Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes the respiratory illness coronavirus disease 2019 (COVID-19) that has infected millions of people worldwide. Soon after detection of spread of SARS-CoV-2 in the United States (US), focus was on developing molecular nucleic acid detection tests (real-time reverse transcriptase polymerase chain reaction [RT-PCR]) for early diagnosis of infection in symptomatic patients, patients with known exposure, and patients who are at risk. Molecular testing is ramping up all over the US, and more than 6.5 million people have been tested by the end of April 2020.1 Different parts of the US have been hit with varying intensity. New York State has been hit hardest, with more than 300,000 confirmed cases and many more presumed to be infected and with a prevalence of COVID-19 expected to be as high as 10% to 15%, similar to that seen in Italy, Spain, and some parts of China.2 Serologic studies in other parts of the US that have a lower COVID-19 attack rate have shown a lower prevalence (<1%-3%).3 With early signs of “flattening of the curve,” as a result of more people adhering to social distancing and staying at home, the focus has now shifted to widespread antibody (serologic) testing of the population.
Antibody tests are blood tests that detect antibodies or immunoglobins (Ig) that are produced as human immune response to SARS-CoV-2 infection. A positive result suggests that the individual has potentially been exposed to SARS-CoV-2. When IgM antibodies are present, they can indicate an active or recent infection. IgG antibodies show up later in infection and can often indicate a past infection but does not exclude recently infected patients who can still be contagious, especially when IgM antibodies are also concurrently detected. For viral infections IgG antibodies usually persist longer than IgM antibodies and provide immunity from reinfection, but this is not known for COVID-19 yet.4 Antibody tests are being developed to detect IgG only, both IgG and IgM, or total antibodies.
The Trump administration and the media have been promoting antibody tests as a screening tool to allow individuals with positive results to get back to work and open our economy. The assumption is that the individuals with positive antibody tests have recovered from COVID-19 (symptomatic or asymptomatic) infection and have developed immunity to the virus. Furthermore, it is assumed that such individuals are thus no longer susceptible to infection and can return to work safely without fear of getting infected or spreading the infection. However, to be used as a robust and successful screening tool, an antibody test should have a high positive predictive value (PPV), ie, positive results can be trusted as true positive with confidence. PPV is dependent on the accuracy of the test (sensitivity and specificity) and the prevalence of disease in the population and can be calculated by using the following formula:
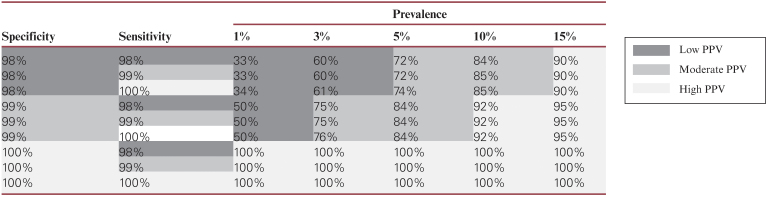
As of April 30, 2020, 10 antibody tests have been approved by the US Food and Drug Administration (FDA) under emergency use authorizations. Average sensitivity and specificity of FDA-approved antibody tests is 84.90% and 98.63%, respectively. The details of FDA-approved tests are shown Table 1.5 Approximately 90 antibody tests offered by various manufactures are still under FDA review but are available in the market, without established sensitivity and specificity, being used in hospitals and clinics as a screening tool. Given variable prevalence of COVID-19 (1%-15%) in different parts of the US and differences in performance characteristics of antibody tests (FDA approved and unapproved), statistically the PPV will vary widely and can be as low as 30% to 50% in areas with low prevalence Table 2.
Table 1.
Manufacturers and Details of Antibody Tests Approved by US Food and Drug Administration as of April 30, 2020
| Manufacturer | Test Type | Antibodies Detected | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| Cellex | Rapid test, LFIA | IgM and IgG | 93.75 | 96 |
| Ortho Clinical Diagnostics | ELISA | Total antibody (IgM and IgG) | 83.33 | 100 |
| Chembio Diagnostic System | Rapid test, LFIA | IgM and IgG | 93.55 | 93.95 |
| Mount Sinai Laboratory | ELISA | IgG only | 92.50 | 100 |
| Autobio Diagnostics | Rapid test, LFIA | IgM and IgG | 88.15 | 99.04 |
| DiaSorin | CLIA | IgG only | 70.90 | 99.27 |
| Ortho Clinical Diagnostics | ELISA | IgG only | 87.50 | 100 |
| Abbott Laboratories | CMIA | IgG only | 89.34 | 99.63 |
| Bio-Rad Laboratories | ELISA | Total (IgM, IgG, and IgA) | 92.16 | 99.56 |
| Wadsworth Center, New York State Department of Health | CMIA | Total (IgM, IgG, and IgA) | 57.84 | 98.85 |
| Average | 84.90 | 98.63 |
CLIA, chemiluminescent immunoassay; CMIA, chemiluminescent microparticle immunoassay; ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunoassay.
Table 2.
Difference in Positive Predictive Value (PPV) of Antibody Tests With Variations in Prevalence of COVID-19 and Accuracy (Sensitivity and Specificity) of Antibody Tests
The antibody tests can be a reliable screening tool in areas with high prevalence of COVID-19, such as the New York City tristate area (New York, New Jersey, and Connecticut) and Massachusetts. However, in areas with lower prevalence, the antibody tests will need to have high accuracy (100% specificity) and consistent performance. This can be hard to achieve when there is limited availability of FDA-approved antibody tests and many hospitals and clinics are using FDA unapproved tests. Prematurely promoting antibody tests as a screening tool all over the US will give individuals, who test positive and are not actually immune to COVID-19, a false sense of protection. These individuals can still get infected and further spread the infection, potentially leading to a second wave of SARS-CoV-2 infections.
Antibody tests are still essential and accurate tests should be developed, as they are critical to diagnose certain cases (negative molecular tests in patients presenting late in illness), identify asymptomatic infections, determine the seroprevalence in a given population, and track progression towards herd immunity over a longer period of time. Antibody tests can also be used along with molecular tests for contact tracing. All these initiatives will help expedite reopening of the economy and returning the US population back to a “new” normal.
References
- 1. Centers for Disease Control and Prevention. Summary of laboratory testing results reported to CDC. COVIDView Week 17, updated May 1, 2020.https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html#virus. Accessed May 3, 2020.
- 2. Signorelli C, Scognamiglio T, Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020;91:175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. MedRxiv. 2020.04.14.20062463; doi: 10.1101/2020.04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Serology testing for COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/lab/serology-testing.html. Accessed May 3, 2020.
- 5. US Food and Drug Administration. Emergency use authorizations. Updated May 1, 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd. Accessed May 3, 2020.