The COVID-19 pandemic has already caused over 400 000 infections and led to over 17 000 deaths worldwide, dramatically disrupted daily life, and is placing extreme demands on healthcare systems [1–3]. Understanding how COVID-19 may impact people with rheumatic diseases is imperative for rheumatology health professionals and people living with rheumatic diseases for three key reasons. First, people with rheumatic disease are often treated with immunomodulating or suppressing medication, which may influence the risk of contracting COVID-19 and its severity [4]. Second, there are reports that medications commonly used to treat rheumatic disease—such as anti-IL-6 agents, anti-IL-1 agents, antimalarials and JAK inhibitors—may have efficacy in treating COVID-19 [5]. Third, there is concern that diverting these medications to prevent or treat COVID-19 infections may stress supply chains and disrupt the availability of these medications for people who depend on them to control serious inflammatory or autoimmune conditions [6, 7]. Traditional approaches to data gathering and evidence synthesis are inefficient in the context of a rapidly evolving pandemic. Here, we describe the rapid mobilization of the international rheumatology community via web-based communication platforms to address key knowledge gaps relevant to the people with rheumatic diseases in the context of the COVID-19 pandemic.
When reports of the international spread of COVID-19 were shared on Twitter, rheumatologists, researchers and people living with rheumatic diseases quickly recognized a need for information about the risk and severity of infection for people with rheumatic diseases, as well as for the potential role for immunosuppressive drugs as treatment for complications of COVID-19 infection. On 11 March 2020, a call went out on Twitter to establish an online registry similar to SECURE-IBD, which is a registry to monitor and report on outcomes for people with inflammatory bowel disease who are infected by COVID-19 [8, 9]. As interest in addressing these knowledge gaps grew, rheumatologists moved the conversation from Twitter to Slack, a web-based instant-messaging platform that allows users to collaborate in real-time by sharing ideas, questions, articles and other resources. In addition to Slack, participants engaged with one another through Zoom, a remote video conferencing service. A priority throughout this process has been inclusivity, welcoming all potential participants with an interest in joining this international effort. Importantly, representatives from different types of practice settings (community and academic), research fields (basic science, translational and clinical research) and patient groups have brought a variety of perspectives to the collaboration. Within the first week, over 250 members joined and participated in the Slack channel, generating over 4000 messages, and almost 100 documents. Over 100 organizations also pledged their support for the effort. Social media has enabled the rheumatology community to exchange ideas in real time, rapidly mobilizing efforts to address the knowledge gaps for people with rheumatic disease in the COVID-19 pandemic.
From these beginnings, the COVID-19 Global Rheumatology Alliance (GRA) took shape as a grassroots organization with activities and priorities identified and led by its members. The collaborators quickly identified several potential avenues of work and investigation, including: (i) development of a physician-reported registry of people with rheumatic disease and COVID-19 infections; (ii) collaborating with patient-facing organizations to develop standardized patient-reported surveys to collect data about the patient experience during the COVID-19 pandemic; (iii) working with insurance payers to query large administrative/claims databases for COVID-19 infections in relevant populations; (iv) addressing knowledge gaps relevant to people with rheumatic disease via evidence synthesis; and (v) the dissemination of resources to patients and health professionals.
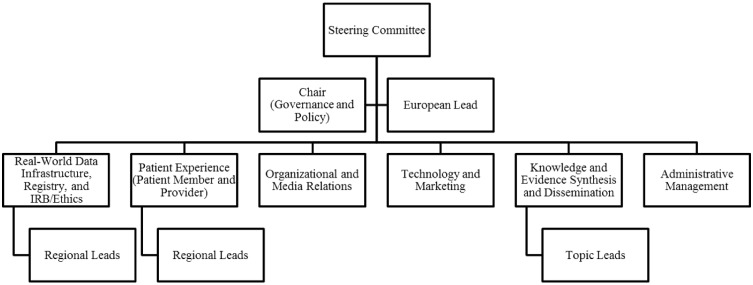
With participation growing exponentially over a few short days, a steering committee was formed. The GRA Steering Committee was assembled with representatives from each of the workgroups (Fig. 1) to champion those efforts, as well as to serve as a leadership body that could establish policies (e.g. authorship, conflicts of interest), develop a website (https://rheum-covid.org), organize volunteers, and serve as liaisons to collaborating groups, which include a growing list of organizations such as the American College of Rheumatology, the European League Against Rheumatology, patient groups and organizations (e.g. universities and industry) offering to provide organizational, administrative or financial support for the effort. The fundamental principle guiding GRA Steering Committee decision-making is to include all interested parties—patients, rheumatology health professionals and patient groups—in a process that is transparent and results in an open platform for data collection and sharing.
Fig. 1.
Current organization flow chart of the COVID-19 Global Rheumatology Alliance
In less than two weeks, the COVID-19 Global Rheumatology Alliance has evolved from a conversation on Twitter into an international collaboration of rheumatology providers and other related specialists, researchers and people with rheumatic disease. The global nature of the COVID-19 pandemic necessitates that the response is supported by scientific contributions from providers, researchers and patients from all nations. The products of these collaborations and contributions will answer fundamental questions relevant to our patients and inform the care of people with rheumatic disease during the evolving COVID-19 pandemic. The rapid mobilization of efforts by leveraging online communications has enabled international co-operation at a time when our usual way of interacting and collaborating has been severely curtailed. These are uncertain times and it is unclear what we might face. The COVID-19 Global Rheumatology Alliance is one way that the rheumatology community is uniting in efforts to gain and disseminate knowledge that might help us in supporting our patients and communities through this unprecedented pandemic.
Acknowledgements
This work was carried out behalf of the COVID-19 Global Rheumatology Alliance. At this time, the COVID-19 Global Rheumatology Alliance does not have a complete list of membership and affiliations, as membership is still being defined. The list will be updated in due course. The views expressed represent those of the author(s), and do not necessarily represent the views of the American College of Rheumatology.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflict of interest.
Global Rheumatology Alliance: rheum-covid.orgrheum.covid@gmail.com, @rheum_covid
References
- 1. Fauci AS, Lane HC, Redfield RR.. Covid-19—navigating the uncharted. N Engl J Med 2020. Feb 28;382:1268–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emanuel EJ, Persad G, Upshur R. et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; doi: 10.1056/NEJMsb2005114. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3. Dong H, Du H, Gardner L.. An Interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; doi: 10.1016/S1473-3099(20)30120-1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao X, Ye F, Zhang M. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; doi: 10.1093/cid/ciaa237. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta P, McAuley DF, Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; doi: 10.1016/S0140-6736(20)30628-0. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas K, Grady D. Trump’s embrace of unproven drugs to treat coronavirus defies science. The New York Times2020; https://www.nytimes.com/2020/03/20/health/coronavirus-chloroquine-trump.html.
- 7.ACR Announcement. Coronavirus Disease (COVID-19). American College of Rheumatology 2020, https://www.rheumatology.org/Announcements.
- 8. Calabrese L. “Totally smart thing to do- Are we doing this in RHEUM? I am unaware @CCalabreseDO @AWIRGROUP @RheumJC @DrBhana @psufka @jasonsknight @DanielHSolomon @BharatKumarMD @RheumNow @ACRheum @eular_org @philipcrobinson @ThrouNeedlesEye @MdWarrington @DrDavidKarp @RheumatologyUK https://t.co/Xit1qspfO0” Date, Time. Tweet.
- 9.SECURE-IBD Registry. 2020. https://covidibd.org/.