Abstract
Background
Chest computed tomography (CT) has been widely used to assess pulmonary involvement in COVID-19. We aimed to investigate the correlation between chest CT and clinical features in COVID-19 suspected patients with or without fever.
Methods
We retrospectively enrolled 211 COVID-19 suspected patients who underwent both chest CT and reverse transcription polymerase chain reaction in Wuhan, China. The performance of CT in patients with relevant onset of symptoms, with fever (n = 141) and without fever (n = 70), was assessed respectively.
Results
The sensitivity of CT for COVID-19 was 97.3%, with area under the curve (AUC) of 0.71 (95% confidence interval [CI], 0.66–0.76). There were 141 suspected patients with fever and 70 without fever. In the fever group, 4 variables were screened to establish the basic model: age, monocyte, red blood cell, and hypertension. The AUC of the basic model was 0.72 (95% CI, 0.63–0.81), while the AUC of the CT-aided model was 0.77 (95% CI, 0.68–0.85), a significant difference (P < .05). In the nonfever group, only dry cough was screened out to establish the basic model. The AUC was 0.76 (95% CI, 0.64–0.88), which was not significantly different than the CT-aided model (P = .08).
Conclusions
Chest CT has a high sensitivity in patients with COVID-19, and it can improve diagnostic accuracy for COVID-19 suspected patients with fever during the initial screen, whereas its value for nonfever patients remains questionable.
Keywords: COVID-19, polymerase chain reaction, reverse transcriptase ROC curve; tomography, x-ray computed
In December 2019, a cluster of patients with acute respiratory disease of unknown etiology occurred in Wuhan, Hubei Province, China, and the initial cases had a history of exposure to the Huanan seafood market [1–3]. The Chinese Center for Disease Control and Prevention (CDC) isolated a novel coronavirus from the throat swab samples of patients; it was formally named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV [4]) by the International Committee on Taxonomy of Viruses (ICTV). The RBD domain of SARS-CoV-2 Spike protein supports strong interaction with human ACE2 molecules [5], indicating the strong prospensity of the virus to infect human respiratory epithelial cells [6, 7]. The pneumonia caused by SARS-CoV-2 is named coronavirus disease 2019 (COVID-19). Most patients with COVID-19 demonstrate mild symptoms with a good prognosis, while some severe patients rapidly develop to acute respiratory distress syndrome (ARDS), acute respiratory failure, and other serious complications that eventually lead to critical outcomes [1, 3, 8]. The China Health Committee has identified COVID-19 as a Class B respiratory infectious disease according to the Law of the People’s Republic of China on the Prevention and Control of Infectious Diseases and taken measures to prevent and control the disease at the Class A level.
According to World Health Organization interim guidance [4], the respiratory tract specimen has been empirically used to diagnose COVID-19 using real-time reverse transcription polymerase chain reaction (RT-PCR) as the gold standard [9]. However, the efficiency of RT-PCR has been reduced due to varying factors in practical applications, including but not limited to the course of infection, the viral loads in body fluids, the sampling site and method, the quality or stability of the RNA isolation kit, and the requirement of specific laboratory environments, which is also time-consuming for promptly obtaining diagnostic results [10].
For patients with COVID-19, chest computed tomography (CT) has been widely practiced as a noninvasive tool for lung condition assessment [11]. Unlike RT-PCR, which requires specific laboratory environments, CT scan is more accessible to Chinese hospitals [12] and can provide timely diagnosis and monitoring of lung lesions. Based on typical CT imaging features of COVID-19, a clinical diagnosis criterion was temporarily adopted in the Chinese guidance document Diagnosis and Treatment Protocol for COVID-19 (Trial Fifth Edition) [13]. Compared with RT-PCR, Ai et al. [14] reported that the sensitivity of chest CT for COVID-19 was 97% (580/601), and the false-positive rate was 75% (308/413, of whom 48% were considered highly suspicious patients). These pilot results suggested the need for further investigation on CT’s potential role as the initial screening method for COVID-19 suspected patients.
This study analyzed the value of chest CT in COVID-19 suspected patients with or without fever, the most common onset symptom [1, 13, 15], and evaluated the diagnostic accuracy of chest CT in a cohort of 211 patients.
METHODS
Patients
This was a retrospective study approved by the Institutional Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and informed consent was waived. Consecutive COVID-19 suspected patients who underwent both chest multislice spiral CT and RT-PCR testing in our hospital (in Wuhan, Hubei Province, China) between January 29, 2020, and February 4, 2020, were retrospectively enrolled. Inclusion criteria: (1) patients with respiratory symptoms but no significant improvement in conventional anti-infective treatment; (2) clinically suspected to have COVID-19 due to contact history with COVID-19 patients (positive real-time RT-PCR assay) within 14 days before symptom onset or due to clustering onset; (3) patients pending invasive operation in need of routine inspection to exclude COVID-19. Exclusion criteria: (1) the first RT-PCR tested >3 days before or after CT scan; (2) incomplete baseline characteristics and laboratory findings. For those patients with negative first-time RT-PCR but suspicious clinical symptoms suggesting COVID-19, a second RT-PCR test was conducted within 3 days after the first, the result of which was taken as the diagnosis gold standard. For patients with only a single RT-PCR test, the test result was taken as the diagnosis gold standard. In addition to the CT imaging and RT-PCR results, laboratory characteristics and signs and symptoms were also retrospectively collected for this study.
Real-time RT-PCR Assay for Detection of SARS-CoV-2
Nasopharyngeal or oropharyngeal swab samples were collected for RT-PCR testing. Total RNA was extracted using the respiratory sample RNA isolation kit (BGI, Wuhan, China). There were 2 target genes simultaneously amplified and tested during the RT-PCR assay, including open reading frame 1ab (ORF1ab) and nucleocapsidprotein (N). Target 1 (ORF1ab): forward primer CCCTGTGGGTTTTACACTTAA; reverse primer ACGATTGTGCATCAGCTGA; and the probe 5’-FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3’. Target 2 (N): forward primer GGGGAACTTCTCCTGCTAGAAT; reverse primer CAGACATTTTGCTCTCAAGCTG; and the probe 5’-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3’. SARS-CoV-2 infection was confirmed only when both genes returned positive results, according to the recommendation of the Chinese National Institute for Viral Disease Control and Prevention [16].
CT Image Acquisition and Analyze
All the suspected patients underwent thin-slice multislice spiral CT scan in a supine position. The CT examinations were performed with a SOMATOM Definition AS+ scanner (Siemens Healthineers, Forchheim, Germany). The scan covered the level of the upper thoracic inlet to the inferior level of the costophrenic angle. The CT protocol was as follows: tube voltage, 120 kV; the tube current was regulated by an automatic exposure control system (CARE Dose 4D); detector collimation width, 64 × 0.6 mm and 64 × 0.6 mm. The images were reconstructed with a thickness of 1 mm and an interval of 1 mm. The total scanning time ranged from 5 to 6 seconds, and then images were transmitted to the workstation and picture archiving and communication systems (PACS) for multiplanar reconstruction (MPR) postprocessing. The images were reviewed independently by 2 radiologists (S.L.S. and F.H.W. with 8 and 4 years of experience in thoracic radiology, respectively) blinded to the RT-PCR results, and the CT diagnosis of viral pneumonia was made according to the main imaging manifestations proposed in recent reports on COVID-19 [17–19]: Multiple bilateral, ill-defined ground glass opacities (GGOs) or mixed consolidation with diffuse peripheral distribution are typical manifestations, besides, bilateral pulmonary consolidation can be presented in severe patients. Nonviral pneumonia on chest CT includes all other pulmonary abnormalities, and normal lungs exclude viral pneumonia. The final diagnosis was reached by consensus if there was a disagreement.
Statistical Analysis
Statistical analyses were performed using SAS software (version 9.4; SAS, Cary, NC, USA). Continuous variables were displayed as mean ± SD, and categorical variables were reported as counts. Patients were divided into fever and nonfever groups, because fever is the most common and objective onset symptom of COVID-19 [1, 2, 15]. Stepwise logistic regression with a significance level of .15 was used to select variables to establish the basic models for COVID-19 diagnosis. The variable of CT result (viral pneumonia/nonviral pneumonia) was force-included as a predictor in all regression models for the fever and nonfever groups during stepwise selection to build CT-aided models. Receiver operating characteristic (ROC) curves were used to analyze these models, and the area under curve (AUC) was calculated in order to compare the differences between the basic model and the CT-aided model. The DeLong test was used to compare the AUCs for all selected models. The sensitivity (Se) and specificity (Sp) of these models for the detection of COVID-19 were calculated. The confidence intervals (CIs) of the analysis above were obtained using the Clopper-Pearson method. All statistical tests were 2-sided probability tests. P values <.05 were considered statistically significant.
RESULTS
Patients
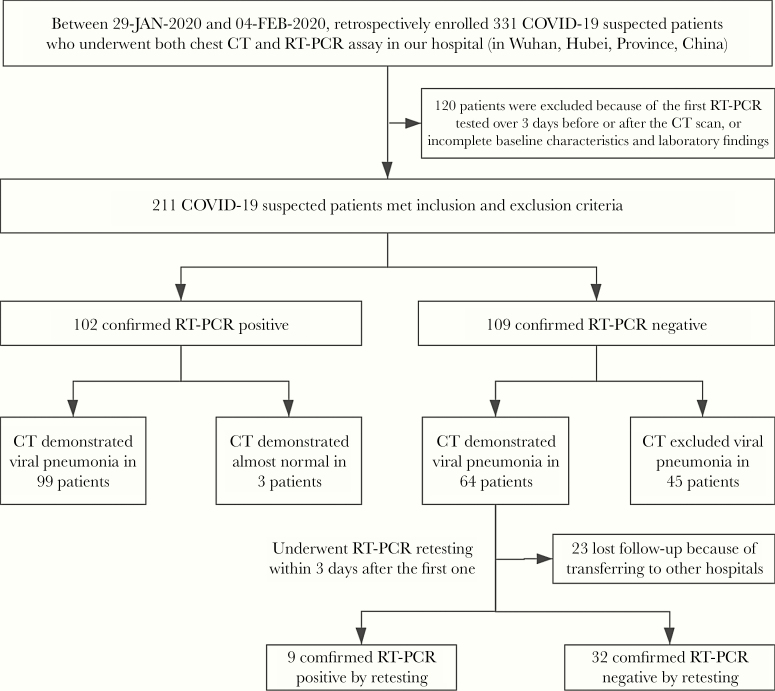
There were 331 patients initially included, of whom 211 met our inclusion and exclusion criteria. The final cohort included 119 males and 92 females with a median age (interquartile range [IQR]; range) of 51 (39–63; 17–86) years. There were 141 patients with fever as the onset symptom, 93 (66.0%) of whom had positive RT-PCR results, while 70 patients had no fever and 18 (25.7%) had positive RT-PCR results. Clinical characteristics and laboratory results of all the suspected patients are summarized in Tables 1 and 2. Figure 1 shows the flowchart of our study. Among 64 patients whose first RT-PCR assay was negative but who had CT suggesting viral pneumonia, 41 underwent repeat RT-PCR assay within 3 days, 9 of which (22.0%) turned positive. Among the remaining 23 patients, 7 patients with other comorbidities were given antiviral treatment in our hospital before RT-PCR retesting within 3 days and 16 patients were transferred to other designated hospitals for treatment and their results were unavailable. Ultimately, there were 111 patients with positive RT-PCR results, including 62 (55.86%) males and 49 (44.14%) females, with a median age (IQR; range) of 55 (44–67; 17–86) years. One hundred patients were RT-PCR negative, including 57 (57.0%) men and 43 (43.0%) women with a median age (IQR; range) of 47.5 (35–61.5; 22–84) years. There was a significant difference in age (P < .01) but no significant difference in sex (P = .89) between the 2 groups. No significant differences were noted in comorbidities between the RT-PCR-positive and RT-PCR-negative groups (P = .35), the 2 most common comorbidities being cardiovascular disease (22 [10.43%]) and hypertension (12 [5.69%]). The 2 most common symptoms at onset were fever (141 [66.82%]) and dry cough (85 [40.28%]), which showed significant differences between the RT-PCR-positive and -negative groups (all P < .01). Patients with positive RT-PCR had significantly higher mean levels of C-reactive protein (21.89 mg/L vs 9.32 mg/L; P < .01) and lower lymphocyte counts (1.05 × 109/L vs 1.21 × 109; P < .01), platelet counts (180 ×109 vs 196 ×109; P = .03), white blood cell counts (4.76 ×109 vs 5.35 ×109; P = .03), and eosinophil (0.01 ×109 vs 0.02 ×109; P < .01) than patients with negative RT-PCR. Other laboratory findings showed no significant difference between patients with positive and negative RT-PCR results.
Table 1.
Baseline Characteristics of Patients Suspected Infected With SARS-CoV-2
| Total (n = 211) | RT-PCR Positive (n = 111) | RT-PCR Negative (n = 100) | P Value | |
|---|---|---|---|---|
| Age, median (IQR), y | 51 (39–63) | 55 (44–67) | 47.5 (35–61.5) | <.01 |
| ≤50 | 101 (47.87) | 45 (40.54) | 56 (56.0) | .03 |
| >50 | 110 (52.13) | 66 (59.46) | 44 (44.0) | |
| Sex | ||||
| Male | 119 (56.40) | 62 (55.86) | 57 (57.0) | .89 |
| Female | 92 (43.60) | 49 (44.14) | 43 (43.0) | |
| Comorbidities | 58 (27.49) | 34 (30.63) | 24 (24.0) | .35 |
| Hypertension | 12 (5.69) | 5 (4.50) | 7 (7.0) | .56 |
| Cardiovascular disease | 22 (10.43) | 12 (10.81) | 10 (10.0) | 1 |
| Diabetes | 12 (5.69) | 8 (7.21) | 4 (4.0) | .38 |
| Malignancy | 7 (3.32) | 5 (4.50) | 2 (2.0) | .45 |
| COPD | 10 (4.74) | 6 (5.41) | 4 (4.0) | .75 |
| Chronic kidney disease | 5 (2.37) | 2 (1.80) | 3 (3.0) | .67 |
| Chronic liver disease | 10 (4.74) | 5 (4.50) | 5 (5.0) | 1 |
| Initial symptom | ||||
| Fever | 141 (66.82) | 93 (83.78) | 48 (48.0) | <.01 |
| Onset of fever to hospital admission, median (IQR), d | 7 (4–10) | 7 (4–10) | 7 (3–10) | .55 |
| Maximum temperature, ℃ | 38.2 (37.8–38.7) (n = 141) | 38.2 (37.8–38.7) (n = 93) | 38.2 (37.65–38.6) (n = 48) | .50 |
| Fatigue | 56 (26.54) | 37 (33.33) | 19 (19.0) | .02 |
| Dry cough | 85 (40.28) | 64 (57.66) | 21 (21.0) | <.01 |
| Runny nose | 4 (1.9) | 1 (0.90) | 3 (3.0) | .35 |
| Myalgia | 30 (14.22) | 21 (18.92) | 9 (9.0) | .05 |
| Dyspnea | 39 (18.48) | 23 (20.72) | 16 (16.0) | .48 |
| Diarrhea | 22 (10.43) | 17 (15.32) | 5 (5.0) | .02 |
| Headache | 15 (7.11) | 11 (9.91) | 4 (4.0) | .11 |
| Vomiting | 5 (2.37) | 4 (3.60) | 1 (1.0) | .37 |
Data are presented as median (IQR) or No. (%). P value for age, days from fever onset to hospital admission, and maximum temperature were calculated using a t test. P values for categorical variables were calcuated from χ 2 analyses.
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; RT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Laboratory Findings of Patients Suspected of Being Infected With SARS-CoV-2 on Admission to Hospital
| Normal Range | Total (n = 211) | RT-PCR Positive (n = 111) | RT-PCR Negative (n = 100) | P Value | |
|---|---|---|---|---|---|
| SpO2, % | 96–100 | 99 (97–100) | 99 (96–100) | 98 (97–99) | .14 |
| C-reactive protein, mg/L | <8.00 | 15.19 (4.63–50.24) | 21.89 (9.94–57.72) | 9.32 (0.67–25.605) | <.01 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 1.115 (0.82–1.49) | 1.05 (0.8–1.34) | 1.21 (0.89–1.64) | <.01 |
| Platelet count, ×109/L | 125–350 | 185 (147–235) | 180 (144–216) | 196 (155.5–256) | .03 |
| White blood cell count, ×109/L | 3.5–9.5 | 5.05 (4.04–6.24) | 4.76 (3.73–6.11) | 5.35 (4.285–6.345) | .03 |
| RBC, ×1012/L | 4.3–5.8 | 4.635 (4.23–5.06) | 4.65 (4.28–5.08) | 4.62 (4.21–5.035) | .31 |
| Hemoglobin, g/L | 130–175 | 138 (124–149) | 138 (124–153) | 138 (124.5–146) | .63 |
| Eosinophil, ×109/L | 0.02–0.52 | 0.01 (0–0.04) | 0.01 (0–0.02) | 0.02 (0–0.07) | <.01 |
| Basophil, ×109/L | <0.06 | 0.01 (0–0.01) | 0 (0–0.01) | 0.01 (0–0.01) | .21 |
| Monocyte, ×109/L | 0.1–0.6 | 0.36 (0.28–0.47) | 0.36 (0.27–0.44) | 0.36 (0.29–0.505) | .26 |
| Neutrophil, ×109/L | 1.8–6.3 | 3.51 (2.52–4.3) | 3.32 (2.39–4.26) | 3.62 (2.645–4.345) | .20 |
Abbreviations: IQR, interquartile range; RBC, red blood cell; RT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SpO2, saturation of peripheral oxygen.
aData are presented as median (IQR). P value was calculated using a t test.
Figure 1.
Flowchart for patient inclusion. Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; RT-PCR, real-time reverse transcription polymerase chain reaction.
Chest CT Results and Features in Suspected COVID-19 Patients
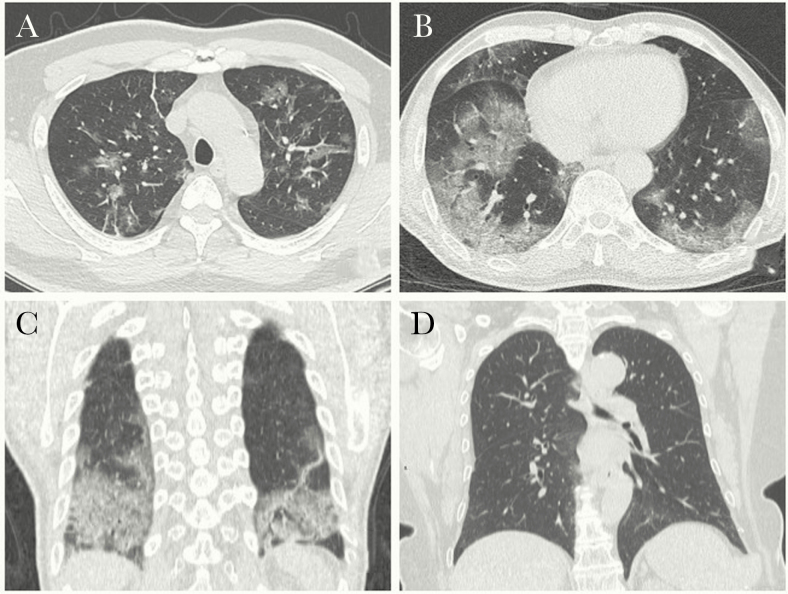
Among all suspected patients, 163 were diagnosed with viral pneumonia by chest CT, 108 (66.3%) of whom were RT-PCR positive and 55 (33.7%) of whom were RT-PCR negative. For those diagnosed with viral pneumonia by CT, the common radiographic manifestations included bilateral multiple ground glass opacities (GGOs) with ill-defined margins, GGOs with consolidation accompanied with interlobular/septal thickening, or mixed pattern with diffuse distribution (Figure 2A–C). Forty-eight patients were found with negative CT findings of viral pneumonia, but 3 (6.3%) of them had a first RT-PCR assay that was positive, all of whom were family members of confirmed COVID-19 patients (representative images demonstrated in Figure 2D).
Figure 2.
Chest computed tomography (CT) images of COVID-19 confirmed patients. A–C, Fifty-six-year-old male presenting with fever. Axial CT image (A, B) demonstrates bilateral, diffuse distribution of ground glass opacities in a lobular configuration with interlobular septal thickening; coronal reformatted CT image (C) shows bilateral, lower lung predominance ground glass opacities with consolidations. D, Forty-nine-year-old female, a family member of the reverse transcription polymerase chain reaction (RT-PCR)–positive patient, presenting with dry cough for 2 days. Her RT-PCR proved positive. The chest CT shows no obvious abnormality in the bilateral lungs.
Diagnostic Value of Chest CT in COVID-19
Of the 111 RT-PCR-positive patients, 108 were diagnosed with viral pneumonia on CT and 3 were excluded by CT. Of the 100 RT-PCR-negative patients, 55 were diagnosed with viral pneumonia by CT, and 45 were excluded by CT. The sensitivity of CT for detection of COVID-19 was 97.3% (108/111 patients), and the specificity was 45.0% (45/100 patients). The AUC was 0.71 (95% CI, 0.66–0.76). We selected 5 variables based on stepwise logistic regression to establish the basic model: age, monocyte, red blood cell (RBC), hypertension, and dry cough. The AUC of each variable was 0.58 (95% CI, 0.51–0.64), 0.52 (95% CI, 0.45–0.58), 0.54 (95% CI, 0.47–0.60), 0.50 (95% CI, 0.46–0.55), and 0.68 (95% CI, 0.62–0.74), respectively. The AUC of the basic model was 0.74 (95% CI, 0.67–0.80). The AUC of the CT-aided (CT+basic) model was 0.81 (95% CI, 0.75–0.87), which was significantly higher than the basic model and CT scan–only model (both P < .01). There were 141 patients in the fever group and 70 patients in the nonfever group. The diagnostic performance of chest CT in fever and nonfever COVID-19 suspected patients is reported in Table 3.
Table 3.
Diagnostic Performance of Basic Model Compared With CT-Aided Model in COVID-19 Suspected Patients
| Model | Sensitivity, % | Specificity, % | AUC (95% CI) | P Value |
|---|---|---|---|---|
| Overall | ||||
| Basic model | 0.74 (0.67–0.80) | <.01 | ||
| Age | 59.46 | 56.00 | 0.58 (0.51–0.64) | <.01 |
| Monocyte | 45.95 | 51.00 | 0.52 (0.45–0.58) | <.01 |
| RBC | 54.05 | 53.00 | 0.54 (0.47–0.60) | <.01 |
| Hypertension | 10.81 | 90.00 | 0.50 (0.46–0.55) | <.01 |
| Dry cough | 57.66 | 79.00 | 0.68 (0.62–0.74) | <.01 |
| CT model | 97.30 | 45.00 | 0.71 (0.66–0.76) | <.01 |
| CT-aided model | 0.81 (0.75–0.87) | |||
| Fever | ||||
| Basic model | 0.72 (0.63–0.81) | .04 | ||
| Age | 64.52 | 45.83 | 0.55 (0.47–0.64) | <.01 |
| Monocyte | 46.24 | 37.50 | 0.58 (0.50–0.67) | <.01 |
| RBC | 54.84 | 62.50 | 0.59 (0.50–0.67) | <.01 |
| Hypertension | 8.60 | 79.17 | 0.56 (0.50–0.63) | <.01 |
| CT model | 100.00 | 16.67 | 0.58 (0.53–0.64) | <.01 |
| CT-aided model | 0.77 (0.68–0.85) | |||
| Nonfever | ||||
| Basic model | .08 | |||
| Dry cough | 55.56 | 96.15 | 0.76 (0.64–0.88) | .08 |
| CT model | 83.33 | 71.15 | 0.77 (0.66–0.88) | .02 |
| CT-aided model | 0.84 (0.72–0.95) |
Abbreviations: AUC, area under curve; CI, confidence interval; CT, computed tomography; COVID-19, coronavirus disease 2019; RBC, red blood cell.
aStepwise logistic regression with a significance level of .15 was used to select variables to establish the basic models for COVID-19 diagnosis; the variable CT result (viral pneumonia/nonviral pneumonia) was force-included as a predictor in all regression models for the fever and nonfever groups during stepwise selection to build CT-aided models.
bThe DeLong test was used to compare the AUCs for all selected models.
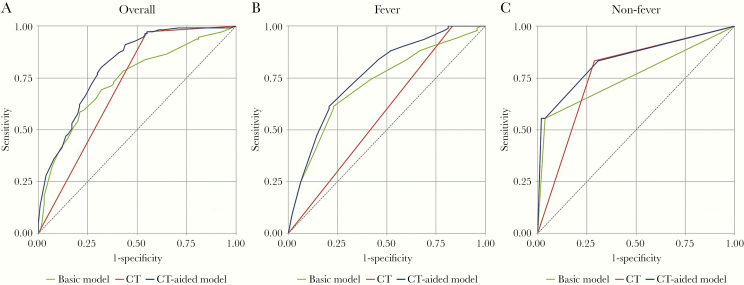
In the fever group, we selected 4 variables based on stepwise logistic regression to establish the basic model: age, monocyte, RBC, and hypertension. The AUC of each variable was 0.55 (95% CI, 0.47–0.64), 0.58 (95% CI, 0.50–0.67), 0.59 (95% CI, 0.50–0.67), and 0.56 (95% CI, 0.50–0.63), respectively. The AUC of the basic model was 0.72 (95% CI, 0.63–0.81). The AUC of CT scan alone was 0.58 (95% CI, 0.53–0.64). The AUC of the CT-aided (CT+basic) model was 0.77 (95% CI, 0.68–0.85), which was significantly higher than the basic model and CT diagnosis alone (both P < .05). In the nonfever group, we selected only dry cough as a variable to establish the basic model; other variables were dropped by the variable selection analysis. The AUC for the basic model was 0.76 (95% CI, 0.64–0.88). The AUC of CT scan alone for nonfever patients was 0.77 (95% CI, 0.66–0.88). The AUC of the CT-aided (CT+basic) model was 0.84 (95% CI, 0.72–0.95), which was significantly higher than CT alone (P < .05), but there was no significant difference when compared with the basic model (P = .08). The ROC curves were used to analyze these models in COVID-19 suspected patients with or without fever (Figure 3).
Figure 3.
The receiver operating characteristic (ROC) curve of COVID-19 suspected patients. A, The ROC curve for all 211 COVID-19 suspected patients. B, The ROC curve for COVID-19 suspected patients with fever. C, The ROC curve for COVID-19 suspected patients without fever.
Discussion
With the rapid increase in the number of patients in epidemic areas, chest CT plays an important role for assessment of lung conditions as it can provide immediate visualization of pulmonary abnormalities and monitor the development of lesions in different stages [17, 18, 20]. A few typical patterns have been identified as the CT imaging features of COVID-19 [18] that are similar to the findings in other viral pneumonias. The lack of specificity in CT manifestations requires diagnosis to be made on a multifactor basis including image demonstration, clinical symptoms, and epidemiological history. In our study, the sensitivity of CT for detection of COVID-19 was 97.3% (108/111 patients), consistent with the 97% (580/601 patients) reported by Ai et al. [14]. On the other hand, the specificity of diagnosis made with CT alone in our cohort was merely 45% (45/100 patients), with RT-PCR results taken as the gold standard, suggesting the importance of patient selection for CT screening to reduce population radiation exposure and to have the scanner resources focused on patients who can benefit from CT scans.
In our cohort, the most common onset symptom was fever, which was significantly more common in RT-PCR-positive patients than in RT-PCR-negative patients (P < .01). In the fever group, although the sensitivity of the CT scan was up to 100% (97/97), the specificity was merely 16.67% (8/48) with an AUC of 0.58. One of the likely reasons might be the presence of patients with high clinical suspicion but negative RT-PCR results. Huang et al. [21] reported a highly suspected case with typical COVID-19 CT findings, whose RT-PCR results were negative on the first 2 tests and turned positive 6 days later. In our study, there were 32 patients who tested negative for 2 consecutive RT-PCR assays within 3 days, for most of whom chest CT showed the typical COVID-19 CT manifestation, including diffuse distributive peripheral GGOs, or GGOs with interlobular or septal thickening, suggesting highly likely viral pneumonia. However, 9 (22%) of the 41 patients underwent repeat RT-PCR within 3 days, which turned positive after the initial negative results, yielding a higher false-positive rate of first-time RT-PCR than that reported by Xie et al. [22]. The false-negative RT-PCR tests could be likely attributed to the following factors: (1) the RNA isolation kit is still under rapid development and is relatively unseasoned for the COVID-19 outbreak; (2) samples from the upper respiratory throat swab could be biased by sampling site [23] and individual operation skills; (3) the virus load is below the detection sensitivity at the sampling time. The specificity calculated in our study may have underestimated the accuracy of CT diagnosis, and well-controlled follow-up studies with RT-PCR tests at multiple time points are needed to find the true specificity of CT diagnosis in suspected patients without fever.
For suspected patients with fever, the AUC of the basic model (considering age, monocyte, RBC, and hypertension) was 0.72. When the CT results were included, the AUC was significantly higher than basic model (P < .05). This observation suggests the importance of patient epidemiological history and laboratory findings in the diagnosis of COVID-19 suspected patients with fever and the added value of CT to enhance confidence in the diagnosis. On the other hand, the AUC was 0.76 for the basic model (dry cough, as screened by logistic regression study), which increased to 0.84 when CT results were taken into account, but there was no significant difference between these 2 models (P = .08), suggesting the questionable role of CT for patients without fever. Dry cough was the second most common symptom in COVID-19 suspected patients; it was also significantly more common in RT-PCR-positive patients than in RT-PCR-negative patients (P < .01). In our study, there were 3 patients with negative CT but positive RT-PCR who showed mild clinical symptoms (dry cough and fatigue) without fever for only 2 days by the time of CT scan, implying that CT may not reflect the occurrence of COVID-19 at its earliest stage [24]. Pan et al. [17] also reported on 4 of 24 COVID-19 confirmed patients who showed negative manifestations on chest CT scan at an early stage (0–4 days after onset of initial symptoms).
Our study had several limitations: (1) the sample size was small for each group (fever and nonfever); (2) lack of CT monitoring of the patients at different disease stages; (3) lack of RT-PCR follow-up after the initial 2 tests to better determine the false-negative RT-PCR test. This study regarded RT-PCR assay as the gold standard for detection of SARS-CoV-2, which leads to COVID-19 diagnosis; however, there was a mild possibility that some patients who were indeed infected by SARS-CoV-2 could not be tested by RT-PCR due to false-negative results. So the diagnostic performance of chest CT compared with RT-PCR may be not accurate. Further studies on false-negative RT-PCR results may reveal the possibility of overestimated sensitivity of chest CT and underestimated specificity.
In conclusion, chest CT has a high sensitivity when compared with the gold standard RT-PCR assay. As part of a comprehensive evaluation of epidemiological history and clinical characteristics of COVID-19 suspected patients, adding CT scan may improve the diagnostic accuracy for patients with fever in initial screening, while the value of CT may not hold for suspected patients without fever. The use of CT in initial screening of suspected patients needs to consider both its capability in detailing lesion characteristics and its benefits to a highly selected cluster of patients.
Acknowledgments
We would like to thank all the authors of the primary studies for helping us during the current study. We appreciate the medical writing support provided by Jiazheng Wang and Dandan Zheng of MSC Clinical & Technical Solutions, Philips Healthcare, Beijing, China.
Financial support. This study was supported by the National Natural Science Foundation of China (grant numbers 81873919, 81801810), the Key Special Project of Ministry of Science and Technology, China (grant number 2020YFC0845700) and the Fundamental Research Funds for the Central Universities (grant number 2020kfyXGYJ021).
Potential conflicts of interest. Songlin Song, Feihong Wu, Yiming Liu, Hongwei Jiang, Fu Xiong, Xiaopeng Guo, Hongsen Zhang, Fan Yang, and Chuansheng Zheng declare that there are no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New Engl J Med. 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected?fbclid=IwAR1_-okdyAjPo9xzd83Cikdklzv3rMtHpULhImX-K5RGL4EDxcWxShvS1uM. Accessed 11 March 2020.
- 5. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabenau HF, Kessler HH, Kortenbusch M, et al. Verification and validation of diagnostic laboratory tests in clinical virology. J Clin Virol 2007;40:93–8. [DOI] [PubMed] [Google Scholar]
- 11. Shi H, Han X, Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology. 2020; 295:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jia B, Scalzo F, Agbayani E, et al. Multimodal CT techniques for cerebrovascular and hemodynamic evaluation of ischemic stroke: occlusion, collaterals, and perfusion. Expert Rev Neurother 2016; 16:515–25. [DOI] [PubMed] [Google Scholar]
- 13. National Health Commision of the People’s Republic of China. Diagnosis and Treatment Protocol for COVID-19 in China (Trial Fifth Edition). Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml. Accessed 8 February 2020.
- 14. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020:10.1111. [DOI] [PubMed] [Google Scholar]
- 16. Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl J Med. 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020; 20:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020; 295:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New Engl J Med. 2020; 382:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng M, Lee EY, Yang J, et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothoracic Imaging. 2020; 2:e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]