Abstract
The recent SARS-CoV-2 outbreak has placed immense pressure on supply chains, including shortages in nasopharyngeal (NP) swabs. Here, we report our experience of using 3D-printing to rapidly develop and deploy custom-made NP swabs to address supply shortages at our healthcare institution.
Keywords: COVID, NP swab, 3D-printing, linical pathology, molecular diagnostics, irology
The recent SARS-CoV-2 pandemic has placed immense strain on global supply chains, including those that produce vital testing equipment and reagents. In an effort to combat these shortfalls, additive manufacturing (eg 3D-printing) is an attractive solution to quickly take raw materials and form them into a functional, useful product. We have designed and developed a nasopharyngeal swab that can be rapidly produced using a filament-based 3D-printer.
In 3D-printing, there are two major printer types: filament-based and resin-based. Filament printers are analogous to a hot-glue gun mounted on XYZ-axes. A software program called a slicer takes a three-dimensional file and develops a series of instructions for the printer that trace the path of the printhead in order to systematically build an object, layer by layer, over time. A resin-based printer uses a vat of UV-reactive monomers (resin) coupled with a liquid crystal display (LCD) to block/transmit UV light. Again, slicer software provides the printer with instructions about what pattern to display on the LCD, the resin is exposed to UV, and then polymerizes to form a layer.
For our swabs, a filament-based printer was chosen over a resin-based printer for a number of reasons: filament printers are readily available and are relatively inexpensive (<$800 USD), the plastics used are well characterized and cost effective (<$30 USD per kg). The actual printing process with filament can be conducted in such a way that the “grain” of the print is parallel along the long-axis of a swab, thereby increasing the tensile strength of the swab to reduce the chance of breaking during a procedure. In examples of resin-printed swabs seen by the authors, all were printed end-to-end, growing perpendicular to the long-axis of the swab—this structure is much more prone to breaking as the “seams” between layers may act as weak points that may shear.
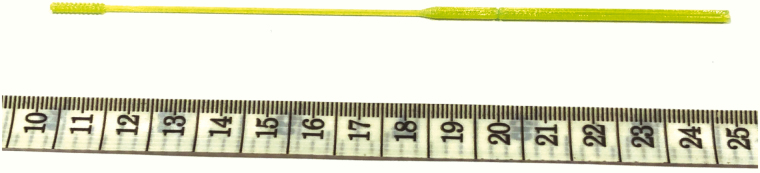
With our printer type in mind, three-dimensional drafting software (Rhinoceros 5.5.5) was used to design a swab 15 cm in length, with a handle 6.5 cm × 2 mm, and a long thin shaft 1 mm in diameter. A “brush” is located over the distal 1 cm of the shaft, consisting of a series of 0.5 mm thick disks spaced every 1mm apart. Additionally, a “score” was incorporated 5 cm up the shaft of the handle to allow the swab to be easily broken to fit into conical bottom tubes containing viral transport medium. The outputted stereolithography (STL) file (available at: www.unemed.com, NIN No.: 20081) was then loaded into PrusaSlicer 2.3.0 software to prepare for printing on a Prusa MK3S printer (www.prusa3d.com). Swabs were printed using polyethylene terephthalate glycol (PETG) filament, a food-grade plastic that is relatively inexpensive and widely available. Further, PETG is a thermoplastic polyester that is durable, chemically inert and well suited for structural applications. A 0.15 mm layer height was used, and default print settings for Prusament PETG were selected (nozzle temperature: 250°C, bed temperature: 90°C). The print time for one swab is approximately 5 minutes (Image 1); for larger-scale production, 50 swabs can be printed at once, with a total print time of 3 hours, 40 minutes. Each swab is approximately 600 mg in weight. Swabs were sterilized via the STERRAD process (Advanced Sterilization Products, Irvine, CA), which uses 58% hydrogen peroxide to coat the surface areas under a vacuum at a temperature range of 54–63°C. After exposure, the hydrogen peroxide is pumped from the chamber, with the entire process lasting approximately 50 minutes. No deterioration of the plastic was noted, and swabs were available for clinical use one day after submission to the sterile core facility.
Image 1.
Photograph of the described 3D-printed nasopharyngeal swab.
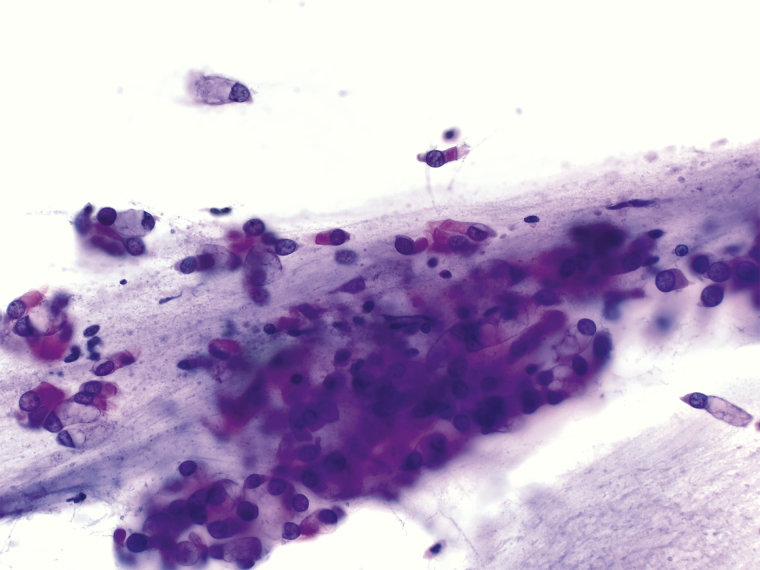
We confirmed the effectiveness of the swabs by performing collection from the nasopharynx from two individuals. Swabs were placed into viral transport medium, and nucleic acids were extracted using our laboratory’s standard operating procedure (Roche MagNA Pure System, Basel, Switzerland). Expression of human RNAse-P was interrogated using our laboratory developed real-time reverse transcription polymerase chain reaction COVID-19 assay, with the RNAse-P serving as our loading control. In this assay, the reaction is run for 45 cycles, with an adequate sample demonstrating logarithmic growth of the RNAse-P transcript by 30 cycles. As expected, RNAse-P was readily detected (26.5 and 27.0 cycles) within the adequate range. An unused swab was also tested, and no RNAse-P was detected. We also performed a smear of collected cells to examine them cytologically (Image 2). We readily observed ciliated respiratory epithelial cells and goblet cells.
Image 2.
Photomicrograph (40X magnification) of air-dried, H&E stained cells collected using the described swab.
Swabs were deployed within our institution and used in a trial run to ensure performance. Twenty-four inpatients were swabbed with either the 3D-printed swab or a commercial swab. No significance difference (P ≅ .35) in the RNAse-P loading control detection cycle (Ct) was noted between the 3D-printed swabs (n = 12, average: 24.91 Ct, standard deviation: 2.08 Ct) and commercial swabs used (n = 12, average: 24.01 Ct, standard deviation: 2.47 Ct). 3D-printed swabs and commercial swabs were collected with similar timing and conditions and were analyzed on the same assay run. Two patients were identified as positive for SARS-CoV-2 using the 3D-printed swabs, and 2 patients were identified as positive using the commercial swabs. Following use, swabs are discarded in the general biohazard waste, per College of American Pathologists sample retention guidelines.
At of the time of this submission, 5500 swabs have been prepared and delivered over a 20-day span, with swabs being integrated into testing kits as needed alongside commercial swabs. Further, the cost of the 3D-printed swabs are comparable to commercial options available at the time of writing.
In conclusion, we were able to rapidly design, iterate and deploy an additive manufacturing process to safely address a shortfall in our testing supply chain, and believe this may be of use to others.