Abstract
Background
Few pediatric cases of coronavirus disease 2019 (COVID-19) have been reported and we know little about the epidemiology in children, although more is known about other coronaviruses. We aimed to understand the infection rate, clinical presentation, clinical outcomes, and transmission dynamics for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in order to inform clinical and public health measures.
Methods
We undertook a rapid systematic review and narrative synthesis of all literature relating to SARS-CoV-2 in pediatric populations. The search terms also included SARS-CoV and MERS-CoV. We searched 3 databases and the COVID-19 resource centers of 11 major journals and publishers. English abstracts of Chinese-language papers were included. Data were extracted and narrative syntheses conducted.
Results
Twenty-four studies relating to COVID-19 were included in the review. Children appear to be less affected by COVID-19 than adults by observed rate of cases in large epidemiological studies. Limited data on attack rate indicate that children are just as susceptible to infection. Data on clinical outcomes are scarce but include several reports of asymptomatic infection and a milder course of disease in young children, although radiological abnormalities are noted. Severe cases are not reported in detail and there are few data relating to transmission.
Conclusions
Children appear to have a low observed case rate of COVID-19 but may have rates similar to adults of infection with SARS-CoV-2. This discrepancy may be because children are asymptomatic or too mildly infected to draw medical attention and be tested and counted in observed cases of COVID-19.
Keywords: coronavirus, SARS-CoV-2, COVID-19, children, infection
Initial reports from China observed low rates of COVID-19 in children compared with adults. Emerging evidence suggests that children may be infected at the same rate as adults but are more likely to experience asymptomatic or mild disease.
(See Editorial Commentary by Creech on pages 2480–1.)
In December 2019, reports emerged of a cluster of cases of pneumonia of unknown cause in Wuhan City, China, culminating in the identification of a novel coronavirus on 12 January 2020, denoted as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the associated disease as coronavirus disease 2019 (COVID-19) [1]. The virus spread rapidly around the world and was declared a pandemic by the World Health Organization (WHO) on 11 March 2020 [2], with many countries adopting unprecedented public health measures to curb its spread.
Coronaviruses are large, lipid-enveloped, single-stranded RNA viruses found in avian and mammalian species. Human coronaviruses commonly cause mild upper respiratory tract infections, accounting for approximately 30% of common colds, although instances of severe disease are described in the elderly, children, and immunocompromised hosts [3]. In addition to SARS-CoV-2, there are 2 other notable exceptions: severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). An epidemic of SARS-CoV in 2003 affected 26 countries and resulted in more than 8000 cases and 774 deaths [4]. MERS-CoV was first reported in 2012 and is now endemic at low levels in the Persian Gulf region, with notable nosocomial outbreaks but otherwise limited human–human transmission [5]. As of 19 November 2019, WHO has reported 2494 cases and 858 deaths [5]. The respective R0 values for SARS-CoV, MERS-CoV, and SARS-CoV-2 (outside nosocomial outbreaks) are 3 [4], <1 [6], and 2–3 [7].
Social-distancing policies have been widely adopted in many countries to limit spread of SARS-CoV-2, at great economic and social cost. Social-distancing policies that apply to children, such as school closures, may have an important role in mitigating the spread of pandemics; for many infectious diseases, such as influenza, children are known to drive transmission in households and communities. However, early reports of SARS-CoV-2 [1], as well as MERS-CoV and SARS-CoV, suggest that children are less likely to be infected and to develop serious disease compared with adults.
We undertook a narrative review of the emerging literature on SARS-CoV-2 to summarize current understanding of the epidemiology and transmission dynamics of this coronavirus in children, in order to inform decisions regarding clinical and public health measures for children in the United Kingdom.
METHODS
We searched MEDLINE, MedRxiv, and the COVID-19 literature resources of WHO and major journals for all English-language papers concerning SARS-CoV, SARS-CoV-2, or MERS-CoV in children up to 9 March 2020.
The MEDLINE search used the following search terms: (“coronavirus OR Severe acute respiratory syndrome OR covid-19 OR nCoV OR COVID OR SARS OR MERS OR middle east respiratory syndrome) AND (Child OR Children OR childhood OR preschool OR infant OR babies OR baby OR neonates OR pediatrics OR pediatric OR pediatrics OR pediatric”). The MedRxiv search used the term “coronavirus.” We searched the WHO COVID-19 literature database using the terms “child, children, childhood, infant, baby, babies, pediatric, pediatric.” To account for papers not yet indexed in databases, we also hand-searched COVID-19 resource centers from the following journals and publishers, by manually trawling the collections for relevant titles: BMJ, Cambridge University Press, Elsevier, JAMA Network, The Lancet, New England Journal of Medicine, Oxford University Press, PLOS, Springer Nature, SSRN (reprints), Wiley.
Inclusion criteria were any study or article that included information about infection risk, transmission, or severity in children (aged <18 years) for SARS-CoV-2. All types of studies and countries of origin were eligible for inclusion. Our broader search also identified papers relating to SARS-CoV and MERS-CoV, to allow for informative comparison, although this was not the focus of the review. We excluded articles that exclusively reported data on adults or other respiratory viruses, that did not relate to the clinical or epidemiological aspects of interest, and that were not written in the English language. Eight reviewers extracted data on infection risk, transmission, and clinical presentation and outcomes into a cloud-based spreadsheet (Google Sheets). We also recorded the case definition, the type of study or report, and important limitations, although quality was not formally assessed, and findings were synthesized narratively. As this was a rapid review, each paper was reviewed by one reviewer.
RESULTS
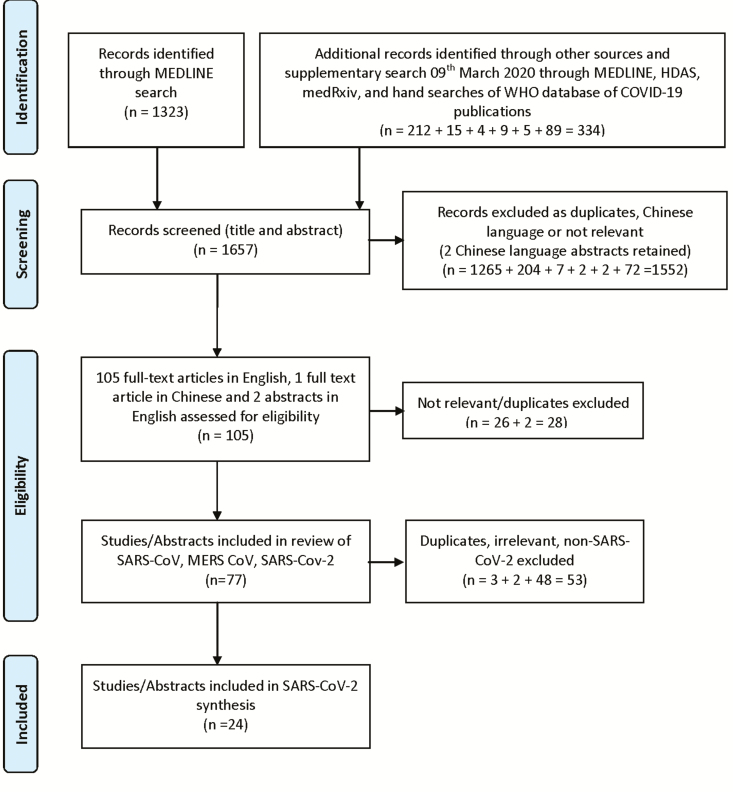
Our search retrieved 1657 records, of which 1552 were excluded via title and abstract screening. A total of 105 full articles were assessed for eligibility and 77 were identified relating to SARS-CoV, MERS-CoV, and SARS-CoV-2. Of these, 24 papers relating to SARS-CoV-2 were identified, from which data were extracted and synthesized (see Figure 1). Sixteen of the included papers were preprints or reports not certain to have been subjected to peer review. All COVID-19 papers drew heavily or exclusively on data and experience from China. A summary of the included papers is provided in Table 1.
Figure 1.
PRISMA 2009 flow diagram [13]. Abbreviations: COVID-19, coronavirus disease 2019; HDAS, Healthcare Databases Advanced Search; MERS-CoV, Middle East respiratory syndrome coronavirus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Table 1.
Summary of Reviewed Papers
| Date; Title of Paper; Author(s) | Study Type and Synopsis | What Does the Paper Tell Us? | |
|---|---|---|---|
| 1 | 30 January 2020; Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement; Shen K et al | Expert consensus statement. Peer reviewed (World Journal of Pediatrics). Development of expert consensus statement on the diagnosis, treatment and prevention of COVID-19 infection in children. | Evidence of infection: Since the outbreak of 2019 novel coronavirus infection (COVID-19) in Wuhan City, China, by 30 January 2020, a total of 9692 confirmed cases and 15 238 suspected cases have been reported. Of these, 28 were children aged 1 month to 17 years (0.29% of confirmed cases). Clinical presentation: Most identified infected children have mild clinical manifestations. They have no fever or symptoms of pneumonia with a good prognosis. Most of them recover within 1–2 weeks after disease onset. Few may progress to lower respiratory infections. Transmission: The majority of cases had close contact with infected cases or were part of a family cluster. Some children “appeared” asymptomatic. This paper regards “silent infection” as those individuals who test positive for coronavirus but have no apparent symptoms. |
| 2 | [Date unspecified] February 2020; Impact assessment of non- pharmaceutical interventions against COVID-19 using influenza transmission as proxy in Hong Kong, February 2020 an observational study; Cowling B et al | Observational modeling study. Not peer reviewed (draft manuscript as of 6 March 2020). Examination of influenza transmission after implementation of control measures and changes in population behaviors in Hong Kong in late January 2020 as a proxy for COVID-19. | Likelihood of infection: No direct evidence of infection in children. Clinical presentation: No direct clinical outcomes recorded. Transmission: No data on transmission of COVID-19. Data on influenza activity as a proxy. There was a 44% reduction in transmissibility in the community (95% CI, 34–53%) and 33% reduction in transmissibility based on pediatric hospitalisation rates (95% CI, 24–43%) following school closures. |
| 3 | 2 February 2020 (Epub ahead of print on 05/02/2020); Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus; Chen ZM et al | Consensus guidelines. Peer reviewed (World Journal of Pediatrics). Expert consensus guidelines developed to standardize the protocol for respiratory infection in children caused by COVID-19 | Evidence of infection: between December (date unspecified) 2019 and 31 January 2020 >20 pediatric cases have been reported in China (including 10 in Zhejiang Province), aged 112 days–17 years. Clinical presentation: At the onset of the disease, infected children mainly present with fever, fatigue and cough which may be accompanied by nasal congestion, runny nose, expectoration, diarrhea and headache. Most children had low to moderate or no fever. Most have good prognosis and in mild cases recover 1–2 weeks after disease onset. No deaths in children. Dyspnea, cyanosis, and other symptoms can occur as the condition progresses, usually after 1 week of the disease, accompanied by systemic toxic symptoms such as malaise, restlessness, poor feeding, appetite, and reduced activity. Transmission: No data reported on transmission. Children mainly belong to family cluster cases. |
| 4 | 5 February 2020; Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue; Shen KL, Yang YH | Opinion piece. Peer reviewed (World Journal of Pediatrics). Editorial on initial 28 pediatric cases reported. | Evidence of infection: 28 confirmed pediatric cases. Several had no obvious clinical symptoms at time of diagnosis, found by screening and CXR suggestive of pneumonia. Clinical presentation: If symptomatic, usually presented with fever, dry cough, fatigue, nasal congestion, runny nose and GI symptoms. Mostly mild symptoms. All had good prognosis and recovered within 1–2 weeks. Only “a few” had lower respiratory tract infections. No severe cases or deaths reported in pediatric population. Transmission: No direct evidence of transmission. All cases were part of familial clusters or close contact history. |
| 5 | 7 February 2020; A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units; Wang J et al | Opinion piece. Peer reviewed (Lancet Child and Adolescent Health). Expert consensus of a contingency plan for the COVID-19 outbreak in neonatal intensive care units, focused on diagnostic and discharge criteria, treatment, prevention, and control strategies. | Evidence of infection: By 5 February 2020, the number of confirmed cases had exceeded 20 000. About 100 children were affected, with the youngest being 30 hours after birth. Clinical presentation: Most adults or children presented with mild flulike symptoms. Disease severity: Concern discussed that neonates might be more susceptible to the virus due to immature immune systems. Advises infected mothers not to breastfeed. Transmission: No direct report of transmission. |
| 6 | 11 February 2020; 2019-nCoV: polite with children! Caselli D, Aricò M. | Opinion piece. Unclear whether peer reviewed. Expert review of selected studies of SARS, MERS, and case study of COVID- 19 data in pediatrics. | Evidence of infection: Report 1 case of an asymptomatic child who tested positive to COVID-19. Clinical presentation: Children are at minimal risk to develop new disease and virtually no risk of a fatal course. SARS data identifies 0% fatality rate in those <18. In MERS data, a 2% fatality rate in children. Transmission: None reported. |
| 7 | 14 February 2020; Novel coronavirus infection in hospitalized infants under 1 year of age in China; Wei M et al | Case series. Unclear whether peer reviewed. Case reports of all infected infants in China. Description of demographic, epidemiologic, and clinical features. | Evidence of infection: Nine infected individuals identified. Clinical presentation: 8 of 9 infants symptomatic. Four had fever, 2 had mild upper respiratory tract symptoms. No ICU, no death, no severe complications. Transmission: All 9 infants had at least 1 adult family member infected with COVID-19. One identified on contact tracing. Seven infants were reported to be either living in Wuhan or having family members who visited Wuhan, 1 had no direct linkage to Wuhan, and 1 had no information available. |
| 8 | 14 February 2020; Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)— China, 2020; The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team | Observational study. Unclear whether peer reviewed. Retrospective cohort, 72 314 cases of COVID-19, all cases identified through China’s Infectious Disease monitoring system up to 2 February 2020. | Evidence of infection: Out of 72 314 cases, 416 (0.9%) were aged 0–9 years and 549 (1.2%) were aged 10–19 years. Clinical presentation: None presented. The case-fatality rate in 0–9 years, 0/416; 10–19 years, 1/549 (0.2%). Severity not reported by age group. For all cases, 80.9% had mild illness. Transmission: None reported. |
| 9 | 16 February 2020; Analysis of CT features of 15 children with 2019 novel coronavirus infection; Feng K et al | Case series—full text only available in Chinese. Unclear whether peer reviewed. A retrospective analysis was performed on clinical data and chest CT images of 15 children diagnosed with COVID-19. Among the 15 children, there were 5 males and 10 females, aged from 4 to 14 years old. | Evidence of infection: 15 confirmed cases of COVID-19, aged from 4 to 14 years old. Clinical presentation: Five of the 15 children were febrile and 10 were asymptomatic on presentation. For their first chest CT images, 6 patients had no lesions, while 9 patients had pulmonary inflammation lesions. Seven cases of small nodular ground- glass opacities and 2 cases of speckled ground-glass opacities were found. Transmission: None reported. |
| 10 | 19 February 2020; Asymptomatic cases in a family cluster with SARS-CoV-2 infection; Pan X et al | Case report. Peer reviewed (The Lancet Infectious Diseases). Case study of clinical characteristics of family cluster of SARS-CoV-2 (mother, 33; father, 35; 3-year-old boy). | Evidence of infection: 1 child in family cluster tested positive for COVID-19. Clinical presentation: Child was asymptomatic. Transmission: No data reported on transmission. |
| 11 | 20 February 2020; Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population- level observational study; Sun K et al | Observational study. Peer reviewed (The Lancet Digital Health). Population-level observational study using data from a healthcare-oriented social network currently streaming news reports on COVID-19 from local and national Chinese health agencies. Trends in the epidemiology of COVID-19 including outbreak progression across China are assessed. | Evidence for infection: Data for 507 patients with COVID-19 reported. Few patients (13, 3%) were younger than 15 years. Age profile of Chinese patients adjusted for baseline demographics confirmed a deficit of infections among children. Relative risk of <0.5 in the under-15-year-olds. Clinical presentation: No data on clinical presentation. No mortality in children. Transmission: None reported. |
| 12 | 25 February 2020; Are children less susceptible to COVID-19? Lee P et al | Opinion piece. Not peer reviewed (Journal preproof). Describes Chinese CDC data, compares infection rates in other pandemics and infections. Speculates as to differences in immune systems and viral receptors by age. | Clinical presentation: None reported. Transmission: No direct evidence of transmission. Authors suggest that lower infection rates in children may be due to them undertaking less international travel and outdoor activities. |
| 13 | 25 February 2020 (medRxiv preprint); Epidemiological characteristics of 1212 COVID-19 patients in Henan, China; Wang P et al | Observational study. Not peer reviewed (medRxiv preprint). Cross-sectional analysis of publicly available data. | Evidence of infection: Out of 1212 COVID-19 there were 24 cases aged 0–10 years (1.98%) and 21 cases aged 11–20 years (1.73%). Clinical presentation: None reported. Transmission: None reported. |
| 14 | 26 February 2020; Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults; Xia W et al | Case series. Peer reviewed (Pediatric Pulmonology). Case series of 20 pediatric patients from Wuhan’s Children’s Hospital, presenting 23 January to 8 February 2020; primarily aimed at description of radiological features. | Evidence of infection: All cases confirmed by pharyngeal swab COVID-19 nucleic acid test. Seven of 20 had underlying conditions (congenital). Clinical presentation: None reported. Noted that 18 of 20 had recovered. Mean length of hospital stay of 13 days. Transmission: No evidence that children transmitted the virus. Thirteen of 20 cases had familial contacts with COVID-19. |
| 15 | 28 February 2020 Report of the WHO- China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16–24 February 2020; WHO | Consensus report. Unclear whether peer reviewed. The Joint Mission consisted of 25 national and international experts over a 9-day period and included workshops and visits to regions around China. | Likelihood of infection: A low attack rate in children was presented (2.4% of all cases). In the absence of serological studies, it is not possible to determine the extent of infection among children. In the included data, infected children were largely identified through household contact tracing of adults. Clinical presentation: No data were presented on clinical outcomes in children. A very small proportion of those aged under 19 years have developed severe (2.5%) or critical disease (0.2%). Transmission: No data were presented on transmission of illness in children. Of note, people interviewed by the Joint Mission Team could not recall episodes in which transmission occurred from a child to an adult. |
| 16 | 1 March 2020 Coronavirus disease (COVID-19) and neonate: what neonatologist need to know; Lu Q, Shi Y | Expert review. Peer reviewed (Journal of Medical Virology). Review of reported case characteristics. | Evidence of infection: From first confirmed child case on 20 January 2020 to 6 February 2020 at least 230 COVID-19 cases in children (≤18 years) have been reported in China. Clinical presentation: SARS-CoV-2 infection can range from asymptomatic infection to severe respiratory distress in neonate and children; respiratory distress occur in children with underlying conditions. The 3 newborns identified had short breath, vomiting of milk, cough, and fever. Vital signs of those neonates were stable. Transmission: There is currently no evidence that SARS-CoV-2 can be transmitted transplacentally from mother to the newborn. |
| 17 | 2 March 2020; Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China; Wang D et al | Case series. Unclear whether peer reviewed. Case series in 31 children aged 6 months to 17 years confirmed with COVID-19 infection. Describes epidemiological history, clinical manifestations, treatment, and the short-term prognosis. | Evidence of infection: Among the 31 children, 28 patients (90%) were family cluster cases. Nine cases (29%) were imported, 21 cases had contact with confirmed infected adults. One case (3%) had contact with asymptomatic returnees from Wuhan. Clinical presentation: Common symptoms were fever (n = 20, 65%), including 1 case of high fever, 9 cases of moderate fever, 10 cases of low fever. Fever lasted from 1 day to 9 days. The fever of 15 cases lasted for ≤3 days, while in the other 5 cases lasted >3 days. Other symptoms included cough (n = 14, 45%), fatigue (n = 3, 10%) and diarrhea (n = 3, 9%). Pharyngalgia, runny nose, dizziness, headache, and vomiting were rare. The clinical types were asymptomatic type in 4 cases (13%), mild type in 13 cases (42%), and common type in 14 cases (45%). No severe or critical types were identified. Among them, 24 children (77%) recovered and were discharged from hospital (unclear if remaining were affected at time of publication or had chronic issues). No death occurred. Transmission: None reported. |
| 18 | 3 March 2020; Coronavirus disease-19 among children outside Wuhan, China; Chuming C et al | Case series. Not peer reviewed (manuscript draft). Prospective follow-up of 31 confirmed cases <18 years of age with SARS-CoV-2 infection in Shenzhen Third People’s Hospital between 16 January and 19 February 2020. | Evidence of infection: All 31 (7.9%) child cases of 291 cases were confirmed as having SARS-CoV-2 Clinical presentation: 12 (38.7%) children had no clinical symptoms, the other 2/3 children had mild cases (no severe cases in children). Most of the children did not have underlying conditions (2 [6.5%] patients had underlying diseases, one of which had asthma, and the other had duplicate kidneys.) Transmission: 29 (93.5%) of the children were in familial clusters. |
| 19 | 4 March 2020; Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts; Bi Q et al | Observational study—full text only available in Chinese. Not peer reviewed (preprint). Shenzhen CDC identified 391 SARS-CoV-2 cases from 14 January to 12 February 2020 and 1286 close contacts. Cases identified through symptomatic surveillance were compared to those identified via contact tracing. | Evidence of infection: The household secondary attack rate was 15%, and children were as likely to be infected as adults. Children reported to be similar risk of infection as the general population. Clinical presentation: Children were reported to have less severe symptoms than adults. Transmission: None reported. |
| 20 | 4 March 2020; Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children; Liu F et al | Case reports. Not peer reviewed (manuscript draft). Clinical and CT data of 59 patients with COVID-19 from 27 January to 14 February 2020 were retrospectively reviewed, including 14 laboratory-confirmed nonpregnant adults, 16 laboratory-confirmed and 25 clinically diagnosed pregnant women, and 4 laboratory-confirmed children. | Evidence of infection: Four laboratory-confirmed cases in children. Clinical presentation: None reported. Imaging had fully resolved in 3 out of the 4 children. Transmission: None reported. |
| 21 | 5 March 2020; Clinical characteristics of COVID-19 in children compared with adults outside of Hubei Province in China; Du W et al | Case series. Not peer reviewed (manuscript draft). Retrospective case series of 67 consecutive hospitalized confirmed cases including 14 children with COVID-19, 23 January to 15 February 2020. | Evidence of infection: There were 14 children confirmed cases among the 67 cases, with a median age of 6.2 years (range, 0–16 years). Clinical presentation: 3 cases (21.4%) of the mild type and 11 cases (78.6%) of the conventional type. No severe or critical cases. Diagnostic criteria for mild cases: mild clinical symptoms, no radiographic findings of pneumonia. Diagnostic criteria for common cases: fever, respiratory symptoms, and radiographic manifestations of pneumonia. Transmission: All the cases in children were familial clusters. |
| 22 | 6 March 2020 (medRxiv preprint); Preliminary epidemiological analysis on children and adolescents with novel coronavirus disease 2019 outside Hubei Province, China: an observational study utilizing crowdsourced data; Henry BM, Santos de Oliveira MH | Observational study. Not peer reviewed (medRxiv preprint). An observational study utilizing crowdsourced data outside of Hubei province (ie, includes mainland China minus Hubei and rest of the world). Defined pediatric cases as patients <19 years of age with a laboratory-confirmed diagnosis. | Evidence of infection: A total of 82 patients were included. Fifty- three children were aged between 0–12 years and 27 adolescents were between 13–19 years. Limited evidence available. Clinical presentation: When clinical features were reported, fever was the most common presentation (68%) followed by cough (36%). Two (8.0%) were asymptomatic. Transmission: A total of 29 (35.4%) patients were noted to have an infected family member. |
| 23 | 6 March 2020 (medRxiv preprint); Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China; Qiu C et al | Case series. Not peer reviewed (medRxiv preprint). Contact investigation was conducted on each patient who admitted to the assigned hospitals in Hunan Province (geographically adjacent to Wuhan) from 22 January to 12 February 2020. | Evidence of infection: 3 children within the 104 confirmed cases. Clinical presentation: No data reported. Transmission: Asymptomatic transmission exists (but example given was adult giving it to their child and to their parent). Family clusters were the major body of patients, with transmission along 3 generations within some families (although direction of transmission not specified). |
| 24 | 10 March 2020 (medRxiv preprint); Data-driven discovery of clinical routes for severity detection in COVID-19 pediatric cases; Yu H et al | Case series. Not peer reviewed (medRxiv preprint). Analysis of 105 cases of COVID-19 in children diagnosed between 1 February to 3 March 2020 from Wuhan Children’s Hospital, Tongji Medical School, Huazhong University of Science and Technology, Wuhan (the sole designated hospital in Wuhan for COVID-19 child patients). | Evidence of infection: 105 cases in children reported. Clinical presentation: Of the 105, 64 were male and 41 were female. Clinical symptoms including shortness of breath, assisted respiration, apnea, cyanosis, dehydration, and progressive increase of lactate were noted. Disease severity: Of the 105 cases, 8 were critically ill. Transmission: None reported. |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest X-ray; ICU, intensive care unit; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
Likelihood of Infection
Children are affected by COVID-19, but extant data (largely case series from China) suggest they are less likely to be affected than adults. A national analysis of the first 72 314 cases in China was reported up to 11 February 2020. Cases included 61.8% laboratory-confirmed, 22.4% suspected, 14.6% clinically diagnosed, and 1.2% asymptomatic cases. They reported 419 cases (0.9% of all cases) in children 0–9 years old and 549 (1.2%) in children aged 10–19 years old. A case series of all 1212 confirmed cases in Henan province, China, from 24 January 2020 to 14 February 2020, reported a similarly low proportion of affected children [8]. Among cases with data on age (n = 1156), 24 (2.1%) cases were in children aged 0–10 years old and 31 cases (2.7%) in children aged 11–20 years old. The youngest patient identified was 28 days old, with several cases reported in neonates [9]. The Report of the WHO-China Joint Mission on COVID-19 [7] concludes that “we cannot determine the extent of infection among children, what role children play in transmission, whether children are less susceptible or whether they simply present differently.”
In an early epidemiological analysis of 507 COVID-19 cases, mainly from China, 13 (2.6%) were younger than 15 years, and the relative risk of COVID-19 in children was less than 0.5 compared with the population average [10]. In contrast, a follow-up study of 1286 close contacts of 391 confirmed cases reported in Shenzhen province, China, reported an infection rate (7.4%) in children under 10 years of age (n = 148) and aged 11–19 years (7.1%) (n = 84) similar to the population average (7.9%). The close-contact cases among children were less likely to be severe and less likely to present with fever compared with adults, demonstrating how milder clinical presentation may lead to undercounting of cases [11].
Clinical Presentation
Symptoms of COVID-19 are nonspecific and disease presentation ranges from asymptomatic to severe pneumonia and death [7]. Evidence so far suggests a milder course of disease in children and some asymptomatic cases, but with evidence of radiological lung changes in both categories. Cai et al [12] reported a case series of 10 pediatric patients (aged 3 months–10 years) with confirmed SARS-CoV-2 infection from 4 cities in China in February 2020. The symptoms reported were fever (8 patients), cough (6), sore throat (4), nasal congestion (3), and sneezing and rhinorrhea (2). None of the patients had dyspnea or diarrhea during the illness, and fever resolved after 24 hours. Chest X-ray revealed unilateral patchy infiltrate in 4 of 10 patients. Wei et al [9] identified all hospitalized infants in China diagnosed with COVID-19 infection between 8 December 2019 and 6 February 2020. Nine infected infants were identified (age range, 1–11 months) with symptoms reported as fever (4 infants), mild upper respiratory tract symptoms (2), no symptoms (1), and 2 with no information on symptoms available. None required intensive care or mechanical ventilation.
Asymptomatic infection in children is reported in 14 individual cases, reported in 5 separate papers [9, 14-17]. Notably, in an early report [14] of a familial cluster in China, an asymptomatic 10-year-old boy was screened as part of the family cluster and chest computed tomography (CT) revealed radiological ground-glass lung opacities. In Singapore, a well 6-month-old infant with COVID-19 and high viral load was detected as part of a cluster and was also asymptomatic throughout admission [16]. Asymptomatic cases are also reported in the English abstract of a Chinese paper, which reports clinical data and CT chest images of 15 children (aged 4–14 years old) diagnosed with COVID-19 in Shenzhen. Five children were febrile and 10 asymptomatic on first visit. The abstract reports radiological changes that are difficult to interpret: “For their first chest CT images, six patients had no lesions, while nine patients had pulmonary inflammation lesions. Seven cases of small nodular ground glass opacities and two cases of speckled ground glass opacities were found” [17].
Clinical Outcomes
While most symptomatic cases in children are described as mild, severe cases of the disease have been reported. The Report of the WHO-China Joint Mission on COVID-19 stated that 2.5% of the cases among those under 19 years old were severe and 0.2% were critical [7]. Among the 105 children admitted to Wuhan’s Children’s Hospital, the sole hospital for treating COVID-19 in children in Wuhan, 8 (7.6%) were reported to have severe disease [18]. Among the large analysis of 72 314 cases from China, there were no deaths reported among the 416 reported cases in children aged 1–9 years and a single death among the 549 cases in children aged 10–19 years. This equates to a case-fatality rate of 0.2% in those aged 10–19 years [19].
Comorbidities
There is a suggestion that children with underlying conditions are at greater risk of infection or more severe disease, although the evidence is limited and mixed. Among 31 pediatric cases of SARS-CoV-2 (12 asymptomatic and 19 described as mild) outside Wuhan, only 2 were reported to have underlying conditions (1 had asthma, 1 had a duplicate kidney) [20]. Among a series of 20 pediatric patients treated at Wuhan Children’s Hospital, 7 were reported as having underlying conditions, although these were not specified [21]. There were no data on underlying conditions from the larger cases series of children from Wuhan Children’s Hospital (n = 105) [18]. While underlying conditions were noted to be an important risk factor in the overall population (particularly respiratory and cardiovascular disease), no pediatric-specific data on underlying conditions were presented in the major analyses of Chinese data [7, 19].
Transmission
There is limited evidence relating to transmission of SARS-CoV-2 by children. Many of the childhood cases are from familial clusters, with the children tending to be identified through contact tracing of adult cases [6, 9, 21, 22]. While people interviewed by the WHO-China Joint Mission could not recall episodes of a child infecting an adult (or another child) [7], there has subsequently been 1 clinical report of probable transmission from a 3-month-old infant to her parents. Both parents developed symptomatic COVID-19 days after they looked after the sick infant without personal protective measures [12].
DISCUSSION
In this study we have reviewed the early data on the likelihood of infection, clinical presentation and outcomes, and transmission of COVID-19 in children. The majority of data were from China and may be subject to ascertainment bias, either due to a higher-than-known proportion of milder cases in both adults and children or due to selective ascertainment and testing bias towards adults with acute respiratory symptoms and limitations in diagnostic capacity. A number of the studies examined are preprints and have not been subjected to peer review.
Nevertheless, we identify that children appear to be less affected by COVID-19 than adults by observed rate of infection; while 17.8% of the population were aged 0–15 years old, only 2.1% of reported cases in a large case series from China were in the 0–19-year age group [23]. However, a preprint paper [11] reports that children under 10 who were potentially exposed to the virus were just as likely to become infected as other age groups in the population. Low observed rates of infection and similar susceptibility to infection could be explained by differences in symptomatic infection rates. Other factors may have included social-distancing methods, such as school closures. It should also be noted that the age profile of the population in China is unusual, in that few children will have siblings but will usually have 2 parents and up to 4 grandparents. Critically, serological data are missing at this stage in the pandemic, without which uncertainty about infection rates is likely to persist.
Reliable evidence about clinical features and outcomes of COVID-19 in children is limited to small case series and case reports. Key emerging themes from the literature include description of generally mild symptoms [12] and several reports of asymptomatic infections [9, 14-17], with some indication from a case series of 105 children in Wuhan that there have been severe cases (7.6%) [18]. Rarely, pediatric deaths have also been reported [19]. We found no detailed studies of transmission of SARS-CoV-2 from children. Many of the childhood cases are from familial clusters with children identified through contact tracing of adult cases [20, 21]. There is only 1 case describing likely transmission from a 3-month-old infant to her parents after they looked after the unwell infant without personal protective measures [12]. Of note is the high frequency of chest radiographic abnormality described in both mild and asymptomatic infections in children. Longitudinal data will be required to understand the duration, persistence, and functional deficit related to these findings.
We detected only a weak signal that children with comorbidities are at increased risk or are overrepresented among pediatric COVID-19 cases. This is broadly in line with a review of 14 pediatric MERS cases, of whom 9 without underlying disease remained healthy and 3 developed mild respiratory symptoms; the remaining 2 cases had significant comorbidities, developed severe respiratory symptoms, were treated in intensive care, and subsequently died. Given that poor COVID-19 outcomes have been seen in adults with underlying conditions, a similar issue in children might be anticipated when sufficient data are available. Typically, a pediatric population may contain approximately 10% of children with 1 or more of the following: prematurity with chronic lung disease, cancer, genetic conditions such as cystic fibrosis and ɑ1-antitrypsin deficiency, chronic asthma, and mucosal immune defects [24]. More evidence is therefore needed about clinical features and outcomes in children with high-risk conditions.
So far there has been only 1 documented case of transmission of SARS-CoV-2 from a child to an adult, although if infection rates are as high in children as in adults, as described by Bi et al [11], it would be highly implausible that children were not infecting adults in a more widespread way. The WHO-China Joint Mission was unable to shed any light on this beyond anecdotally reporting no transmission from children [7]. One study attempts to assess the impact of Hong Kong’s public health measures and population behavior change on COVID-19 transmission, using routine influenza surveillance data as a proxy, assuming that influenza and COVID-19 are likely transmitted in similar ways [25]. The authors demonstrate the substantial impact that familiar public health measures (including school closure), social distancing, and behavioral change could have in slowing down COVID-19 transmission, although questions remain about whether these measures can avoid fatigue and be sustained in the long term.
While the evidence we reviewed related mainly to data from China, as the COVID-19 pandemic spreads worldwide data are beginning to emerge from other countries, including Italy [26], South Korea [27], Iceland [28], and the United States [29]. These reports corroborate our findings of lower observed rates of infection in children [25–28], a milder disease course [29], and asymptomatic cases having a role in transmission [28, 29]. Varying public health measures and testing policies between countries should be noted in such comparisons.
It is informative to compare our findings with the literature for SARS-CoV and MERS-CoV, while data on SARS-CoV-2 are emerging. The majority of documented cases of SARS-CoV occurred in adults, in whom SARS was a severe respiratory disease characterized by fever and dyspnea, which could progress rapidly to acute respiratory distress syndrome and death [30]. Pediatric infection rates in SARS-CoV were low (135 cases in patients aged <18 years; 0.01%) with no deaths reported. The evidence is clear that young children (<12 years of age) generally had a milder course that had resolved by day 7, and that symptoms in teenagers were more akin to those in adults [31–37]. Follow-up studies in 47 children with SARS-CoV who were asymptomatic detected abnormal imaging high-resolution computed tomography (HRCT) in 16 of 47 and abnormal lung function in 4 of 47 [11], and another study that followed up 34 affected children revealed reduced aerobic capacity at both 6 and 15 months postdiagnosis [38]. It will be important to be alert for similar long-term consequences for children infected by SARS-CoV-2.
There is little high-quality evidence relating to transmission of SARS-CoV in children. Public awareness of infection was high in the community, and parents were instructed to keep their child out of school if they had fever or respiratory symptoms, and so there was no documented spread in schools [34]. One seroprevalence study for SARS-CoV in children compared those residing in a district with a point source outbreak compared with a low-risk area [39]. Seroprevalence in both areas was low (0.6% in the high-risk area, 0% in the low-risk area, with positive children having reported no SARS-compatible symptoms). This would suggest that positive serology for SARS-CoV in healthy asymptomatic children was very uncommon (0.57%), suggesting a low rate of asymptomatic infection (in possible contrast to SARS-CoV-2), and that community transmissibility of this virus was low.
There are even fewer data relating to MERS-CoV in children. The majority of documented cases of MERS-CoV were in adults. There were 31 reported cases of MERS in children out of a global total of 2449 cases to June 2019 (0.01%). Notably, 13 of 31 (42%) were asymptomatic, identified through contact tracing. Of the 31 children, 2 who had underlying comorbidities died [40]. There is no evidence of transmission of MERS-CoV from children to adults.
Conclusions
From large datasets in China, COVID-19 has been observed in children and young people at a low rate relative to the adult population. Limited data on attack rate suggest that children under 10 are infected with SARS-CoV-2 at approximately the same rates as adults. Children may be asymptomatic or too mildly infected to draw medical attention and be tested and counted in observed cases of COVID-19. Societal measures to limit the spread of the outbreak and household configuration in China (as a legacy of the “1-child” policy) may limit generalizability to other countries or settings.
Evidence suggests that the clinical course in young children is milder than in adults, although there is a paucity of data and less is known about the course in adolescents. There are several reports of asymptomatic infection in children, which would appear to be consistent with emerging data relating to infection rates. There are, as yet, inadequate data on transmissibility of SARS-CoV-2 from children to other children. More evidence is required on all aspects of COVID-19 in pediatric populations including seroprevalence studies when an assay is available and long-term follow-up of silent radiographic abnormalities.
Notes
Author contributions. N. S. M., O. T. M., E. W. S. M., T. A. F., C. L. F., O. B. M., W. J. P. J., and J. S. N.-V.-T. wrote the paper. N. S. M., W. J. P. J., and O. B. M. devised and coordinated the literature search and data extraction. N. S. M., O. T. M., E. W. S. M., T. A. F., C. L. F., C. L., and D. H. E. extracted the data. J. S. N.-V.-T. critically reviewed the data. All authors reviewed and contributed towards revising the final manuscript for important intellectual content.
Acknowledgments. The authors acknowledge Claire Blackmore, Alicia Demirijan, David Evans, Adam Finn, Clare Gregory, Mike Linney, Helen Mactier, Simon Nadel, Andrew Oakes, Andrew Pollard, John Reynolds, Calum Semple, Russell Viner, and Hongxin Zhao.
Disclaimer. All authors are, or have been, affiliated with, seconded to, or employed by the Department of Health and Social Care (DHSC), England. The views in this manuscript are those of the authors and do not necessarily represent the official views of DHSC or Her Majesty’s Government.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020:1–9. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report–51. World Health Organization, 11 March 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf. Accessed 12 March 2020. [Google Scholar]
- 3. Ison MG, Lee N. Noninfluenza respiratory viruses. In: Cohen J, Powderly W, Opal S, eds. Infectious diseases. 4th ed. Vol. 22017 London: Elsevier, 2017:1472–82. [Google Scholar]
- 4. World Health Organization, Department of Communicable Disease Surveillance and Response. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) World Health Organization, 2003. Available at: https://www.who.int/csr/sars/en/WHOconsensus.pdf. Accessed 9 March 2020. [Google Scholar]
- 5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV), MERS Monthly Summary, November 2019 World Health Organization, 2019. Available at: https://www.who.int/emergencies/mers-cov/en/. Accessed 9 March 2020. [Google Scholar]
- 6. Choi S, Jung E, Choi BY, Hur YJ, Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect 2018; 99:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), 16–24 February 2020. World Health Organization, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 9 March 2020. [Google Scholar]
- 8. Wang P, Lu J, Jin Y, et al. Epidemiological characteristics of 1212 COVID-19 patients in Henan, Chin a. medRxiv. 2020. [Preprint]. doi: 10.1101/2020.02.21.20026112v2. Available at: https://www.researchgate.net/publication/339456629_Epidemiological_characteristics_of_1212_COVID-19_patients_in_Henan_China. Accessed 12 March 2020. [DOI] [Google Scholar]
- 9. Wei M, Yuan J, Liu Y, et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA [Preprint] 2020; 323:1313‐4. doi: 10.1001/jama.2020.2131. Available at: https://pubmed.ncbi.nlm.nih.gov/32058570/. Accessed 28 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit Heal 2020; 2:e201–08. Available at: https://www.thelancet.com/action/showPdf?pii=S2589-7500%2820%2930026-1. Accessed 28 February 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. medRxiv [Preprint] 2020. doi: 10.1101/2020.03.03.20028423v1.full.pdf. Available at: https://www.researchgate.net/publication/339716563_Epidemiology_and_Transmission_of_COVID-19_in_Shenzhen_China_Analysis_of_391_cases_and_1286_of_their_close_contacts. Accessed 12 March 2020. [DOI] [Google Scholar]
- 12. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. Published online February 28, 2020. doi: 10.1093/cid/ciaa198/5766430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6:e1000097. doi: 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infec t Dis 2020. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kam K, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis 2020:ciaa201. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi (Chinese Journal of Pediatrics) 2020;58:E007. [DOI] [PubMed] [Google Scholar]
- 18. Yu H, Shao J, Guo Y, Xiang Y, et al. Data-driven discovery of clinical routes for severity detection in COVID-19 pediatric cases. MedRixv 2020. doi: 10.1101/2020.03.09.20032219. Available at: https://www.researchgate.net/publication/339845989_Data-driven_discovery_of_clinical_routes_for_severity_detection_in_COVID-19_pediatric_cases. Accessed 12 March 2020. [DOI] [Google Scholar]
- 19. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team Vital Surveillances. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly 2020;2:113–22. [PMC free article] [PubMed] [Google Scholar]
- 20. Chuming C, Cao M, Peng L, et al. Coronavirus disease-19 among children outside Wuhan, China. Lancet Child Adolesc. MedRxiv 2020. Available at: https://ssrn.com/abstract=3546071. Accessed 9 March 2020.
- 21. Xia W, Jianbo S, Guo Y, et al. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol 2020; 55:1169–74. 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologist need to know. J Med Virol 2020; 92:564–7. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Statista Research Department. Population distribution in China in 2019, by broad age group Statista Research Department, March 5, 2020. Available at: https://www.statista.com/statistics/251524/population-distribution-by-age-group-in-china/. Accessed 9 March 2020. [Google Scholar]
- 24. The March of Dimes Foundation, The Partnership for Maternal, Newborn & Child Health, Save the Children, World Health Organization; Howson CP, Kinney MV, Lawn JE, eds. Born too soon: the global action report on preterm birth. New York, NY: World Health Organization, 2012. [Google Scholar]
- 25. Cowling B, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against COVID-19 using influenza transmission as proxy in Hong Kong, February 2020 an observational study. Lancet 2020; Draft Manuscript (February 2020), Medrxiv [Preprint] 16 March 2020. doi: 10.1101/2020.03.12.20034660. Available at: https://www.medrxiv.org/content/10.1101/2020.03.12.20034660v1.full.pdf+html. Accessed 28 February 2020. [DOI] [Google Scholar]
- 26. Epicentro - L’epidemiologia per la sanità pubblica, Istituto Superiore di Sanità. The COVID-19 Task Force of the Department of Infectious Diseases and the IT Service Istituto Superio re di Sanità. Integrated surveillance of COVID-19 in Italy Available at: https://www.epicentro.iss.it/en/coronavirus/bollettino/Infografica_7aprile%20ENG.pdf. Accessed 9 March 2020.
- 27. Statista Research Department. Age distribution of coronavirus (COVID-19) cases in South Korea as of April 16, 2020 Statista Research Department; Available at: https://www.statista.com/statistics/1102730/south-korea-coronavirus-cases-by-age/. Accessed 9 March 2020. [Google Scholar]
- 28. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. CDC COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12– April 2, 2020. Morb Mortal Wkly Rep 2020;69:422–6. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donnelly CA, Ghani AC, Leung GM, et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003; 361:1761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Bever HP, Chng SY, Goh DY. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr Allergy Immunol 2004; 15:206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung CW, Kwan YW, Ko PW, et al. Severe acute respiratory syndrome among children. Pediatrics 2004; 113:e535–43. [DOI] [PubMed] [Google Scholar]
- 33. Bitnun A, Read S, Tellier R, Petric M, Richardson SE. Severe acute respiratory syndrome-associated coronavirus infection in Toronto children: a second look. Pediatrics 2009; 123:97–101. [DOI] [PubMed] [Google Scholar]
- 34. Wong GW, Li AM, Ng PC, Fok TF. Severe acute respiratory syndrome in children. Pediatr Pulmonol 2003; 36:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeng QY, Liu L, Zeng HS, et al. Clinical characteristics and prognosis of 33 children with severe acute respiratory syndrome in Ghangzhou area. Chinese J Pediatr 2003;41:408–12. [PubMed] [Google Scholar]
- 36. Ng PC, Leung CW, Chiu WK, Wong SF, Hon EK. SARS in newborns and children. Biol Neonate 2004; 85:293–8. [DOI] [PubMed] [Google Scholar]
- 37. Chiu WK, Cheung PC, Ng KL, et al. Severe acute respiratory syndrome in children: experience in a regional hospital in Hong Kong. Pediatr Crit Care Med 2003; 4:279–83. [DOI] [PubMed] [Google Scholar]
- 38. Yu CC, Li AM, So RC, et al. Longer term follow up of aerobic capacity in children affected by severe acute respiratory syndrome (SARS). Thorax 2006; 61:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee PPW, Wong WHS, Chiu SS, et al. Risk-stratified seroprevalence of SARS coronavirus in children residing in a district with point-source outbreak compared to a low-risk area. Hong Kong Med J. 2008;14(Suppl 4):17–20. [PubMed] [Google Scholar]
- 40. Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J 2014; 33:904–6. [DOI] [PubMed] [Google Scholar]