [DOI] [PubMed] [Google Scholar]
- 11. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of sssan outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145–51.32064853 [Google Scholar]
- 12. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21(4):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21(4):e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrò L, Peters S, Soria JC, et al. Challenges in lung cancer therapy during the COVID-19 pandemic [published online ahead of print, 2020 Apr 9]. Lancet Respir Med 2020;S2213–2600(20)30170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddy PD, Nguyen SA, Deschler D. Bronchoscopy, laryngoscopy and esophagoscopy during the COVID-19 pandemic [published online ahead of print, 2020 Apr 29]. Head Neck 2020. doi: 10.1002/hed.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Passaro A, Peters S, Mok TSK, Attili I, Mitsudomi T, de Marinis F. Testing for COVID-19 in lung cancer patients. Ann Oncol 2020;S0923-7534:39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Liu M, Zhao Q, et al. Preliminary recommendations for lung surgery during 2019 novel coronavirus disease (COVID-19) epidemic period. Zhongguo Fei Ai Za Zhi 2020;23(3):133–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salazar MC, Rosen JE, Wang Z, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol 2017;3(5):610–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang L, Xu HY, Wang Y. Diagnostic and therapeutic strategies of lung cancer patients during the outbreak of 2019 novel coronavirus disease (COVID-19). Zhonghua Zhong Liu Za Zhi 2020;42:E006. [DOI] [PubMed] [Google Scholar]
- 21. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139–48. [DOI] [PubMed] [Google Scholar]
- 22. Lung Cancer Study Group CTS, Chinese Medical Association, Chinese Respiratory Oncology Collaboration Expert recommendations on the management of patients with advanced non-small cell lung cancer during epidemic of COVID-19 (trial version). Zhonghua Jie He He Hu Xi Za Zhi 2020;43:E031. [DOI] [PubMed] [Google Scholar]
- 23. Zhang H, Xie C, Huang Y. Treatment and outcome of a patient with lung cancer infected with severe acute respiratory syndrome Coronavirus-2. J Thorac Oncol 2020;15:e63–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382(19):1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know [published online ahead of print, 2020 Mar 14]. Gastrointest Endosc 2020;S0016–5107(20)30245–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Xu JM. Medical diagnosis and treatment strategies for malignant tumors of the digestive system during the outbreak of novel coronavirus pneumonia. Zhonghua Zhong Liu Za Zhi 2020;42:E005. [DOI] [PubMed] [Google Scholar]
- 28. Yu GY, Lou Z, Zhang W. Several suggestion of operation for colorectal cancer under the outbreak of corona virus disease 19 in China. Zhonghua Wei Chang Wai Ke za Zhi 2020;23:9–11. [DOI] [PubMed] [Google Scholar]
- 29. Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol 2018;19:660–71. [DOI] [PubMed] [Google Scholar]
- 30. Liu BL, Ma F, Wang JN, Fan Y, Mo HN, Xu BH. Health management of breast cancer patients outside the hospital during the outbreak of 2019 novel coronavirus disease. Zhonghua Zhong Liu Za Zhi 2020;42:E002. [DOI] [PubMed] [Google Scholar]
- 31. Coronavirus and Haematopoietic Stem Cell Transplantation Worldwide Network for Blood & Marrow Transplantation (WBMT), 2020.
- 32. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Qahtani AA. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Emergence, History, Basic and Clinical Aspects [published online ahead of print, 2020 Apr 23]. Saudi J Biol Sci 2020. doi: 10.1016/j.sjbs.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang JZ, Zhou P, Han DB, et al. Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:E055. [DOI] [PubMed] [Google Scholar]
- 35. Pang KH, Osman NI. Asymptomatic COVID-19 infection in a patient evaluated for ureteric colic: radiological findings and impact on management. Urology 2020;S0090-4295:30440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online ahead of print, 2020 Feb 19]. Radiology 2020;200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al-Tawfiq JA, Memish ZA. Diagnosis of SARS-CoV-2 infection based on CT scan vs. RT-PCR: reflecting on experience from MERS-CoV [published online ahead of print, 2020 Mar 5]. J Hosp Infect 2020. doi: 10.1016/j.jhin.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing [published online ahead of print, 2020 Feb 12]. Radiology 2020;200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [published online ahead of print, 2020 Feb 26]. Radiology 2020;200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masjedi H, Omidi R, Zamani H, Perota G, Zare MH. Radiation dose and risk of exposure-induced death associated with common computed tomography procedures in Yazd Province. Eur J Radiol 2020;126:108932. [DOI] [PubMed] [Google Scholar]
- 41. National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin 2015;65:30–54. [DOI] [PubMed] [Google Scholar]
- 43. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online ahead of print, 2020 Mar 21]. Clin Infect Dis 2020;ciaa310. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary Care Hospital in Wuhan, China [published online ahead of print, 2020 Mar 25]. JAMA Oncol 2020;e200980. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]


