Abstract
Aims
To compare demographic characteristics, clinical presentation, and outcomes of patients with and without concomitant cardiac disease, hospitalized for COVID-19 in Brescia, Lombardy, Italy.
Methods and results
The study population includes 99 consecutive patients with COVID-19 pneumonia admitted to our hospital between 4 March and 25 March 2020. Fifty-three patients with a history of cardiac disease were compared with 46 without cardiac disease. Among cardiac patients, 40% had a history of heart failure, 36% had atrial fibrillation, and 30% had coronary artery disease. Mean age was 67 ± 12 years, and 80 (81%) patients were males. No differences were found between cardiac and non-cardiac patients except for higher values of serum creatinine, N-terminal probrain natriuretic peptide, and high sensitivity troponin T in cardiac patients. During hospitalization, 26% patients died, 15% developed thrombo-embolic events, 19% had acute respiratory distress syndrome, and 6% had septic shock. Mortality was higher in patients with cardiac disease compared with the others (36% vs. 15%, log-rank P = 0.019; relative risk 2.35; 95% confidence interval 1.08–5.09). The rate of thrombo-embolic events and septic shock during the hospitalization was also higher in cardiac patients (23% vs. 6% and 11% vs. 0%, respectively).
Conclusions
Hospitalized patients with concomitant cardiac disease and COVID-19 have an extremely poor prognosis compared with subjects without a history of cardiac disease, with higher mortality, thrombo-embolic events, and septic shock rates.
Keywords: COVID-19, Cardiovascular disease, Pneumonia, Mortality
See page 1830 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa415)
Introduction
New pneumonia cases related to the severe acute respiratory syndrome coronavirus (SARS-CoV-2), or coronavirus disease 2019 (COVID-19), rapidly spread worldwide, establishing critical challenges for the public health and medical communities. The World Health Organization has declared COVID-19 a public health emergency of international concern, with a global estimate of laboratory-confirmed cases of 1 696 588, and 105 952 deaths as of 12 April 2020.1
A high proportion of COVID-19 patients have comorbidities.2–5 Studies from China show that 15–40% of them have a history of cardiac disease2–5 and 10–30% show laboratory signs of cardiac injury and cardiovascular involvement, associated with a more severe clinical course.6,7 These values are probably higher in COVID-19 patients from other areas, such as Europe and the USA, because of the older age of their population. In a report from the USA, including 21 critically ill patients with COVID-19, heart failure (HF) was present in 43% of cases at baseline and complications leading to HF occurred in a third of these patients.8 Another series of 24 patients admitted to the intensive care unit (ICU) in the Seattle area for hypoxaemic respiratory failure showed a prevalence of hypotension requiring vasopressors of 71%, with a 50% in-hospital mortality. No data regarding history of cardiovascular disease were given.9
Thus, although patients with a history of cardiac disease seem more likely to be infected and to have a more severe clinical course with COVID-19, their clinical characteristics and outcomes have not yet been described. In the Brescia area of the Lombardy region, North Italy, the COVID-19 outbreak has caused a major burden on healthcare services, with major changes in in-hospital medical specialties, leading to a transformation of otherwise specialized units, such as cardiology units, into specialized COVID-19 units.10 In this report, we describe the demographic characteristics, clinical presentation, and outcomes of consecutive patients with COVID-19 and cardiac disease, and compare them with patients with COVID-19 and no history of cardiac disease, hospitalized at the same hospital during the same time interval.
Methods
Study population
The study population comprises two groups of consecutive patients hospitalized for COVID-19 pneumonia at Civil Hospitals of Brescia, Lombardy, Italy, between 4 March and 25 March 2020. The first group included all patients with a history of cardiac disease admitted to our Cardiology Unit, the second all patients with no history of cardiac disease, admitted to a COVID-19 unit of our hospital (director M.B.). All patients had a diagnosis confirmed by positive results of PCR testing of a nasopharyngeal swab. Only patients with a complete follow-up at 14 days were included.
Data collection and definitions
Demographic, clinical, laboratory, instrumental, treatment, and outcome data were extracted from the in-hospital medical records. Chronic kidney disease (CKD) was defined by the presence of an estimated glomerular filtration rate (eGFR), calculated by the CKD-EPI equation, of ≤60 mL/min/m2. Fever was defined as axillary temperature of at least 37.5°C. The sequential organ failure assessment (SOFA) score was calculated for all patients.11 Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition.12
Radiological assessments included chest radiography or computed tomography (CT). An internal radiological score was developed (see Supplementary material online, Text S1). Venous blood samples and arterial samples for blood gas analysis were collected at the time of hospitalization and thereafter based on clinical indications. High sensitivity troponin T (hsTnT) plasma levels were defined as normal when below the 99th percentile of normal values, e.g. 14 ng/L. N-terminal probrain natriuretic peptide (NT-proBNP) plasma levels were defined as normal when <125 pg/mL for patients aged 0–74 years and <450 pg/mL for those older.13
Statistical analysis
Continuous variables are expressed as mean (SD) or median [interquartile range (IQR)] values when they did not show a normal distribution. Categorical data were expressed as absolute values and proportions. Variables were compared between patients with and without concomitant cardiac disease as well as between survivors and non-survivors and between patients with different causes of inclusion in the cardiac group by using the Fisher exact test or χ2 test for categorical variables, and the t-test or the Mann–Whitney U test, as appropriate, for continuous variables. The overall trend difference in laboratory markers during the hospitalization course among patients with cardiac disease, stratified by mortality, was assessed using a mixed-effects longitudinal analysis model. Survival curves were plotted using the Kaplan–Meier method and compared between patients with and without cardiac disease by the log-rank test. Analyses were performed with Stata, version 14 (Stata Corp., College Station, TX, USA). For all the statistical analyses, P <0.05 was considered significant.
Results
Characteristics on admission
Demographic and clinical features are shown in Table 1. Among the cardiac patients, HF, atrial fibrillation (AF), coronary artery disease (CAD), and CKD were present in 40, 36, 30, and 28% of the patients, respectively. Cardiac patients were more likely to receive chronic treatment with an angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB), or angiotensin receptor–neprilysin inhibitor (ARNI), anticoagulants, and statins. Fever on admission was present in 42% of patients. Chest X-ray showed pneumonia in all patients. No differences were found in any variable except for a lower blood pressure in cardiac patients.
Table 1.
Demographic and clinical findings
| Variable | Total (N = 99) | Cardiac disease (N = 53) | No cardiac disease (N = 46) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 67 ± 12 | 68 ± 12 | 66 ± 12 | 0.51 |
| Sex (male), n (%) | 80 (81) | 45 (85) | 35 (76) | 0.27 |
| Body mass index (kg/m2) ≥30 kg/m2, n (%) | 18 (23) | 13 (26) | 5 (18) | 0.41 |
| Clinical history, n (%) | ||||
| Smoker | 17 (20) | 11 (21) | 6 (18) | 0.77 |
| Hypertension | 63 (64) | 40 (75) | 23 (51) | 0.012 |
| Dyslipidaemia | 29 (30) | 23 (43) | 6 (13) | <0.001 |
| Diabetes | 30 (31) | 16 (30) | 14 (31) | 0.92 |
| Heart failure | 21 (21) | 21 (40) | 0 (0) | <0.001 |
| Atrial fibrillation | 19 (19) | 19 (36) | 0 (0) | <0.001 |
| Coronary artery disease | 16 (16) | 16 (30) | 0 (0) | <0.001 |
| Prior cardiac surgery | 9 (9) | 9 (17) | 0 (0) | 0.003 |
| Prior percutaneous valve treatment | 3 (3) | 3 (11) | 0 (0) | 0.10 |
| Chronic obstructive pulmonary disease | 9 (9) | 6 (11) | 3 (6) | 0.41 |
| Chronic kidney disease | 15 (15) | 15 (28) | 0 (0) | <0.001 |
| Cancer | 17 (18) | 13 (24) | 4 (9) | 0.05 |
| Prior ACEi/ARB/ARNI therapy | 30 (31) | 28 (53) | 2 (4) | <0.001 |
| Prior anticoagulant therapy | 17 (18) | 16 (30) | 1 (2) | <0.001 |
| Prior statin therapy | 25 (26) | 23 (44) | 2 (4) | <0.001 |
| Data on admission | ||||
| Temperature, °C | 37.3 ± 1.0 | 37.3 ± 1.1 | 37.2 ± 0.9 | 0.53 |
| Fever, n (%) | 39 (42) | 24 (46) | 15 (37) | 0.35 |
| Systolic blood pressure, mmHg | 132 ± 23 | 126 ± 23 | 140 ± 20 | 0.003 |
| Heart rate, b.p.m. | 87 ± 20 | 86 ± 21 | 90 ± 18 | 0.33 |
| Oxygen saturation (ambient air), % | 91 ± 89 | 92 ± 5 | 90 ± 12 | 0.30 |
| PaO2/FiO2 | 273 ± 88.5 | 272 ± 98.5 | 274 ± 75 | 0.91 |
| PaO2/FiO2 <300, n (%) | 50 (64) | 27 (61) | 23 (68) | 0.57 |
| SOFA score | 2.2 ± 1.2 | 2.2 ± 1.3 | 2.2 ± 0.9 | 0.85 |
| COVID score peak | 10.2 ± 4.4 | 10.9 ± 4.7 | 9.4 ± 3.9 | 0.13 |
| Left ventricular ejection fraction, % | 48 ± 14 | 47 ± 14 | 57 ± 3 | 0.25 |
Continuous variable are reported as mean ± SD.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; FiO2, fraction of inspired oxygen; PaO2, oxygen partial pressure at arterial gas analysis; SOFA, sequential organ failure assessment; COVID, coronavirus disease.
Laboratory parameters are reported in Table 2. The only differences between the two groups regarded higher values of serum creatinine, NT-proBNP, and hsTnT in cardiac patients. NT-proBNP plasma levels were increased in 25 of the 28 cardiac patients (88%) and in 26 of the 45 non-cardiac patients (58%) in whom they were measured. hsTnT levels were increased in 31 of the 40 cardiac patients (71%), and in 17 of 36 non-cardiac patients (47%).
Table 2.
Laboratory findings of patients stratified by concomitant cardiac disease
| Variable | Reference range | Total (N = 99) | Cardiac (N = 53) | Non-cardiac (N = 46) | P-value |
|---|---|---|---|---|---|
| Red blood cell count, ×106/μL | 4.0–5.2 | 4.6 (4.0–5.0) | 4.4 (3.7–4.9) | 4.7 (4.3–5) | 0.035 |
| Haemoglobin, g/dL | 12.0–16.0 | 13.8 (12.3–14.7) | 13.2 (11.1–15) | 13.9 (13.2–14.4) | 0.45 |
| White blood cell count, per μL | 4000–10800 | 6620 (4870–8750) | 6370 (4660–8880) | 6750 (5010–8700) | 0.58 |
| Neutrophils, per μL | 1500–8000 | 4625 (2855–6725) | 4150 (2830–6650) | 5108 (3170–6880) | 0.64 |
| Lymphocytes, per μL | 900–4000 | 920 (700–1250) | 850 (640–1160) | 1040 (750–1290) | 0.15 |
| Platelet count, ×103/μL | 130–400 | 188 (155–242) | 183 (155–233) | 188 (158–247) | 0.73 |
| Creatinine, mg/dL | 0.60–1.00 | 1.0 (0.9–1.3) | 1.1 (0.9–1.4) | 1.0 (1–1.1) | 0.037 |
| Sodium, mEq/L | 136–145 | 137 (135–139) | 137 (134–139) | 138 (136–140) | 0.15 |
| Potassium, mEq/L | 3.4–4.5 | 3.9 (3.6–4.3) | 4.1 (3.6–4.4) | 3.8 (3.5–4.1) | 0.04 |
| Chloride, mEq/L | 98–107 | 99 (97–102) | 99.5 (96.5–101.5) | 101 (97–103) | 0.23 |
| Baseline CRP, mg/dL | <5.0 | 65 (21–133) | 50 (11–113) | 99 (48–138) | 0.07 |
| Peak CRP, mg/dL | <5.0 | 113 (65 –169) | 113 (59–176) | 107 (72–160) | 0.9 |
| Procalcitonin, ng/mL | <0.5 | 0.3 (0.1–0.8) | 0.3 (0.1–1.4) | 0.1 (0.1–0.2) | 0.022 |
| Ferritin, μg/L | 30–400 | 1392 (745–2733) | 1574 (745–2754) | 1373 (753–2245) | 0.62 |
| D-dimer, ng/mL | <232 | 576 (330–985) | 640 (204–1873) | 573 (332–868) | 0.85 |
| High sensitivity troponin T, ng/L | <14 | 18 (10–43) | 34 (14–105) | 16 (7–21) | <0.001 |
| NT-proBNP, pg/mL | <93 | 311 (107–1536) | 2584 (206–4546) | 180 (86–458) | <0.001 |
| Aspartate transaminase, U/L | 18–39 | 46 (34–68) | 46 (35–63) | 44 (34–69) | 0.96 |
| Alanine transaminase, U/L | 10–50 | 34 (24–58) | 31 (24–49) | 41.5 (24–67) | 0.06 |
| Lactate dehydrogenase, U/L | 135–225 | 351 (251–481) | 321 (242–448) | 365 (289–490) | 0.15 |
| Creatine phosphokinase, U/L | 39–308 | 153 (64–335) | 151 (64–353) | 158 (64–302) | 0.59 |
| Albumin, g/L | 45–52 | 33 (29.4–36) | 35 (30–37) | 32 (28–34) | 0.041 |
| Lactate, mmol/L | 0.5–2.2 | 1.1 (0.9–1.5) | 1.3 (0.9–1.6) | 1.0 (0.8–1.53) | 0.06 |
Values are reported as median (interquartile range).
CRP, C-reactive protein, NT-proBNP, N-terminal probrain natriuretic peptide.
In-hospital management
Data regarding in-hospital management are shown in Supplementary material online, Table S1 and in Table 3. Chronic therapy with an ACEi, ARB, or ARNI was discontinued in 77% of cases because of severe hypotension. Oxygen support with FiO2 ≥50% was needed in half of patients; non-invasive ventilation was used in 19% of patients; two patients needed intubation. Ventilatory support or intubation were excluded in seven cardiac patients and in three non-cardiac patients for their age and/or comorbidities.
Table 3.
In-hospital management and outcomes of the study population stratified by concomitant cardiac disease
| Variable | Total (N = 99) | Cardiac (N = 53) | Non-cardiac (N = 46) | P-value |
|---|---|---|---|---|
| Changes in ongoing treatment | ||||
| ACE/ARB/ARNI interruption, n (%)* | 23 (77) | 21 (75) | 2 (100) | <0.001 |
| Needed ventilatory support | ||||
| Oxygen support with FiO2 <50%, n (%) | 54 (57.4) | 31 (58.5) | 23 (56.1) | 0.82 |
| Oxygen support with FiO2 ≥50%, n (%) | 47 (50) | 29 (54.7) | 18 (43.7) | 0.3 |
| Non-invasive ventilation, n (%) | 18 (19.1) | 10 (18.9) | 8 (19.5) | 0.94 |
| Intubation, n (%) | 2 (2) | 2 (3.8) | 0 (0) | 0.19 |
| Outcomes | ||||
| Intensive care unit admission, n (%) | 12 (12) | 10 (19) | 0 (0) | <0.001 |
| Hospital length of stay, days | 11.4 ± 6.5 | 11.8 ± 8.3 | 10.8 ± 3.4 | 0.48 |
| ARDS, n (%) | 19 (19) | 12 (23) | 7 (15) | 0.35 |
| Venous thrombo-embolism, n (%) | 12 (12) | 9 (17) | 3 (6) | 0.11 |
| Arterial thrombo-embolism, n (%) | 3 (3) | 3 (6) | 0 (0) | 0.1 |
| Septic shock/sepsis, n (%) | 6 (6) | 6 (11) | 0 (0) | 0.019 |
| Death, n (%) | 26 (26) | 19 (36) | 7 (15) | 0.02 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; FiO2, fraction of inspired oxygen; ARDS, acute respiratory distress syndrome.
The proportion of patients who underwent ACEi/ARB/ARNI interruption was calculated relative to the number of patients on chronic therapy.
Outcomes
The most frequent complications of the clinical course are shown at Table 3 and included acute respiratory distress syndrome (ARDS), venous thrombo-embolism, arterial thrombo-embolism, and sepsis or septic shock in 19, 12, 3, and 6% of the patients, respectively. Mortality was significantly higher in cardiac compared with non-cardiac patients (35.8% vs. 15.2%; log-rank P = 0.019; relative risk 2.35; 95% confidence interval 1.08–5.09) (Take home figure). Death occurred during the hospitalization at a median time of 8 days (IQR 5–14) in cardiac patients and at a median time of 10 days (IQR 6–12) among non-cardiac patients. Causes of death of cardiac patients were ARDS in 11 patients, septic shock in five patients and acute pulmonary thrombo-embolism in the other three. Cause of death among non-cardiac patients was ARDS in all but one subject who died for pulmonary embolism. Even after excluding patients who were denied intubation due to comorbidities or age, the mortality rate remained higher in cardiac compared with non-cardiac patients (26% vs. 9%; P = 0.039).
Take home figure.
Top: Kaplan–Meier 14-day survival rates for the patients with and without concomitant cardiac disease. Bottom: 14-day outcomes (major complications and deaths) of all patients and the patients without and with concomitant cardiac disease. ARDS, acute respiratory distress syndrome; CI, confidence interval; RR, relative risk; TE, thrombo-embolism.
Clinical, laboratory, and outcome characteristics of the overall study population stratified by mortality are reported in Supplementary material online, Tables S2–S4.
Clinical characteristics of survivors and non-survivors among cardiac patients
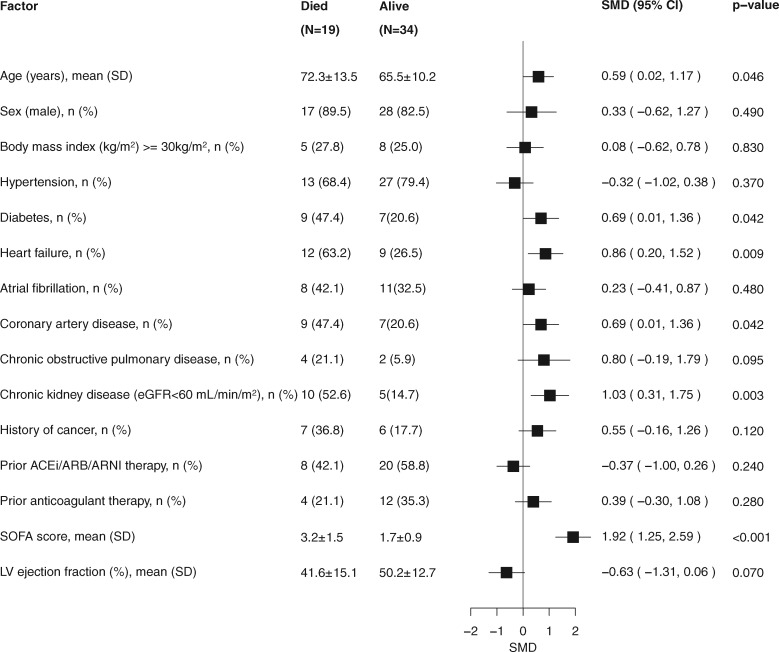
Compared with patients who were alive, those who died were older and more likely to have a history of HF, diabetes, CAD, and CKD. Moreover, they had lower systolic blood pressure, higher SOFA score, and were more likely to have a PaO2/FiO2 ratio <300 mmHg at the time of hospitalization (Figure 1; Supplementary material online, Table S5).
Figure 1.
Clinical features of cardiac patients stratified by vital status. Standardized forest plot comparing selected clinical variables between survivors and non-survivors among cardiac patients. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; eGFR, estimated glomerular filtration rate; LV, left ventricular; SMD, standardized mean difference; SOFA, sequential organ failure assessment.
With regard to laboratory parameters, non-survivors had lower lymphocyte count and higher serum creatinine and procalcitonin levels at the time of hospitalization. D-dimer, C-reactive protein (CRP), NT-proBNP, and hsTnT levels were numerically higher at the time of hospitalization in non-survivors compared with survivors (Figure 2; Supplementary material online, Table S6). All nine patients with normal troponin levels did not have complications or death during hospitalization. Plasma levels of sodium, potassium, and chloride tended to be lower in non-survivors vs. survivors during the whole clinical course. Platelets were lower at admission and increased during the hospitalization, reaching higher values in the subjects who died (Figure 3).
Figure 2.
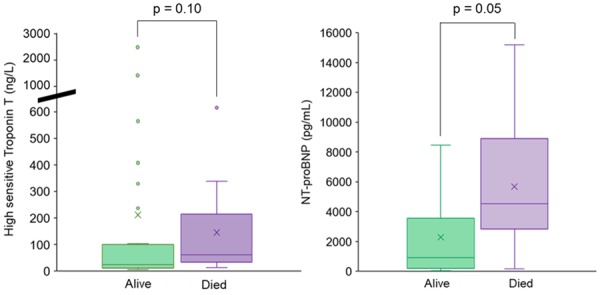
Cardiac biomarkers on admission among cardiac patients. Box plot showing high sensitivity troponin T and N-terminal probrain natriuretic peptide (NT-proBNP) on admission in alive (green) and dead (purple) patients.
Figure 3.
Temporal changes in laboratory markers during the hospitalization in cardiac patients. Median and interquartile range changes over time in lymphocytes, platelets, serum creatinine, sodium, potassium, and chloride in patients stratified by in-hospital mortality (alive, green; dead, purple).
Non-survivors needed oxygen support with FiO2 >50% more frequently than survivors. Among complications, ARDS and septic shock were more common in patients who died compared with those who were alive, whereas thrombo-embolic events were similar between the two groups (Supplementary material online, Table S7).
Primary causes of hospital admission of cardiac patients are shown in Supplementary material online, Text S1 and Tables S8–S10.
Discussion
This is the first study describing the clinical characteristics and outcome of patients with a history of cardiac disease and COVID-19 pneumonia. Our results showed a high rate of in-hospital mortality and complications in cardiac patients compared with those without a history of cardiac disease. Mortality of cardiac patients was high regardless of their main cause of hospitalization, COVID-19 pneumonia vs. acute cardiac conditions. More than a third of our cardiac patients died in hospital and more than half developed severe complications such as ARDS, septic shock, or thrombo-embolic events. Mortality and major event rates of cardiac patients were higher compared with those of non-cardiac patients admitted for COVID-19 pneumonia (36% vs. 15% for mortality and 57% vs. 21% for overall major complications). Several conditions (i.e. history of HF, severe aortic stenosis, and CKD), and laboratory abnormalities (i.e. lymphocytopenia, high levels of D-dimer, procalcitonin, TnT, and NT-proBNP on admission) were associated with poor outcomes.
The outcomes reported for our patients are worse than those reported in series from China,2–5,7 but are consistent with those reported from the USA.8,9 The poor outcomes of our patients were probably related to their older age and higher burden of cardiac comorbidities, as shown by the comparison with non-cardiac COVID-19 patients. Older age was consistently shown to be a major risk factor for poor outcomes3,14,15 and/or myocardial injury.6,7 Also a history of cardiovascular disease was associated with poorer outcomes. A higher prevalence of hypertension and CAD in non-survivors, compared with survivors, was shown by Zhou et al.,3 and hypertensive patients were more likely to develop ARDS in the study by Wu et al.14
Patient selection may have influenced our results. However, we describe a series of consecutive patients admitted in a limited time interval because of COVID-19 and concomitant cardiac disease. These patients were selected at the time of their presentation to our emergency department and the reason for admission to our institute of cardiology was considered upon their concomitant cardiac disease requiring specialized treatment, regardless of whether their primary cause of hospitalization was COVID-19 pneumonia or a cardiac disorder. Consistently, the primary cause of admission had no relationship to the characteristics our cardiac patients (Supplementary material online, Tables S8–S10). Thus, our data point out the major impact of a clinically relevant cardiac comorbidity on the outcomes of COVID-19 patients.
The mechanism of poor outcomes in patients with COVID-19 and concomitant cardiac disease may be multiple and cannot be ascertained by our data. An exaggerated inflammatory activation with hypercytokinaemia (cytokine storm) and multiorgan failure seem to be the main mechanisms of the high mortality of COVID-19 pneumonia and ARDS.16 This is consistent with the increase in inflammatory markers, such as CRP, D-dimer, ferritin, and interleukin plasma levels, in the patients developing ARDS and/or not surviving COVID-19 infection.2–4,14,15,1,17 Inflammatory activation may have a prominent role in the patients with HF and/or CAD, and this may explain their susceptibility to COVID-19 and their poorer clinical course.18,19,20
Severe hypoxaemia, inflammatory activation, and hypotension may all contribute to myocardial injury during COVID-19, and patients with concomitant CAD or HF may be at higher risk.21 Recent data show that cardiac injury may occur in 10–30% of unselected patients with COVID-19 and be associated with a severe clinical course and high mortality, independently of a history of cardiovascular disease.3,6,7 More than 70% of our cardiac patients had increased hs-TnT levels, and high hsTnT levels were also found in almost half of those with a non-cardiac history, consistent with greater severity of COVID-19 patients hospitalized in our area. Plasma troponin levels tended to be higher at entry in non-survivors, compared with survivors. This difference did not reach statistical significance probably because of the small size of our study group. Consistent with previous studies,7 our patients who had low troponin levels at the time of hospitalization had an uneventful clinical course and all survived. Similar to what was proposed for the patients with acute HF,22 the detection of normal troponin levels at the time of hospitalization might therefore be considered as a criterion for early discharge from hospital even in a high-risk population such as our patients.
The rate of thrombo-embolic events was high in our patients, with 15% of all our patients and 23% of our cardiac patients showing major thrombo-embolic events. Such events were likely to be favoured by the marked inflammatory activation associated with COVID-19.23 Elevated plasma levels of coagulation parameters have been associated with poorer outcomes in COVID-19 patients.3,4,17,24 Anticoagulation was not routinely performed in our patients in sinus rhythm. However, based on such a high event rate, a wider use of anticoagulation, or at least thrombo-embolic prophylaxis, seems warranted. Better outcomes were shown with low molecular weight heparin treatment in COVID-19 patients with high D-dimer plasma levels or meeting sepsis-induced coagulopathy criteria.25
It is controversial whether treatment with ACEi/ARB/ARNI may favour coronavirus infection or be protective from COVID-19 pneumonia.26–29 To date, it is considered that these drugs ‘should be continued in patients in otherwise stable condition who are at risk for Covid-19.’28 Our data indirectly support this statement as the prevalence of patients on ACEi/ARB/ARNI was similar between non-survivors and survivors. It is, however, noteworthy that 78% of our patients had to have these drugs temporarily withdrawn during the hospitalization because of persistent hypotension. High rates of hypotension were also shown in another series of critically ill patients.9
Antiviral and antinflammatory agents were given to our patients based on recommendations by an infective disease specialist. Many of these therapies have been associated with untoward effects in cardiac patients. Based on our observational data, it is not possible to ascertain whether they contributed to the poor outcomes of our patients. However, as similar outcomes are reported in other series of patients of similar age and with similar comorbidities,8,9 we may consider that such poor outcomes are caused by COVID-19 and not by concomitant treatment. Despite its hypothesized untoward effects in viral pneumonia, corticosteroid therapy has been associated with more favourable outcomes in COVID-19 pneumonia in an observational study.14
The main limitation of our study is the relatively small size of our study group. However, the extreme burden of the COVID-19 outbreak on the healthcare system and the high major event rates of our patients offset such a limitation. A larger study group would have probably allowed more sophisticated analyses aimed at finding independent prognostic variables. Given the logistical limitations at the onset of this emerging outbreak, some laboratory data (such as troponin and NT-proBNP) were not collected in all patients. Similarly, detailed echocardiographic data were not collected. Data from larger cardiovascular populations and multiple centres are warranted. However, our data are from one of the two areas, Brescia and Bergamo, which were firstly and more severely affected by the COVID-19 outbreak in Italy.
In conclusion, patients with concomitant cardiac disease and COVID-19 have an extremely poor prognosis, compared with subjects without a history of cardiac disease, with higher mortality, septic shock, and thrombo-embolic event rates. Better prevention of COVID-19 and possibly better evidence-based treatment of COVID-19 is warranted in these patients.
Conflict of interest: M.M. has received personal honoraria for participation in trial committees, advisory boards, or speeches at sponsored symposia from Abbott Vascular, Amgen, Astra Zeneca, Bayer, and Vifor Pharma. All other authors have no conflicts to declare.
Supplementary Material
References
- 1.World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report 44. Published 4 March 2020.
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,, Xiang J,, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J,, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M.. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;doi: 10.1001/jama.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim M, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PM, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O’Mahony S, Mikacenic C.. Covid-19 in critically ill patients in the Seattle region—Case Series. N Engl J Med 2020;doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasselli G, Pesenti A, Cecconi M.. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020;doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 11. Lambden S, Laterre PF, Levy MM, Francois B.. The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC.. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes-Genis A, Mueller T, Richards M, Januzzi JL Jr; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. [DOI] [PubMed] [Google Scholar]
- 14. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y.. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y.. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;doi: 10.1016/S2213-2600(20)30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Healh Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q.. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, Veldhuisen DJ, Kakkar R, Voors AA, Meer P.. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail 2019;21:965–973. [DOI] [PubMed] [Google Scholar]
- 19. Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG.. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020; doi:10.1093/eurheartj/ehz961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawler PR, Bhatt DL, Godoy LC, Luscher TF, Bonow RO, Verma S, Ridker PM.. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J 2020; doi:10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 21. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O.. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020. [DOI] [PubMed] [Google Scholar]
- 22. Pang PS, Teerlink JR, Voors AA, Ponikowski P, Greenberg BH, Filippatos G, Felker GM, Davison BA, Cotter G, Kriger J, Prescott MF, Hua TA, Severin T, Metra M.. Use of high-sensitivity troponin T to identify patients with acute heart failure at lower risk for adverse outcomes: an exploratory analysis from the RELAX-AHF Trial. JACC Heart Fail 2016;4:591–599. [DOI] [PubMed] [Google Scholar]
- 23. Glynn RJ, Ridker PM.. Inflammation, venous thromboembolism, and what we can do about it. Eur Heart J 2018;39:3615–3617. [DOI] [PubMed] [Google Scholar]
- 24. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z.. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaduganathan M VO, Michel T, McMurray JJV, Pfeffer MA, Solomon SD.. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danser AHJ, Epstein M, Batlle D.. Renin–angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin–angiotensin system blockers. Hypertension 2020;doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.