Abstract
Recent EACVI recommendations described the importance of limiting cardiovascular imaging during the COVID-19 pandemic in order to reduce virus transmission, protect healthcare professionals from contamination, and reduce consumption of personal protective equipment. However, an elevated troponin remains a frequent request for cardiac imaging in COVID-19 patients, partly because it signifies cardiac injury due to a variety of causes and partly because it is known to convey a worse prognosis. The present paper aims to provide guidance to clinicians regarding the appropriateness of cardiac imaging in the context of troponin elevation and myocardial injury, how best to decipher the mechanism of myocardial injury, and how to guide patient management.
Keywords: COVID-19, Echocardiography, Computed tomography, Lung ultrasound, Cardiac magnetic resonance, Troponin, Myocardial injury
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the pandemic disease, coronavirus disease 2019 or COVID-19, resulting in important morbidity and mortality worldwide. The risk of fatalities has been shown to be increased by 15% in patients with a previous history of cardiovascular disease (CVD) and/or with CVD risk factors.1,2 Additionally, an increase in troponin above the 99th percentile upper reference limit during the course of COVID-19, suggesting cardiac injury, is associated with higher risk of in-hospital mortality.3 In many countries, the risk of overwhelmed critical care capacity has led to the need to better stratify patient risk and to thereby determine who would benefit most from required intensive therapy and, conversely, those patients best treated conservatively. The interpretation of elevated cardiac troponin levels in COVID-19 is challenging because it may be due to a wide range of cardiac insults, occurring at various stages of the disease, with variable implications for prognosis and clinical decision-making. As a consequence, complementary imaging examinations are frequently requested in order to clarify the diagnosis and prognosis of these patients. Such imaging requests need to be carefully considered in light of the recent EACVI recommendations on cardiovascular imaging during the COVID-19 pandemic. These insisted upon limiting cardiac imaging in COVID-19 patients to only those situation where ‘it is likely to substantially change patient management or be lifesaving’,4 in order to reduce virus transmission, protect heathcare professionals from contamination, and limit consumption of personal protective equipment (PPE). The aim of the present paper is to provide guidance to clinicians regarding the appropriateness of cardiac imaging in COVID-19 patients with suspected or established myocardial injury, and how cardiac imaging has the potential to influence therapy.
Myocardial injury: definition, prevalence, mechanisms, and timing
Definition
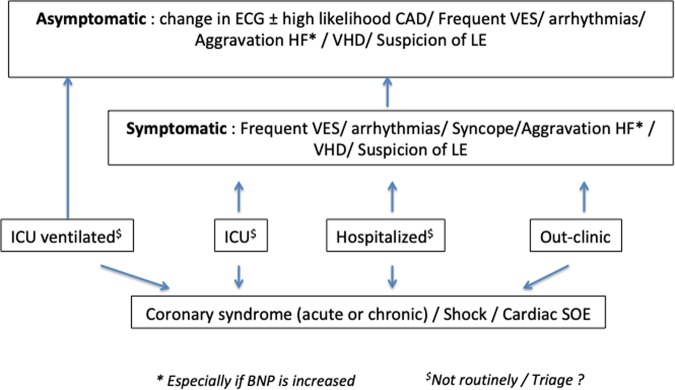
Myocardial injury in the context of COVID-19 is usually defined as an increase of troponin above the 99th percentile of the upper reference limit during the course of the disease. It is difficult to define absolute threshold values due to local variations in the methods used in different laboratories, and in the context of some clinical conditions (i.e. chronic renal failure). The interpretation of increased troponin requires careful integration with a range of other clinical factors [symptoms, history of CVD and other co-morbidities, electrocardiogram (ECG) changes, etc.]. The amplitude of the troponin increase as well as its dynamic nature (e.g. an acute rise and fall vs. more persistent elevations) and comparison with historical measurements may also be informative. A mild increase in troponins is often found in patients with COVID-19 and pre-existing cardiac disease.5 This multiparametric approach will inform indications for cardiac imaging (Figure 1).
Figure 1.
Indications for cardiac imaging in COVID-19 patients with myocardial injury. CAD = coronary artery disease; VES = ventricular extrasystoles; HF = heart failure; VHD = valvular heart disease; LE = lung embolism; ICU = intensive care unit; SOE = source of embolism.
Prevalence of myocardial injury
The prevalence of myocardial injury in COVID-19 is estimated to be between 23% and 28%, but it is difficult to be precise as evidence is based only on previous reports from China, which have limitations. According to these reports, troponin levels were not available in 25–32% of these patients and were probably an overestimate given the potential selection bias as troponin levels were probably requested in those who were more critically unwell or in cases where myocardial ischaemia or myocardial dysfunction was suspected. Patients with high troponin levels showed a higher incidence of complications such as acute respiratory syndrome, malignant arrhythmias, acute renal injury, and acute coagulopathy. The presence of both CVD and elevated troponin was associated with the highest mortality rate, while patients without elevated troponin levels, even in the presence of CVD, had a lower mortality risk.6
Mechanisms
In addition to classic acute coronary syndromes (ACS) and other commonly encountered conditions such as myocarditis, Takotsubo cardiomyopathy, and cardiac dysrhythmia, four putative and direct mechanisms of acute cardiac injury due to COVID-19 have been proposed: (i) viral angiotensin-converting enzyme 2 (ACE-2)-mediated direct damage; (ii) hypoxia-induced myocardial injury; (iii) cardiac microvascular damage due to perfusion defects, vessel hyperpermeability, or angiospasm; and (iv) systemic inflammatory response syndrome including cytokine storm, dysregulated immunocytes, and uncontrolled inflammation.7,8
Timing
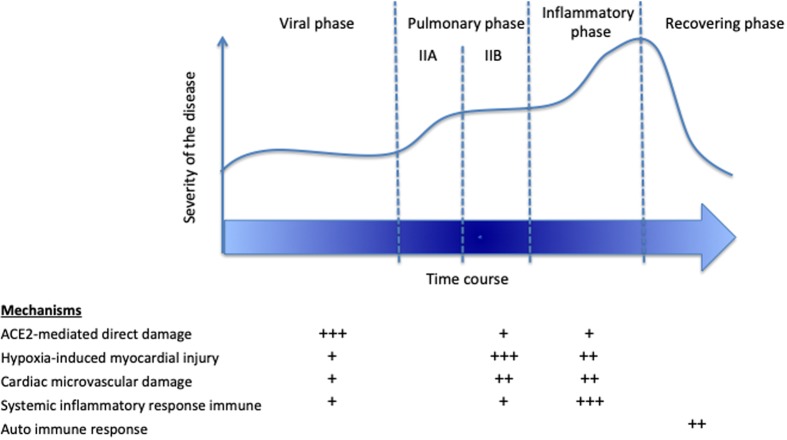
Myocardial injury could be multifactorial and may occur at different phases of the COVID-19 disease, even late after the onset of symptoms9 (Figure 2). In most series, myocardial injury occurs ∼7 days after the onset of symptoms. Depending on the clinical context, the suspected mechanisms, the timing, and the potential management implications, the most efficient imaging modality should be selected. Table 1 summarizes the potential roles of the various imaging modalities according to this proposed approach.
Figure 2.
Additional possible mechanisms to classical coronary syndromes in COVID-19 with myocardial injury.
Table 1.
Role of cardiac imaging modalities in COVID-19 patients with myocardial injury
| High troponin | Echocardiography | Lung ultrasound | CCTA | CTT | CMR | Invasive angio + ventriculo |
|---|---|---|---|---|---|---|
| Suspected CAD | ||||||
| Very low probability | − | − | − | − | − | − |
| Intermediate probability | + | − | ++ | − | + | − |
| Very high probability | − | − | − | − | ++ in high risk patients | |
| Suspected HF/unexplained haemodynamic instability | ++ | ++ | + | − | − | − |
| Ventricular arrhythmias | ++ | − | + | − | + | − |
| Suspected myocarditis | ++ | − | − | − | ++ | − |
| Suspected pericarditis | ++ | − | − | + | + | − |
| Suspected LE | + | − | − | ++* | − | − |
| Suspected SOE | ++ | − | − | + | − | − |
| Suspected IE | ++ | − | − | + | − | − |
The degree of suspicion is related to the timing in the course of COVID-19, the profile of troponin elevation, symptoms, signs, and ECG changes (see text for details).
CAD = coronary artery disease; HF = heart failure; LE = lung embolism; SOE = source of embolism; CCTA, cardiac computed tomography angiography; CTT = thoracic computed tomography; CMR = cardiac magnetic resonance; IE = infective endocarditis.
Myocardial injury: differential diagnoses
A rise in troponin concentration may have a wide range of underlying causes other than ACS and may occur without significant angiographic coronary artery disease (CAD).10 The potential causes are summarized in Table 2. Imaging is well placed to decipher the mechanism of injury in COVID-19 patients, and we here provide guidance to clinicians using different clinical scenarios regarding the indication for cardiac imaging and also which modality to use.
Table 2.
Recognized associations with troponin elevation
| • | Myocardial infarction (MINOCA) |
| • | Heterophile antibodies, such as in rheumatoid arthritis (troponin I) |
| • | Renal impairment (troponin T) |
| • | Congestive heart failure (severe) |
| • | Aortic stenosis |
| • | Aortic dissection |
| • | Severe pulmonary hypertension |
| • | Pulmonary embolism |
| • | Tachycardia with haemodynamic compromise |
| • | Direct injury to the heart (accidental trauma, ablation, cardiac surgery) |
| • | Toxins (e.g. adriamycin, 5-fluorouracil) |
| • | Percutaneous coronary intervention |
| • | Myocarditis, pericarditis, infective endocarditis |
| • | Cerebrovascular accident |
| • | Sepsis, critical illness |
| • | Extensive burns |
| • | Stroke, subarachnoidal haemorrhage |
| • | Prolonged strenuous endurance exercise |
Suspicion of coronary artery disease
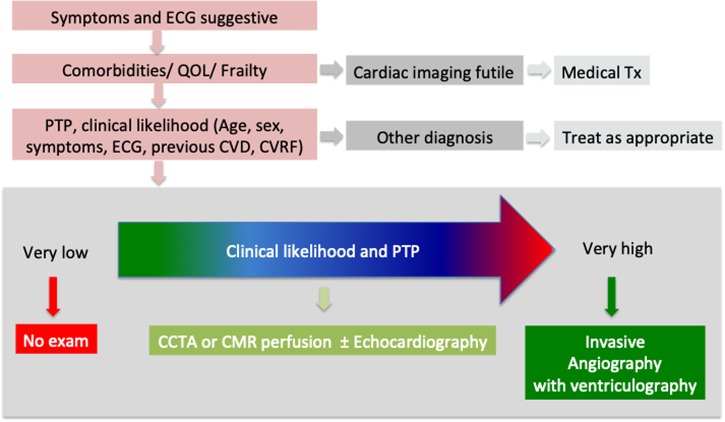
Acute and chronic coronary syndromes may occur in COVID-19 patients. First of all, a high level of comorbidities, poor quality of life, and frailty may render additional examinations futile. The likelihood of CAD based on symptoms, ECG, age, sex, previous history, and CVD risk factors informing the pre-test probability have to be evaluated.11 If the pre-test probability is low and/or an alternative explanation for the troponin release is found, further cardiac imaging is not required. If the suspicion for an ACS is very high and patients are deemed at high risk, an invasive coronary angiogram should be proposed, especially in ST-segment elevation myocardial infarction (STEMI), high-risk non-STEMI, and crescendo angina. In an intermediate risk ACS population, a coronary computed tomography (CCT) angiography is recommended.11 Cardiovascular magnetic resonance (CMR) is a potential alternative. If obstructive CAD is present, revascularization should be discussed, although this may be deferred during the COVID-19 pandemic if symptoms settle and in the absence of high-risk markers.12 In the absence of significant obstructive stenoses, different potential differential diagnoses should be considered, and further investigations and treatments pursued as necessary (see below). A summary of this stepwise approach is provided in Figure 3.
Figure 3.
Stepwise approach in COVID-19 with myocardial injury and suspicion of CAD. QOL = quality of life; Tx = treatment; PTP = pre-test probability; CVD = cardiovascular disease; CVRF = cardiovascular risk factors; CCTA = cardiac computed tomography angiography. Adapted from Knuuti et al.11
Myocarditis is a well-recognized mimic of ACS and remains an important differential diagnosis in the COVID-19 pandemic. Other clinical manifestations ranging from asymptomatic presentations to cardiogenic shock have also been described in COVID-19 patients with suspected myocarditis. Only a few COVID-19 patients with a definitive diagnosis of myocarditis have so far been reported. In some of them, the presence of SARS-CoV-2 in myocardial biopsies has been described;13 in others, no virus was observed, but inflammatory infiltrates were documented.14 During the recovery period, 1 month after the onset of COVID-19 symptoms, a patient presented with acute heart failure with a pattern of ischaemic reperfusion injury pattern at biopsies. It seems that myocarditis represents a heterogeneous condition in COVID-19 patients with different underlying mechanisms to explain the myocardial injury and consequently different conceptual therapeutic approaches: ranging from antiviral therapy to anti-inflammatory treatment. In this context, the role of cardiac imaging in guiding management may be limited (most of the patients also have multiple organ damage, which deserves global treatment),15 although there still remains an important role in diagnosis and the evaluation and monitoring of myocardial function. Echocardiography is the first-line imaging test, that may demonstrate myocardial thickening, wall motion abnormalities, and a pericardial effusion; however, confirmation may require further imaging. In order to identify myocarditis, some have proposed to use a late iodine enhancement or extracellular mapping with CCT to complement CCT angiography (exclusion of CAD).16 The best test for myocarditis is, however, CMR if practical to perform. It may show diffuse myocardial oedema causing pseudo wall hypertrophy, non-infarct patterns of late gadolinium enhancement, and increased signal on short T1 inversion recovery (STIR), T1 mapping, and T2 mapping sequences.17 Pericarditis can also present in COVID-19 patients. Diagnosis should be based on the classic criteria for acute pericarditis,18 the exclusion of CAD, and it should be treated as such if the diagnosis is confirmed.
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is also a possible diagnosis19 and seems to be more frequent in COVID-19.20 In these patients, this may be due to rupture of a non-obstructive plaque or embolus. Continuous ECG monitoring is recommended in such patients taking into account the higher risk for potential life-threatening arrhythmias. Takotsubo or stress cardiomyopathy is reported in COVID-19, associated with a variable degree of ventricular dysfunction. In patients where this is suspected, repeat echocardiography after several weeks to confirm recovery of function should be planned.
CMR is well placed to differentiate myocardial infarction, myocarditis, and stress cardiomyopathy, and should be considered in patients that are well enough to be scanned and in whom establishing a clear diagnosis is of clinical importance. However, CMR protocols should be shortened to focus on addressing the key clinical questions and to avoid long acquisition times that may be challenging for COVID-19 patients. It is acknowledged that not all hospitals treating COVID-19 patients will have easy access to a dedicated CMR service without prolonged transport and, in such circumstances, CMR may be indicated but not practical.
COVID-19 patients with thoracic pain and elevation of troponin due to pulmonary embolism are common. Due to inflammation, a high thrombogenicity is observed in this population, with a frequent concomitant elevation of D-dimer levels. A thoracic computed tomography (CT) should be performed in the case of high suspicion, especially in patients with an unexplained aggravation of the dyspnoea and/or a desaturation. Silent deep venous thrombosis should also be assessed by venous Doppler.
Finally, chest CT (ideally ECG gated) can be used to investigate other causes of thoracic pain, such as acute aortic syndromes, that will still present during the COVID-19 pandemic. Although transoesophageal echocardiography (TOE) is an alternative for these patients, especially if they are critically ill patients or cannot be transported for other reasons, its use should be restricted, given that it is an aerosol-generating procedure. TOE might also be indicated in very rare cases of ACS when ventricular septal rupture or papillary muscle rupture are suspected and not well assessed by transthoracic echocardiography or CT, before emergent surgery, irrespective of COVID-19 status.
Suspicion of heart failure
In COVID-19 patients with potential myocardial injury, symptoms compatible with heart failure are quite challenging to elicit. Dyspnoea is frequent and may be associated with the need for oxygen support. Its origin may be due to the evolution of lung pathology or to the presence of associated myocardial dysfunction. Systemic capillary leak syndrome (SCLS) is a paroxysmal permeability disorder that leads to an abrupt massive shift of fluids and proteins from the intravascular to the interstitial compartment. In some cases of COVID-19, tissue oedema may involve the myocardium and can induce transient myocardial dysfunction, potentially contributing to the pathogenesis of shock.21,22 An increase in myocardial extracellular volume detected using CMR T1 and T2 mapping techniques may serve as a diagnostic marker of SCLS in COVID-19 patients, although, as mentioned, the logistics of CMR scanning need to be carefully considered.23 Pulmonary embolism is quite frequent and may be difficult to diagnose given that in many patients D-dimer is increased in association with systemic inflammation.
In symptomatic patients with high troponin and simultaneous high brain natriuretic peptide (BNP)/N-terminal pro brain natriuretic peptide (NT-proBNP), the diagnosis of heart failure is likely and should be established with a bedside echocardiogram. In the case of low BNP/NT-proBNP, the likelihood of myocardial dysfunction related to myocardial injury is very low and echocardiography should not be performed systematically. Lung echography can also be very helpful in distinguishing between lung and cardiac pathology and can be rapidly performed at the bedside.24 In this setting of COVID-19 with high troponin, patients presenting with shock, signs of acute heart failure, suspicion of right ventricular dysfunction, haemodynamic instability without clear explanation, and cardiac murmur, bedside echocardiography is also indicated. It is clearly helpful for the evaluation of myocardial function, the detection of regional wall contraction abnormalities, acute valvular disease, and for non-invasive haemodynamic assessments. The role of strain imaging is extremely limited due to the moderate quality of echocardiography imaging in these patients and the absence of ECG recordings in most of them. Pulmonary embolism should be excluded in the case of unexplained right ventricular dysfunction or pulmonary hypertension, as indicated in the section on chest pain.
The patient with arrhythmias or syncope
In COVID-19 patients with myocardial injury, the occurrence of ventricular arrhythmias or syncope requires the exclusion of myocarditis, ACS, advanced valve disease, underlying cardiomyopathies, or ventricular dysfunction. The latter may occur secondary to acute (i.e. hydroxychloroquine) or chronic medical treatments (i.e. anthracyclines in oncology patients). Bedside echocardiography is the first-line examination, with CMR and CT performed as required and appropriate, as discussed above.
The asymptomatic patient
In COVID-19, pneumonia often predominates, and cardiac symptoms may be missed or absent. Critically ill intubated patients usually do have not the opportunity to complain. However, routine cardiac imaging is not indicated. In patients with elevated troponins and myocardial injury, imaging may be considered but should not be performed routinely if the ECG is not suggestive of CAD/cardiomyopathy/myocarditis and if the patient is haemodynamically stable. In some rare patients with a previous CVD history, echocardiographic assessments of myocardial function can help risk-stratify patients, and provide guidance for triage.
Late follow-up
In all hospitalized patients with myocardial injury that are finally discharged, careful follow-up encompassing cardiac imaging should be systematically offered to evaluate for residual cardiac damage and dysfunction. Lessons learnt from previous influenza pandemics are that CVD is a major source of complications post-infection.25 Follow-up of COVID-19 patients should ideally be organized when the contamination risk is minimal.
Conclusions
A key message from the recommendations for cardiovascular imaging in COVID-19 was the need to avoid unnecessary cardiac imaging examinations in order to reduce transmission of the virus, protect healthcare professionals, and conserve PPE. In COVID-19 hospitalized patients, myocardial injury represents a frequent request for cardiac imaging, partly because it is known to convey a worse prognosis. The present paper aims to provide guidance to clinicians regarding the appropriateness of cardiac imaging modalities in these patients, and how best to decipher the mechanism of myocardial injury and thereby guide patient management.
Conflict of interest: none to declare; S.E.P. provides consultancy to Circle Cardiovascular Imaging, Inc. (Calgary, Alberta, Canada).
References
- 1. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O.. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 2. Chapman AR, Bularga A, Mills NL.. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation 2020; doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 3. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi:10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo G, Di Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz-Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich-Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T.. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Hear J Cardiovasc Imaging 2020;doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M.. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Hao G.. The role of angiotensin converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. SSRN Electron J 2020;doi: 10.1093/cvr/cvaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakshi TK, Choo MKF, Edwards CC, Scott AG, Hart HH, Armstrong GP.. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002;32:520–525. [DOI] [PubMed] [Google Scholar]
- 11. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 12. Daniels MJ, Cohen MG, Bavry AA, Kumbhani DJ.. Reperfusion of STEMI in the COVID-19 era—business as usual? Circulation 2020;doi/10.1161/CIRCULATIONAHA.120.047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E.. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C.. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruan Q, Yang K, Wang W, Jiang L, Song J.. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esposito A, Palmisano A, Barbera M, Vignale D, Benedetti G, Spoladore R, Ancona MB, Giannini F, Oppizzi M, Del Maschio A, De Cobelli F.. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imaging 2019;12:745–748. [DOI] [PubMed] [Google Scholar]
- 17. Demirkiran A, Everaars H, Amier RP, Beijnink C, Bom MJ, Götte MJW, van Loon RB, Selder JL, van Rossum AC, Nijveldt R.. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. Eur Heart J Cardiovasc Imaging 2020;20:723–734. [DOI] [PubMed] [Google Scholar]
- 18. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: The European Association for Cardio-Thoracic Surg. Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:E891–E908. [DOI] [PubMed] [Google Scholar]
- 20. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS.. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med 2020;doi:10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu MA, Catena E, Cogliati C, Ottolina D, Castelli A, Rech R, Fossali T, Ippolito S, Brucato AL, Colombo R.. Myocardial edema in paroxysmal permeability disorders: the paradigm of Clarkson’s disease. J Crit Care 2020;57:13–18. [DOI] [PubMed] [Google Scholar]
- 22. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ertel A, Pratt D, Kellman P, Leung S, Bandettini P, Long LM, Long LM, Young M, Nelson C, Arai AE, Druey KM.. Increased myocardial extracellular volume in active idiopathic systemic capillary leak syndrome. J Cardiovasc Magn Reson 2015;17:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerin C, Gattinoni L.. Assessment of oxygenation response to prone position ventilation in ARDS by lung ultrasonography. Intensive Care Med 2016;42:1601–1603. [DOI] [PubMed] [Google Scholar]
- 25. Madjid M, Miller CC, Zarubaev V V, Marinich IG, Kiselev OI, Lobzin YV, Filippov AE, Casscells SW 3rd.. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. Eur Heart J 2007;28:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]