Abstract
Aims
The current pandemic coronavirus SARS-CoV-2 infects a wide age group but predominantly elderly individuals, especially men and those with cardiovascular disease. Recent reports suggest an association with use of renin–angiotensin–aldosterone system (RAAS) inhibitors. Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for coronaviruses. Higher ACE2 concentrations might lead to increased vulnerability to SARS-CoV-2 in patients on RAAS inhibitors.
Methods and results
We measured ACE2 concentrations in 1485 men and 537 women with heart failure (index cohort). Results were validated in 1123 men and 575 women (validation cohort).
The median age was 69 years for men and 75 years for women. The strongest predictor of elevated concentrations of ACE2 in both cohorts was male sex (estimate = 0.26, P < 0.001; and 0.19, P < 0.001, respectively). In the index cohort, use of ACE inhibitors, angiotensin receptor blockers (ARBs), or mineralocorticoid receptor antagonists (MRAs) was not an independent predictor of plasma ACE2. In the validation cohort, ACE inhibitor (estimate = –0.17, P = 0.002) and ARB use (estimate = –0.15, P = 0.03) were independent predictors of lower plasma ACE2, while use of an MRA (estimate = 0.11, P = 0.04) was an independent predictor of higher plasma ACE2 concentrations.
Conclusion
In two independent cohorts of patients with heart failure, plasma concentrations of ACE2 were higher in men than in women, but use of neither an ACE inhibitor nor an ARB was associated with higher plasma ACE2 concentrations. These data might explain the higher incidence and fatality rate of COVID-19 in men, but do not support previous reports suggesting that ACE inhibitors or ARBs increase the vulnerability for COVID-19 through increased plasma ACE2 concentrations.
Keywords: Men, Heart failure, Coronavirus disease (COVID-19), ACE2
See page 1818 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa414)
Introduction
The world is currently faced with the outbreak of a new severe acute respiratory syndrome coronavirus (SARS-CoV). The new virus, SARS-CoV-2, emerged in December 2019 in the city of Wuhan, China, and is the causative agent of a respiratory syndrome now known as coronavirus disease 2019 (COVID-19).1 , 2 Efforts aimed at curbing the spread of SARS-CoV-2 and finding effective treatments are ongoing.
Early epidemiological observations indicate that SARS-CoV-2 infects all age groups, but older men with chronic illnesses may be more severely affected. There is a preponderance of men (58.1%) compared with women (41.9%) testing positive for COVID-192 and, in the previous SARS-CoV epidemic in 2003, men had a higher mortality than women (21.9% vs. 13.2%; P < 0.0001)3. Whether men with the current SARS-CoV-2 virus also have a worse mortality outcome is becoming apparent as recent report indicate that 70% of patients that died of COVID-19 in Italy were men,4 and mainly elderly.
The increased vulnerability of older people with cardiovascular disease and comorbid conditions could be related to increased concentrations of angiotensin-converting enzyme 2 (ACE2),5 , 6 and ACE2 is known to be increased in heart failure.7 ACE2 is not only an enzyme but also a functional receptor on cell surfaces for both SARS-CoV and SARS-CoV-2, and is highly expressed in the heart, testis, kidneys, and lungs,8–12 and shed into the plasma. Some reports have suggested that inhibitors of the renin–angiotensin–aldosterone system (RAAS) increase plasma ACE2 concentrations,5 , 13 although these speculations are not supported by a substantial body of research.
We therefore investigated plasma concentrations of ACE2 in two large and independent cohorts of men and women with heart failure according to the use of RAAS inhibitors.
Methods
Study participants
From the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT-CHF),14 we measured ACE2 concentrations in 1485 men and 537 women with heart failure in 11 European countries. Results were validated in another, independent BIOSTAT-CHF cohort consisting of 1123 men and 575 women with heart failure enrolled in Scotland. Only participants with sufficient plasma samples were used for this research. The design and baseline characteristics of both cohorts of BIOSTAT-CHF have been published elsewhere.14 Inclusion criteria were the same in the index and validation cohorts; the only exception was that when the left ventricular ejection fraction (LVEF) was >40%, patients had to have a B-type natriuretic peptide BNP >400 ng/L or N-terminal proBNP (NT-proBNP) >2000 ng/L in the index cohort, but not in the validation cohort. The study complied with the Declaration of Helsinki and was approved by the medical ethics committees of participating centres.14
ACE2 was measured using the Olink Proseek analysis service (Olink Proteomics, Uppsala, Sweden). The Olink platform15 utilizes a high-throughput multiplex immunoassay based on a proprietary proximity extension assay (PEA) technology, where each biomarker is addressed by a matched pair of antibodies, coupled to unique, partially complementary oligonucleotides, and measured by quantitative real-time PCR. Results are expressed in the form of relative quantification (Normalized Protein eXpression or NPX) which are logarithmically related to protein concentration but cannot be converted to absolute protein concentrations. Interpretations are therefore relative and not absolute. Analytical validation of the sensitivity and specificity of the Olink assay for this study was achieved by comparing available routine laboratory measurements of two protein biomarkers, growth differentiation factor 15 (GDF-15) (pg/mL) and NT-proBNP (pg/mL), with those measured using Olink (NPX). NT-proBNP is a canonical biomarker in cardiovascular disease biology.16
Statistical analyses
All statistical analyses were performed using R17 version 3.6.2. In group comparisons, categorical variables were depicted as numbers with percentages. Normally distributed variables were depicted as means ± SD, and non-normally distributed variables as median and interquartile range (IQR) defined as the first and third quartile (Q1–Q3). The means for continuous variables were compared by one-way analysis of variance (ANOVA) or the Kruskal–Wallis test, while categorical variables were compared by the χ2 test. Multivariate models were based on backward stepwise regression. Baseline tables were made using the R-based CompareGroups18 package. In general, a two-tailed P-value of <0.05 was considered statistically significant.
Results
Clinical characteristics
Baseline characteristics of the index and validation cohort are presented in Table 1 and Supplementary material online, Table S1, respectively. In the index cohort (n = 2022), the median age was 69 years in men (IQR, 60–76), and 75 years in women (IQR 64–81; P < 0.001 between men and women). In the validation cohort (n = 1698), the median age for men was 74 years (IQR 66–81) and for women 76 years (IQR 69–82; P < 0.001 between men and women). In the index cohort, patients with higher concentrations of ACE2 were more often men, and were more likely to have atrial fibrillation, a higher heart rate, and lower systolic blood pressure (Table 1). In the validation cohort, patients with higher concentrations of ACE2 were more often men, and were more likely to have atrial fibrillation, diabetes, a higher heart rate, and a lower systolic blood pressure (Supplementary material online, Table S1). In the index cohort, only 0.3% (6/2022) of patients received both an ACE inhibitor and an angiotensin receptor blocker (ARB). In the validation cohort, only 0.4% (7/1691) received both an ACE inhibitor and an ARB.
Table 1.
Baseline characteristics according to quartiles of plasma ACE concentrations (index cohort)
| Q1 (2.78–4.80) | Q2 (4.80–5.25) | Q3 (5.25–5.76) | Q4 (5.76–8.72) | P-value | N | |
|---|---|---|---|---|---|---|
| n = 506 | n = 505 | n = 506 | n = 505 | |||
| ACE2 plasma concentrations (NPX) | 4.49 (4.25–4.65) | 5.03 (4.92–5.14) | 5.48 (5.35–5.62) | 6.17 (5.97–6.52) | 0.000 | 2022 |
| Sex: | <0.001 | 2022 | ||||
| Men | 329 (65.0%) | 353 (69.9%) | 81 (75.3%) | 422 (83.6%) | ||
| Women | 177 (35.0%) | 152 (30.1%) | 125 (24.7%) | 83 (16.4%) | ||
| Age (years) | 69.0 (61.0–77.0) | 71.0 (62.0;79.0) | 71.0 (62.0;78.0) | 70.0 (61.0;78.0) | 0.394 | 2022 |
| Body mass index (kg/m2) | 27.2 (24.5–31.1) | 27.1 (24.0;30.9) | 26.6 (23.8–29.8) | 27.2 (24.2;31.0) | 0.148 | 1990 |
| Heart rate (b.p.m.) | 74.0 (65.0–84.0) | 76.0 (68.0;90.0) | 77.0 (67.0;90.0) | 78.0 (69.0;90.0) | <0.001 | 2017 |
| Systolic blood pressure (mmHg) | 125 (110–140) | 121 (110–140) | 120 (110–136) | 120 (108–132) | <0.001 | 2018 |
| Left ventricular ejection fraction | 30.0 (25.0–38.0) | 30.0 (25.0–36.0) | 30.0 (24.0–37.0) | 30.0 (23.0–35.0) | <0.001 | 1804 |
| New York Heart Association (NYHA) class: | <0.001 | 1961 | ||||
| I | 17 (3.44%) | 11 (2.25%) | 11 (2.23%) | 3 (0.62%) | ||
| II | 216 (43.7%) | 198 (40.5%) | 164 (33.3%) | 138 (28.5%) | ||
| III | 214 (43.3%) | 230 (47.0%) | 239 (48.5%) | 278 (57.3%) | ||
| IV | 47 (9.51%) | 50 (10.2%) | 79 (16.0%) | 66 (13.6%) | ||
| History of atrial fibrillation | 196 (38.7%) | 197 (39.0%) | 242 (47.8%) | 283 (56.0%) | <0.001 | 2022 |
| Renal disease | 136 (26.9%) | 133 (26.3%) | 145 (28.7%) | 161 (31.9%) | 0.199 | 2022 |
| Diabetes | 160 (31.6%) | 169 (33.5%) | 150 (29.6%) | 166 (32.9%) | 0.574 | 2022 |
| Hypertension | 330 (65.2%) | 308 (61.0%) | 322 (63.6%) | 286 (56.6%) | 0.029 | 2022 |
| Chronic obstructive pulmonary disease | 99 (19.6%) | 92 (18.2%) | 76 (15.0%) | 79 (15.6%) | 0.178 | 2022 |
| Myocardial infarction | 184 (36.4%) | 199 (39.4%) | 173 (34.2%) | 197 (39.0%) | 0.276 | 2022 |
| Ischaemic heart failure aetiology | 257 (51.7%) | 275 (55.6%) | 260 (52.8%) | 290 (58.1%) | 0.175 | 1983 |
| Coronary artery disease | 211 (41.7%) | 232 (45.9%) | 205 (40.5%) | 245 (48.5%) | 0.037 | 2022 |
| Coronary artery by-pass graft | 73 (14.4%) | 85 (16.8%) | 73 (14.4%) | 117 (23.2%) | <0.001 | 2022 |
| Percutaneous coronary intervention | 101 (20.0%) | 110 (21.8%) | 98 (19.4%) | 112 (22.2%) | 0.632 | 2022 |
| Use of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) | 374 (73.9%) | 365 (72.3%) | 351 (69.4%) | 366 (72.5%) | 0.434 | 2022 |
| Beta-blockers | 427 (84.4%) | 423 (83.8%) | 407 (80.4%) | 423 (83.8%) | 0.325 | 2022 |
| ACE inhibitors | 304 (60.1%) | 315 (62.4%) | 310 (61.3%) | 315 (62.4%) | 0.857 | 2022 |
| ARBs | 72 (14.2%) | 59 (11.7%) | 49 (9.68%) | 56 (11.1%) | 0.150 | 2022 |
| Mineralocorticoid receptor antagonist (MRA) | 256 (50.6%) | 249 (49.3%) | 259 (51.2%) | 299 (59.2%) | 0.007 | 2022 |
| ACE inhibitors and MRA: | 0.043 | 2022 | ||||
| ACE inhibitor with MRA | 154 (30.4%) | 168 (33.3%) | 172 (34.0%) | 189 (37.4%) | ||
| ACE inhibitor without MRA | 150 (29.6%) | 147 (29.1%) | 138 (27.3%) | 126 (25.0%) | ||
| MRA without ACE inhibitor | 102 (20.2%) | 81 (16.0%) | 87 (17.2%) | 110 (21.8%) | ||
| No ACE inhibitor and no MRA | 100 (19.8%) | 109 (21.6%) | 109 (21.5%) | 80 (15.8%) |
Among patients that were not treated with RAAS inhibitors, men were predominant in the uppermost quartile of ACE2 (Supplementary material online, Table S2 and S3). ACE2 concentrations were higher in men than in women in 9 out of 11 countries but were similar by ACE inhibitor/ARB use (Supplementary material online, Figures S1 and S2).
Analytical validation of the Olink assay
In both study cohorts, routine lab concentrations of two golden standard biomarkers (GDF-15 and NT-proBNP) showed a strong correlation with those measured using Olink (Spearman’s rho 0.77–0.92, P < 0.001; Supplementary material online, Figure S3).
ACE2 concentrations in men and women according to the use of RAAS inhibitors
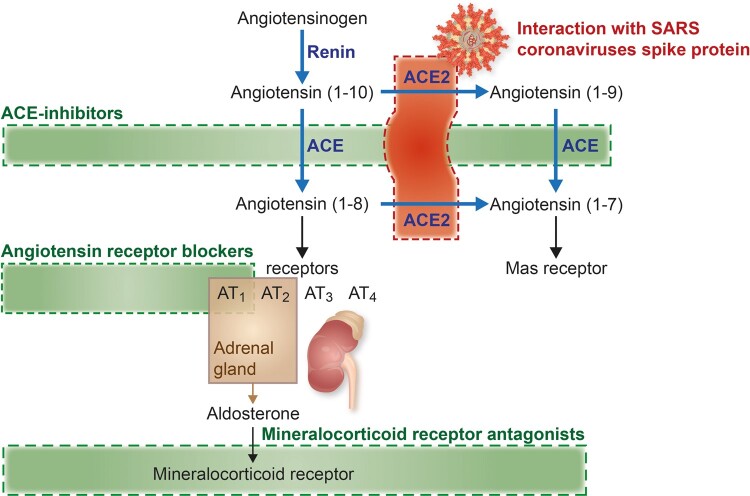
The ACE2–RAAS–COVID-19 axis is summarized in Figure 1. In both cohorts, plasma ACE2 concentrations (in NPX units) were higher in men than in women. In the index cohort, the mean plasma concentration was 5.38 in men compared with 5.09 in women (P < 0.001). In the validation cohort, the mean plasma concentration was 5.46 in men compared with 5.16 in women (P < 0.001).
Figure 1.
Summary of the RAAS pathway.
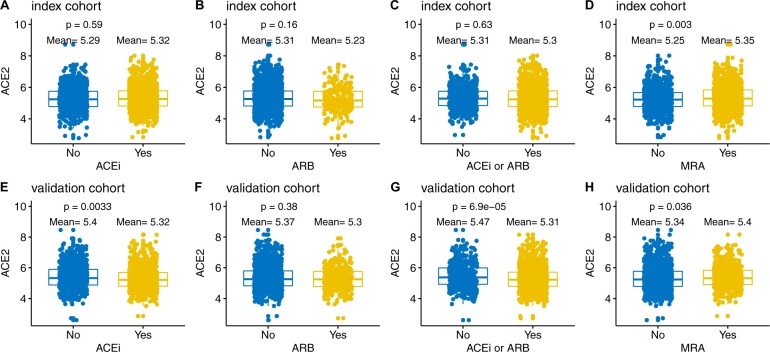
Figure 2 shows plasma ACE2 concentrations in those treated with or without blockers of the RAAS. In the index cohort, mean plasma concentration was 5.32 in patients who used an ACE inhibitor compared with 5.29 in those who did not (P = 0.59. In the validation cohort, the mean plasma concentration was 5.32 in those who used an ACE inhibitor vs. 5.4 in those who did not (P = 0.0033). In the index cohort, the mean plasma concentration was 5.23 in patients who used an ARB compared with 5.31 in those who did not (P = 0.16). In the validation cohort, the mean plasma concentration was 5.3 in those who used an ARB vs. 5.37 in those who did not (P = 0.38). In the index cohort, mean plasma concentration was 5.35 in patients who used an MRA compared with 5.25 in those who did not (P = 0.003). In the validation cohort, the mean plasma concentration was 5.4 in those who used an MRA vs. 5.34 in those who did not (P = 0.036).
Figure 2.
ACE2 concentrations in patients with and without use of an ACE inhibitor, ARB, and MRA (index and validation). ACEi, use of an angiotensin-converting enzyme (ACE) inhibitor; ARB, use of an angiotensin receptor blocker; MRA, use of a mineralocorticoid receptor antagonist.
Age and sex interaction analyses indicated that men who used MRA have an increased ACE2 concentration (P ≤ 0.01, for unadjusted models, and those adjusted for ACE inhibitor use, ARB use, age, diabetes, and atrial fibrillation). This was statistically significant only in the index cohort. In the validation cohort, men who used an ACE inhibitor had lower ACE2 concentrations (P < 0.05; for unadjusted models, and those adjusted for ACE inhibitor use, ARB use, age, diabetes, and atrial fibrillation). All similar interaction tests were not statistically significant.
Variables associated with plasma ACE2 concentrations
The strongest predictor of elevated plasma concentrations of ACE2 in the index and validation cohort was male sex (estimate = 0.26, P < 0.001; and 0.19, P < 0.001, respectively). In the index cohort, neither ACE inhibitors, ARBs, nor MRAs were associated with plasma ACE2 concentrations (Table 2). In the validation cohort, ACE inhibitors (estimate = –0.17, P = 0.002) and ARBs (estimate = –0.15, P = 0.03) were associated with lower plasma ACE2 concentrations, but MRAs (estimate = 0.11, P = 0.04) were associated with higher concentrations (Supplementary material online, Table S4).
Table 2.
Multivariable predictors of ACE2 concentrations (index cohort)
| Predictor | Coefficient | Exponentiated coefficient (95% CI) | P-value |
|---|---|---|---|
| ACE inhibitors: yes | 0.016 | 1.02 (0.94–1.1) | 0.685 |
| ARBs: yes | –0.068 | 0.93 (0.83–1.05) | 0.258 |
| Chronic obstructive pulmonary disease: yes | –0.156 | 0.86 (0.78–0.94) | <0.001 |
| Coronary artery by-pass graft: yes | 0.115 | 1.12 (1.02–1.23) | 0.014 |
| Heart rate (b.p.m.) | 0.003 | 1 (1–1) | 0.007 |
| History of atrial fibrillation: yes | 0.166 | 1.18 (1.1–1.27) | <0.001 |
| Left ventricular ejection fraction (%) | –0.004 | 1 (0.99–1) | 0.033 |
| MRA: yes | 0.051 | 1.05 (0.98–1.13) | 0.154 |
| New York Heart Association (NYHA) class: II | 0.124 | 1.13 (0.88–1.46) | 0.339 |
| NYHA class: III | 0.272 | 1.31 (1.02–1.69) | 0.035 |
| NYHA class: IV | 0.325 | 1.38 (1.06–1.81) | 0.017 |
| Sex: male | 0.26 | 1.3 (1.2–1.41) | <0.001 |
| Systolic blood pressure (mmHg) | –0.003 | 1 (1–1) | 0.002 |
For yes–no variables, only the ‘yes’ group is shown as the ‘no’ group is the reference. NYHA class I was the reference for NYHA.
Discussion
In two large independent cohorts of patients with heart failure, we found that plasma ACE2 concentrations were higher in men than in women. In addition, those receiving ACE inhibitors or ARBs did not have higher concentrations of ACE2, and an increase in those taking MRA in the validation cohort was not confirmed in the index cohort.
There is an increased susceptibility of elderly people with chronic comorbidities to SARS coronaviruses, and men appear to be especially vulnerable to SARS-CoV-2.1 , 2 , 19 Given that a typical heart failure patient belongs to this high-risk group, we sought to uncover factors that could explain the sex-based susceptibility to SARS-CoV-2 in this vulnerable population.
Baseline characteristics of the two cohorts presented are typical for patients with heart failure and confirm that these are elderly patients that often have comorbidities, including diabetes, hypertension, renal disease, and COPD. The spectrum of comorbidities involves most of the organs affected in COVID-19, including the heart, lungs, kidneys, and liver.1
COVID-19 patients and other patients with such underlying diseases are in a hyperinflammatory state. As such, it might well be that patients with various kidney diseases have high endothelial ACE2,20 making ACE2 a damage marker. Furthermore, plasma ACE2 activity is increased in patients with heart failure.21
Post-transcriptional events of ACE2 in the testis
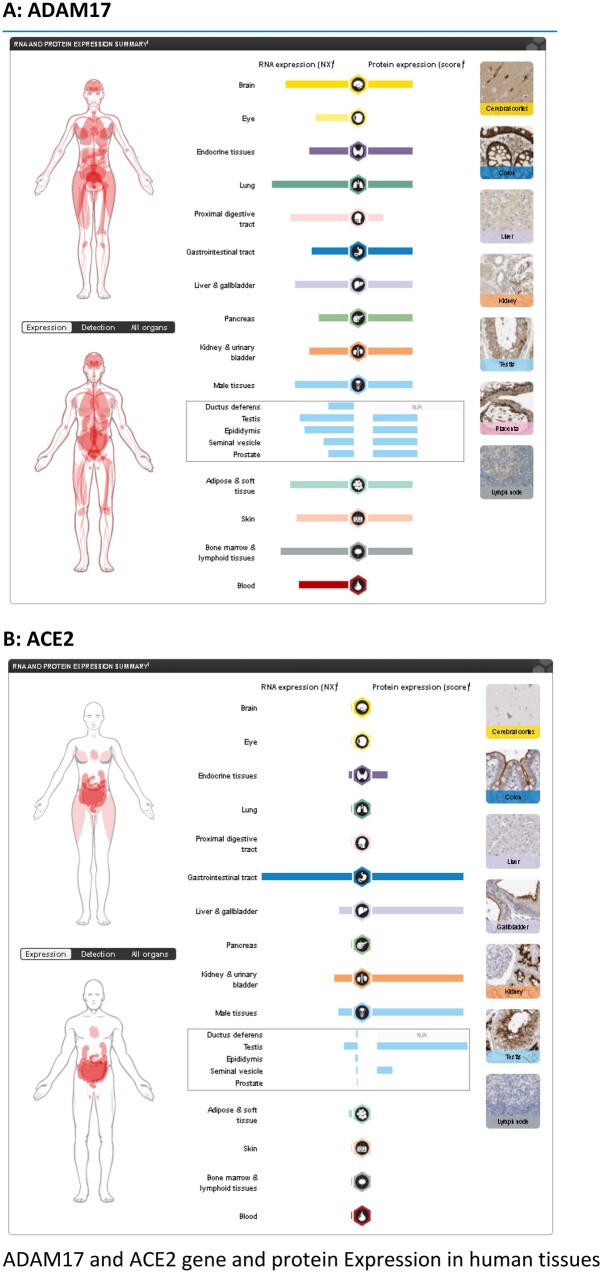
ACE2 is widely distributed in tissues, including lung alveolar epithelial cells, vascular endothelium, heart, kidney, and testis.8 , 11 , 20 , 22 For the readers’ convenience, we provide Supplementary material online, Figures S4 and S5 showing the gene structure of ACE2, and its isoforms and tissue distribution. ACE2 protein and, interestingly, also the non-coding isoforms are highly expressed in the testis22 (Take home figure, Supplementary material online, Figure S6). Isoform transcription could possibly affect protein translation in this male-specific tissue, e.g. via microRNA (miRNA) competition. Previous studies indicate that ACE2 may be subject to post-transcriptional regulation via miR-42123 which could be exploited as a novel potential therapeutic target to modulate ACE2 expression in disease. How the testis-expressed ACE2 protein, or that expressed in other organs, enters the circulation is largely unknown. The tissue-specific transcriptional regulation of ACE2 could partially explain higher ACE2 protein concentrations and why a coronavirus would flourish in men.
Take home figure.
ADAM17 (A) and ACE2 (B) gene and protein Expression in human tissues. (Source A : https://www.proteinatlas.org/ENSG00000151694-ADAM17/tissue and B : https://www.proteinatlas.org/ENSG00000130234-ACE2/tissue).
ACE2 plasma concentrations and use of RAAS inhibitors
Recently, it was suggested that the higher prevalence and fatality rate in patients with cardiac diseases, such as hypertension or diabetes, was related to the concomitant use of ACE inhibitors and ARBs that were suggested to increase ACE2 concentrations. The authors speculated that this might be due to increased expression of ACE2, but offered no evidence for this.5 , 6 , 24 , 25 In animal models, selective blockade of either angiotensin II synthesis or activity induced increases in cardiac ACE2 gene expression and cardiac ACE2 activity;13 whether this translates to humans needs to be validated.
To the best of our knowledge, this is the first substantial study to examine the association between plasma ACE2 concentrations and the use of RAAS blockers in patients with cardiovascular disease. In contrast to previous reports,5 , 6 , 24 , 25 ACE inhibitors and ARBs were not associated with increased plasma concentrations of ACE2 in the present study. Indeed, if anything, the use of ACE inhibitors and ARBs predicted lower concentrations of ACE2 in the validation cohort, although these findings were not replicated in the index cohort. Taken together, these data do not support withholding ACE inhibitors or ARBs in patients at risk for SARS-CoV-2 infection.
In the validation cohort, MRA use was associated with a weak but statistically significant increase in plasma ACE2 concentrations. Univariate and multivariate-adjusted analyses indicated a significant sex-based interaction, with men on MRA having higher ACE2 concentrations. A similar association was not found in the index cohort. The effect of an MRA on plasma ACE2 is therefore not clear. One study found a trend (P = 0.07) towards increased plasma ACE2 activity in patients treated with an MRA.21 In addition, one mechanistic study using macrophages reported an increase of ACE2 activity after MRA,26 but further data are not available. Clearly, our findings do not suggest that MRAs should be discontinued in patients with heart failure, in whom SARS-CoV-2 infection is found. Moreover, even if MRAs are consistently found to increase plasma ACE2 concentrations, it still needs to be established whether their use is associated with higher vulnerability to or more severe consequences of SARS-CoV-2 infection. MRAs are a very effective treatment for heart failure, and these hypothetical effects on viral infection should be weighed carefully against their proven benefits.
The equilibrium between soluble and membrane-bound ACE2 might influence COVID-19 pathogenesis and treatment options. Previous studies indicate ADAM17-mediated ACE2 shedding,27 , 28 but how this would affect coronavirus infectivity during concomitant use of RAAS inhibitors warrants separate research. A study on dogs with heart disease indicated that ACE2 shedding is not an important factor in the total extent of tissue-bound ACE2 activity, but rather a loss of tissue ACE2 into the circulation would tend to decrease the overall compensatory potential of ACE2.29 Further work is required to show whether this translates to humans.
Conclusion
In two large cohorts of patients with heart failure, plasma ACE2 concentrations were higher in men than in women, possibly reflecting higher tissue expression of this receptor for SARS coronavirus infections. This could explain why men might be more susceptible to infection with, or the consequences of, SARS-CoV-2. Patients receiving ACE inhibitors or ARBs did not have higher plasma concentrations of ACE2, and any effect of MRAs was small and inconsistent, supporting the continued use of these agents in patients with heart failure during the current SARS-CoV-2 pandemic.
Limitations
The conclusions drawn in this analysis are mainly restricted to heart failure, albeit a group of patients at high risk for COVID-19. Secondly, since our patients are not coronavirus infected, we cannot provide a direct link between the course of COVID-19 disease in patients with low vs. high plasma ACE2 concentrations, and the influence of age and RAAS blockers on the course of the disease. Thirdly, we measured plasma ACE2 concentrations and not membrane-bound ACE2. We can only speculate that circulating concentrations are associated with tissue concentration, since there is no compelling evidence for this.
Funding
This work was supported by a grant from the European Commission (FP7-242209-BIOSTAT-CHF).
Conflict of interest: B.T.S reports grants from the Dutch Heart Foundation (2019T094), during the conduct of this study. J.G.F.C. reports grants and personal fees from Amgen, Novartis, Pharmacosmos, and Vifor, and personal fees from Servier, outside the submitted work. S.D.A. reports personal fees from Bayer, Boehringer Ingelheim, Cardiac Dimension, Impulse Dynamics, Novartis, Servier, St. Jude Medical, and Vifor Pharma, and grant support from Abbott Vascular and Vifor Pharma, outside the submitted work. G.F. reports being a Committee Member in trials sponsored by Medtronic, Vifor, Servier, Novartis, and BI, outside the submitted work. M.M. reports personal fees from Consulting honoraria for participation to trials' committees or advisory boards from Abbott vascular, Amgen, Astra-Zeneca, Bayer and Vifor pharma in the last 3 years, personal fees from Fees for public speeches in sponsored symposia from Abbott vascular and Edwards Therapeutics, outside the submitted work. All other authors have no conflicts to declare.
Supplementary Material
Contributor Information
Iziah E Sama, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Alice Ravera, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Cardiology, Department of Medical and Surgical Specialties, Radiologic Sciences and Public Health, University of Brescia, Brescia, Italy.
Bernadet T Santema, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Harry van Goor, Department of Pathology and Medical Biology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Jozine M ter Maaten, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
John G F Cleland, Robertson Centre for Biostatistics & Clinical Trials Unit, University of Glasgow and National Heart & Lung Institute, Imperial College, London, UK.
Michiel Rienstra, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Alex W Friedrich, Department of Medical Microbiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Nilesh J Samani, Department of Cardiovascular Sciences, University of Leicester, Glenfield Hospital, and NIHR Leicester Biomedical Research Centre, Leicester, UK.
Leong L Ng, Department of Cardiovascular Sciences, University of Leicester, Glenfield Hospital, and NIHR Leicester Biomedical Research Centre, Leicester, UK.
Kenneth Dickstein, University of Bergen, Bergen, Norway; Stavanger University Hospital, Stavanger, Norway.
Chim C Lang, Division of Molecular and Clinical Medicine, School of Medicine, University of Dundee Ninewells Hospital and Medical School, Dundee, UK.
Gerasimos Filippatos, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece; University of Cyprus, School of Medicine, Nicosia, Cyprus.
Stefan D Anker, Department of Cardiology (CVK) and Berlin Institute of Health Center for Regenerative Therapies (BCRT), Germany; German Centre for Cardiovascular Research (DZHK) partner site Berlin, Charité Universitätsmedizin Berlin, Germany.
Piotr Ponikowski, Department of Heart Diseases, Medical University, Military Hospital, Wrocław, Poland.
Marco Metra, Cardiology, Department of Medical and Surgical Specialties, Radiologic Sciences and Public Health, University of Brescia, Brescia, Italy.
Dirk J van Veldhuisen, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Adriaan A Voors, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
References
- 1. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,, Xiang J,, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J,, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 2004;159:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020;doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020;doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 2020; doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med 2004;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1–E9. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sluimer JC, Gasc JM, Hamming I, van Goor H, Michaud A, van den Akker LH, Jütten B, Cleutjens J, Bijnens AP, Corvol P, Daemen MJ, Heeneman S. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J Pathol 2008;215:273–279. [DOI] [PubMed] [Google Scholar]
- 12. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020;5:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 14. Voors AA,, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 15. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA,, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 18. Subirana I, Sanz H, Vila J. Building bivariate tables: the compareGroups Package for R. J Stat Softw 2014;57:1–16.25400517 [Google Scholar]
- 19. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 2004;204:587–593. [DOI] [PubMed] [Google Scholar]
- 21. Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin–angiotensin–aldosterone system. J Am Coll Cardiol 2008;52:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 23. Lambert DW, Lambert LA, Clarke NE, Hooper NM, Porter KE, Turner AJ. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin Sci (Lond) 2014;127:243–249. [DOI] [PubMed] [Google Scholar]
- 24. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res 2005;97:946–953. [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical relevance and role of neuronal AT. Circ Res 2017;121:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 2005;280:30113–30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larouche-Lebel É, Loughran KA, Oyama MA, Solter PF, Laughlin DS, Sánchez MD, Assenmacher CA, Fox PR, Fries RC. Plasma and tissue angiotensin-converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J Vet Intern Med 2019;33:1571–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.