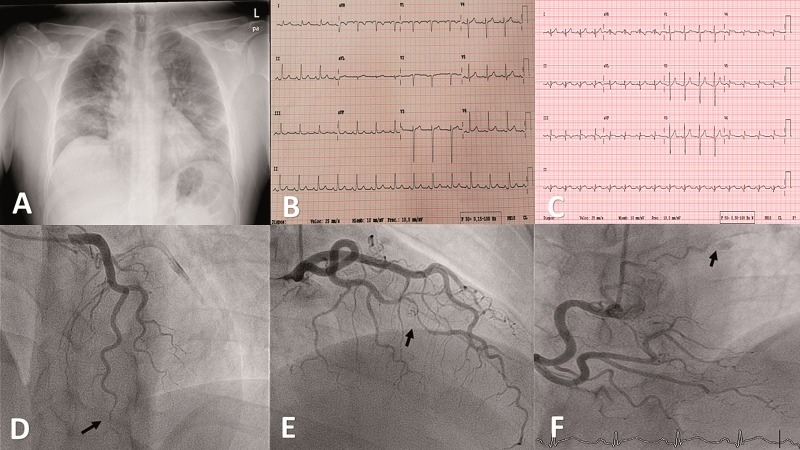
A 39-year-old-man presented to the Emergency Department after 5 days of fever, cough, myalgia, oppressive chest pain, and dyspnoea at rest. He had no cardiovascular risk factors or relevant past medical history. On admission, he had a temperature of 38.9°C, blood pressure of 127/71 mmHg, heart rate of 112 b.p.m., and oxygen saturation of 89%. Physical exam revealed decreased breath sounds at the base of the lungs. Chest X-ray showed multifocal bilateral patchy airspace opacities consistent with bilateral viral pneumonia (Panel A). Nasopharyngeal and oropharyngeal swabs confirmed SARS-CoV-2. In-hospital ECG showed normal ST-segment and repolarization (Panel B). Laboratory analysis revealed: ferritin 967 μg/L (normal 30–400), C-reactive-protein 26 mg/dL (0.10–0.50 mg/dL), D-dimer 322 ng/mL (<500), lymphocyte count 800/μL, lactate dehydrogenase (LDH) 297 U (135–225), and normal troponin. He was started with hydroxychloroquine, azithromycin, lopinavir/ritonavir, and tocilizumab. Over the course of the next 2 days, his respiratory status rapidly deteriorated, requiring intubation for hypoxaemic respiratory failure and mechanical life support. After 8 days, the patients was extubated and progressively improved. In a routine blood test 13 days after admission, a high creatinine kinase of 573 U/L (34–171) and high sensitivity troponin T of 2204 ng/L (normal <14) was seen. ECG (Panel C) showed mild ST-elevation in leads II, III, aVF, and V4–V6; appearance of a dominant R wave in V1–V4; and Q wave in leads II, III, aVF, and V5–V6. A point-of-care ultrasound showed mild left ventricular systolic dysfunction (LVEF: 50–55%); severe basal and middle inferoseptal, inferolateral, and inferior wall hypokinesis; and basal inferior and lateral hypokinesis of the right ventricle. Although the patient was asymptomatic, a coronary angiogram was performed. It did not reveal any atherosclerotic lesions; however, it did show a large and severe lumen narrowing in the first obtuse marginal branch artery bordered by normal segments compatible with type 2A in the Saw classification of spontaneous coronary artery disease (SCAD) (Panel D, arrow); moderate lumen narrowing in the distal left anterior descending artery that extended to the end of the artery compatible with type 2B SCAD (Panel E, arrow); and a mild fistula between the proximal segment of the right coronary and the pulmonary artery (Panel F, arrow) (Supplementary material online, Videos S1–S4). Regarding the typical type 2 SCAD appearance, no intravascular imaging was performed because of the potential risks of extending the dissection with coronary wires or contrast injections. Following current recommendations, as the patient was stable with normal coronary flow in the affected vessels, a conservative therapy was decided on. Autoimmune and rheumatological disease was ruled out. The final diagnosis was myocardial infarction due to multivessel acute SCAD associated with severe acute respiratory syndrome SARS-CoV-2. SCAD has been described in case reports of patients with various systemic inflammatory diseases but has been rarely described in relation to SARS-CoV-2 infection. Recently, it has been suggested that the pathogenesis of SARS-CoV-2 is mediated by disproportional immune responses and the ability of the virus to circumvent innate immunity that induces a proinflammatory cytokine generation. This could be the predisposing factor in the development of SCAD. Although the use and duration of antiplatelet therapies in SCAD are controversial, because of the myocardial infarction and the hypercoagulable state related to the infection, a longer dual antiplatelet therapy with aspirin and an ADP receptor antagonist was decided on.
Supplementary material is available at European Heart Journal online.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.