Abstract
Objective:
To measure the association between receipt of specific infection prevention interventions and procedure-related CIED infections.
Design:
Retrospective cohort with manually-reviewed infection status.
Setting:
National, multicenter Veterans Health Administration (VA) cohort.
Participants:
Sampling of procedures entered into the VA Clinical Assessment Reporting and Tracking-Electrophysiology (CART-EP) database from FY 2008–15.
Methods:
A sample of procedures entered into the CART-EP database underwent manual review for occurrence of CIED infection as well as other clinical/procedural variables. The primary outcome was 6-month incidence of CIED infection. Measures of association were calculated using multivariable generalized estimating equations logistic regression.
Results:
101 procedure-related CIED infections were identified among 2,098 procedures (4.8% of sample); factors associated with increased odds of infections included wound complications (adjusted OR (aOR) 8.74; 95% CI: 3.16 – 24.20) and revisions including generator changes (aOR 2.4; 95% CI: 1.59 – 3.63) as well as elevated INR >1.5 (aOR 1.56; 95% CI: 1.12 – 2.18) and methicillin-resistant Staphylococcus colonization (aOR 9.56; 95% CI: 1.55 – 27.77). Clinically effective prevention interventions included pre-procedural skin cleaning with chlorhexidine versus other topical agents (aOR 0.41; 95% CI: 0.22 – 0.76) and receipt of beta-lactam antimicrobial prophylaxis versus vancomycin (aOR 0.60; 95% CI: 0.37 – 0.96). The use of mesh pockets and continuation of antimicrobial prophylaxis after skin closure were not associated with reduced infection risk.
Conclusions:
These findings about the real-world clinical effectiveness of different prevention strategies can be applied to develop evidence-based protocols and infection prevention guidelines specific to the electrophysiology laboratory.
Introduction
Infections of Cardiac Implantable Electronic Devices (CIEDs) are a major cause of morbidity and mortality. As the patient population receiving these invasive devices is increasingly older and more medically complex, CIED infection incidence has doubled over the past decade.2,3 At the same time, the number of patients receiving CIEDs continues to increase, with over 200,000 new devices placed annually in the United States. Yet, despite the growing burden of these infections, little is known about the most clinically effective methods for preventing them.16
The majority of data on the effectiveness of preventative measures for CIED infections is extrapolated from cardiac surgery, rather from studies specific to the electrophysiology (EP) laboratory. Among the most commonly applied strategies, high-quality evidence supports pre-incisional antimicrobial prophylaxis.8 Other measures—such as combination prophylactic regimens and methicillin-resistant Staphylococcus aureus (MRSA) screening and decolonization— have limited data supporting their effectiveness.38 Evidence supporting topical application of antimicrobials, either through the use of antibacterial-impregnated envelopes (TYRX™, TYRX-A™) or antimicrobial washes is also limited.39 Bundled approaches, which include a collection of infection prevention measures performed simultaneously, have demonstrated promising effectiveness; however, due to the nature of the study designs, it remains unclear which bundle components are effective and which increase costs and complexity without improving care.40–42
Host factors that drive infection risk are generally not modifiable or only partially modifiable; examples include smoking, MRSA colonization, diabetes, renal impairment, and increased risk of bleeding due to underlying disease or medications.43 Although bleeding is not on the direct causal pathway to CIED infections, bleeding complications increase rates of hematomas and wound dehiscence, which, in turn increase infection risk by providing microorganisms with a portal of entry (in the case of wound dehiscence) and a source of growth (in the case of hematoma).44 Some procedural factors associated with increased risk – such as re-operation procedures where the generator and/or leads need to be revised or replaced—are also not modifiable.
Thus, to identify the most effective prevention strategies for reducing procedure-related CIED infections performed in the EP laboratory, we sought to measure the association between individual infection prevention interventions and procedure-related CIED infections among a large, multi-center, national cohort.
Methods
Databases
The VA Clinical Assessment Reporting and Tracking (CART) program is a national quality program supporting all VA cardiac catheterization laboratories where invasive procedures are performed. A key feature of this program is a clinical software application designed to collect standardized procedure data. Although use is mandatory for coronary angiograms and percutaneous coronary interventions, it is optional for EP procedures; an estimated 30% of EP procedures performed throughout the national VA are captured by CART.15 Prospectively collected data included procedure type and date, patient demographics (age, sex), and co-morbidities (diabetes, renal disease, heart disease). Procedural complications, such as wound complications and hematomas, are also captured in real-time from the electronic medical record (EMR). CART data are then combined with other data from the VA Corporate Data Warehouse including pharmacy and administrative data to create a longitudinal cohort.
Cohort Development
Cardiac device procedures, including implantations and revisions of permanent pacemakers, implantable cardioverter-defibrillators (ICDs), biventricular pacemaker-ICDs and biventricular pacemakers entered into the CART application during the period from 10/2008 – 9/2015 were considered for inclusion (N=6,497). Manual EMR review was necessary to accurately determine infection status as CIED infection outcomes are not electronically captured. Given the low expected incidence rate of CIED infections (estimated 1–3%3) cases with higher CIED infection probability were over-sampled to enhance case ascertainment. Specifically, all procedures with a potentially relevant ICD9/10 code (Supplemental Material 1) and/or a blood culture order and/or wound culture order within 90 days of the index device procedure underwent manual review by a trained infectious diseases clinician (AA, WBE) applying standard definitions for CIED infection.6 Cases in the enriched sample were then matched 1:3 with cases without potentially relevant ICD9/10 codes and without potentially relevant microbiology orders. The unenriched sample was stratified by facility to the high-probability sample, then a random number generator was used to select cases for manual review. If a facility lacked sufficient CART-EP-entered procedures to complete the matching process, then other low-probability procedures were selected randomly from the cohort so that three unenriched procedures were matched for every one procedure in the enriched sample. Cases without documented cardiac device procedures and/or without clinical notes entered into the VA EMR were excluded.
Outcomes and Exposures
The primary outcome measure was incidence of CIED infection during the 6-month period following the device procedure. These infections were categorized into superficial if they involved only the subcutaneous tissues or incision or deep if the infection spread to involve the pacemaker leads and/or resulted in bacteremia and/or endocarditis. They were identified during the manual review as definite, possible and probable. Definite infections met standard definitions of CIED infections.6,45,46 Cases were classified as possible if there was diagnostic uncertainty about etiology of symptoms (e.g., warmth, tenderness, erythema, but disagreement about infection versus hematoma), but the patient received treatment for an infection. Probable infections were cases where patients were treated with antimicrobial therapy for CIED infection due to signs of an infection (e.g., warmth, tenderness, erythema, purulence, fever) however confirmatory testing was not ordered or completed. These were all combined as the outcome of presumed CIED infection.
Risk factors were categorized into patient variables, procedural variables and infection prevention variables (Supplemental Material 2). Some variables (e.g. demographic characteristics) were extracted from the CART-EP database using electronic definitions, however, variables not collected by CART-EP were extracted and/or validated using manual review to optimize detection and to ensure accuracy of electronic data entry. Although MRSA screening is mandated for inpatient admissions, MRSA screening is not required pre-procedure or for outpatients. Thus, screening results were not available for the entire cohort. Management of patients identified with MRSA colonization is at the discretion of each individual VA medical center and is not standardized nation-wide.
Statistical Analysis
Demographic and clinical characteristics among patients with and without CIED infections were evaluated. Chi-squared tests were used to compare categorical variables and Mann–Whitney Wilcoxon tests for continuous variables. Epidemic curves of the CIED infection outcomes were constructed using the R-package epitools.23 Generalized estimating equations were used to evaluate the relationships between risk factors and the outcome of CIED infection within 6 months following the procedure. CIED infection was evaluated as a binary outcome (described above) with an assumed binomial distribution, logistic link function, and an exchangeable structure, to allow for clustering by facility. Due to the limited number of CIED infections in the cohort, the number of risk factors included in the multivariable regression models were restricted and included age, antibiotic solution, chlorhexidine skin cleaning, Elixhauser comorbidity index,47 MRSA status, revision procedure, wound complication, INR, and type of pre-procedure antibiotic (any vancomycin as referent, beta-lactam, and other). These adjustment variables were chosen a priori based on prior clinical research. A secondary analysis was performed to evaluate the impact of including fever in place of the preventive measure with the weakest statistical association with the outcome, i.e. antibiotic solution.
Analyses were completed using SAS software version 9.4 (SAS Institute, Cary, NC) or R v3.4.0.24 The study was approved by the Colorado Multiple Institutional Review Board and the VA Boston Healthcare System Institutional Review Board prior to data collection and analysis.
Results
In total, 2,098 procedures at 39 different VA medical centers underwent detailed manual review, representing 32% of the N=6,497 procedures entered into the CART-EP application during the study period. The final analytic cohort included 2,059 unique patients (Table 1a). Patients were predominantly male (97.9%) with a median age of 71.7 years (IQR 64.4 – 81.0) A large proportion had medical comorbidities, the most common being tobacco use (50.3%), diabetes (47.1%), and chronic kidney disease (32.5%). 39% (819/2098) of the cohort were prescribed anticoagulants and 13.2% (276/2098) were on antiplatelet medications; in 13.2% (276/2098) of procedures INR was >1.5 at time of incision. Amongst all procedures, 13.7% (287/2098) had complications, including hematoma formation (6.2%, 131/2098) and wound complications (1.0%, 21/2098; e.g. dehiscence).
Table 1a:
Cohort characteristics (by patient)
| Variable | Median (IQR) (Total N=2059) | No CIED Infection (N = 1960) | CIED infection (N = 99) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 71.7 (64.4–81.0) | 71.8 (64.5–81.1) | 68.6 (62.2–79.6) | 0.078 |
| Sex | 2,015 (97.9%) | 1,918 (97.9%) | 97 (98.0%) | 1 |
| Race | ||||
| White | 1,778 (86.4%) | 1,688 (86.1%) | 90 (90.9%) | 0.25 |
| Black | 245 (11.9%) | 236 (12.0%) | 9 (9.1%) | |
| Other | 36 (1.7%) | 36 (1.8%) | 0 (0.0%) | |
| Hispanic Ethnicity | 146 (7.1%) | 138 (7.0%) | 8 (8.1%) | 0.69 |
| Co-morbidities | ||||
| Elixhauser Risk Score (Median (IQR)) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (5.0–8.0) | 0.13 |
| BMI (Median (IQR)) | 28.3 (25.0–32.7) | 28.3 (25.1–32.8) | 27.7 (24.4–32.6) | 0.55 |
| Diabetes | 970 (47.1%) | 921 (47.0%) | 49 (49.5%) | 0.63 |
| Tobacco Use | 1,036 (50.3%) | 982 (50.1%) | 54 (54.5%) | 0.39 |
| Chronic Obstructive Pulmonary Disease | 628 (30.5%) | 590 (30.1%) | 38 (38.4%) | 0.081 |
| Cerebrovascular Disease | 480 (23.3%) | 449 (22.9%) | 31 (31.3%) | 0.054 |
| Peripheral Arterial Disease | 470 (22.8%) | 432 (22.0%) | 38 (38.4%) | 0.0002 |
| Chronic Kidney Disease | 670 (32.5%) | 638 (32.6%) | 32 (32.3%) | 0.96 |
| Dialysis | 66 (3.2%) | 62 (3.2%) | 4 (4.0%) | 0.56 |
| Beta-Lactam Allergy | 300 (14.6%) | 287 (14.6%) | 13 (13.1%) | 0.68 |
| MRSA pre-procedure | 13 (0.6%) | 10 (0.5%) | 3 (3.0%) | 0.022 |
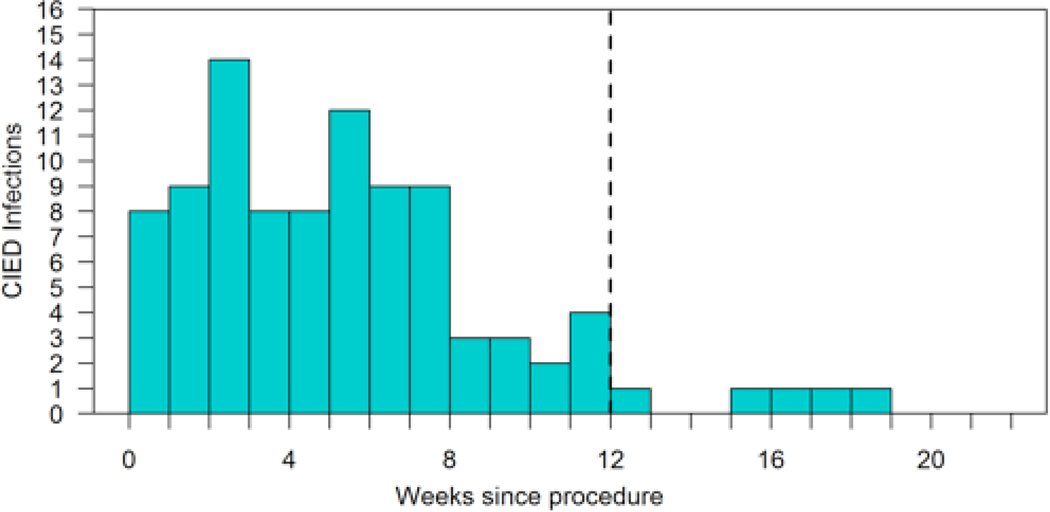
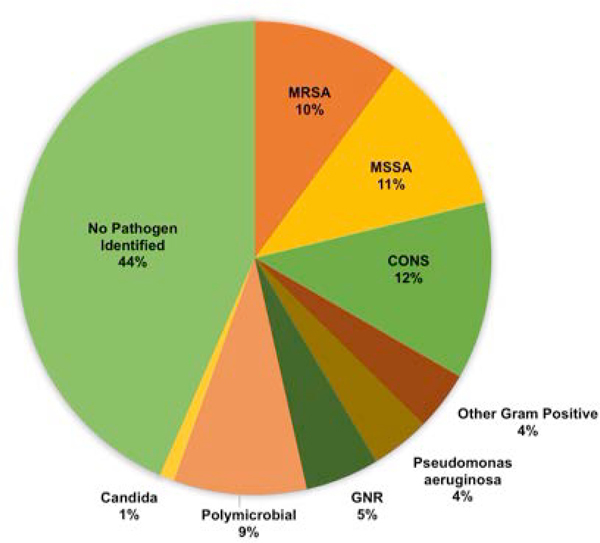
One hundred and one definite, probable and possible CIED infections (4.5%) among 99 unique patients were identified within 6 months of the device procedures; the majority occurred within the 90-day National Healthcare Surveillance Network (NHSN) window period (Figure 1). Among these cases, the majority of infections were deep and/or endocarditis (N = 68/101, Table 1b). The plurality of infections (44%) did not have a pathogen identified, but among cases with positive clinical microbiology results, the most common organisms cultured were gram-positive bacteria, particularly Staphylococcal species (Figure 2).
Figure 1:
Epidemiologic curve of incidence of CIED infections following electrophysiology procedure
Table 1b:
Cohort characteristics (by procedure)
| Variable | Median (IQR) (Total N=2098) | No CIED Infection (N = 1997) | CIED infection (N = 101) | P-value |
|---|---|---|---|---|
| CIED infection | ||||
| No CIED infection | 1,997 (95.2%) | 1,997 (100.0%) | 0 (0.0%) | |
| CIED infection | 101 (4.8%) | 0 (0.0%) | 101 (100%) | |
| Definite | 94 (4.5%) | 0 (0.0%) | 94 (93.1%) | |
| Possible | 5 (0.2%) | 0 (0.0%) | 5 (5.0%) | |
| Probable | 2 (0.1%) | 0 (0.0%) | 2 (2.0%) | |
| Type of CIED infection | ||||
| Superficial + cellulitis | 33 (33%) | 0 (0.0%) | 33 (33.0%) | |
| Deep | 68 (67%) | 0 (0.0%) | 68 (67.0%) | |
| Device and Procedural characteristics | ||||
| Bi-Ventricular Pacemaker | 36 (1.7%) | 34 (1.7%) | 2 (2.0%) | 0.69 |
| Bi-Ventricular Pacemaker-ICD | 278 (13.3%) | 262 (13.1%) | 16 (15.8%) | 0.43 |
| Permanent Pacemaker | 1,199 (57.1%) | 1,152 (57.7%) | 47 (46.5%) | 0.027 |
| ICD | 600 (28.6%) | 564 (28.2%) | 36 (35.6%) | 0.11 |
| Repeat device procedure | 41 (2.0%) | 34 (1.7%) | 2 (2.0%) | 0.69 |
| Revisiona | 781 (37.2%) | 262 (13.1%) | 16 (15.8%) | 0.43 |
| Medications & Pre-procedure | ||||
| Anti-coagulant medications | 819 (39.0%) | 775 (38.8%) | 44 (43.6%) | 0.34 |
| Anti-platelet medications | 276 (13.2%) | 263 (13.2%) | 13 (12.9%) | 0.93 |
| INR >1.5% | 276 (13.2%) | 256 (12.8%) | 20 (19.8%) | 0.043 |
| INR not measured | 219 (10.4%) | 212 (10.6%) | 7 (6.9%) | 0.24 |
| Feverb | 173 (8.2%) | 145 (7.3%) | 28 (27.7%) | <.0001 |
| Infection prevention measures | ||||
| Pre-procedure chlorhexidine | 597 (28.5%) | 581 (29.1%) | 16 (15.8%) | |
| Pre-procedure mupirocin | 13 (0.6%) | 12 (0.6%) | 1 (1.0%) | 0.47 |
| Antimicrobial mesh pocket | 122 (5.8%) | 117 (5.9%) | 5 (5.0%) | |
| Antibiotic solution | 1,727 (87.4%) | 1,648 (87.1%) | 79 (92.9%) | 0.7 |
| Pre-procedure antibiotics | 2,034 (96.9%) | 1,935 (96.9%) | 99 (98.0%) | 0.77 |
| None | 64 (3.1%) | 62 (3.1%) | 2 (2.0%) | |
| Other | 74 (3.5%) | 72 (3.6%) | 2 (2.0%) | |
| Beta-lactam | 957 (45.6%) | 916 (45.9%) | 41 (40.6%) | |
| Vancomycin | 927 (44.2%) | 873 (43.7%) | 54 (53.5%) | |
| Vancomycin plus Beta-lactam | 76 (3.6%) | 74 (3.7%) | 2 (2.0%) | |
| Post-procedure antibiotics | 1,649 (78.6%) | 1,577 (79.0%) | 72 (71.3%) | 0.066 |
| Duration of post-procedure antibiotics | ||||
| 0 | 476 (22.7%) | 445 (22.3%) | 31 (30.7%) | |
| <24 hours | 641 (30.6%) | 613 (30.7%) | 28 (27.7%) | |
| 24–48 hours | 37 (1.8%) | 34 (1.7%) | 3 (3.0%) | |
| >48 hours | 944 (45.0%) | 905 (45.3%) | 39 (38.6%) | |
| Complications | ||||
| Any complication | 287 (13.7%) | 249 (12.5%) | 38 (37.6%) | <.0001 |
| Wound complication | 21 (1.0%) | 15 (0.8%) | 6 (5.9%) | <.0001 |
| Hematoma | 131 (6.2%) | 111 (5.6%) | 20 (19.8%) | <.0001 |
| Other complication | 149 (7.1%) | 130 (6.5%) | 19 (18.8%) | <.0001 |
Includes generator/battery changes
Fever was defined as a temperature ≥100.4°F (38°C) within 24 hours post-procedure
Figure 2:
Distribution of microbiology results among patients with CIED Infections
MRSA: Methicillin-resistant Staphylococcus aureus
MSSA: Methicillin-sensitive Staphylococcus aureus
CONS: Coagulase-negative Staphylococcus
GNR: Gram-negative rod
Fever (T ≥100.4°F) within 24 hours post-procedure was found in 27.7% of CIED infection cases on univariate analysis (28/101 vs 145/1997, p <0.0001). Revision procedures including generator changes (16/101 vs 262/1997, p = 0.0002) and complications (any complication, 38/101 vs 249/1997, p <0.0001) were higher in the CIED infection group.
Infection prevention interventions included documented pre-procedural chlorhexidine skin cleaning in 597 of 2098 (28.5%) manually reviewed procedures and use of antimicrobial mesh pocket in 122 (5.8%) procedures. Pre-procedural antibiotics were used in nearly all manually reviewed procedures (96.9%, 2034/2098); administration of antimicrobials after skin closure was common (78.6%, 1649/2098). 46.8% (981/2098) of reviewed procedures received post-procedural antibiotics for greater than 24 hours’ post-device placement. The most commonly used prophylactic agents included beta-lactam antibiotics (45.6%, 957/2098) and vancomycin (44.2%, 927/2098). Pocket irrigation with an antibiotic solution prior to skin closure was applied in 87.4% (1727/2098) of procedures.
Among infection prevention measures identified, chlorhexidine skin cleaning (adjusted OR (aOR) 0.41; 95% CI 0.22 – 0.76) and pre-procedure antimicrobial prophylaxis with a beta-lactam versus vancomycin (aOR 0.6; 95% CI 0.37 – 0.96) were associated with reduced odds of infection after controlling for other factors (Table 2). Other factors associated with increased odds of infection included pre-procedure MRSA status (aOR 6.56; 95% CI 1.55 – 27.77), INR > 1.5 prior to the procedure (aOR 1.56; 95% CI 1.12 – 2.18), revision procedures (aOR 2.4, 95% CI 1.12 – 2.18) and wound complications (aOR 8.74, 95% CI 3.16 – 24.40). Neither antibiotic irrigation of the pocket intra-procedure (aOR 2.01, 95% CI 0.81 – 5.11) nor prolonged post-procedural antibiotics >24 hours (aOR 0.65, 95% CI 0.41 – 1.05) were associated with significantly reduced risk of infection. The results were generally consistent in the sensitivity analysis that included fever rather than antibiotic solution (Supplemental Material 3) Fever within 24 hours post-procedure substantially increased the odds of developing a CIED infection (aOR 5.04, 95% CI 3.03 – 8.39).
Table 2:
Multivariable logistic regression of CIED infection risk by patient and procedural variables as well as infection prevention measures
| Parameter | aOR | Confidence Interval | p-value | ||
|---|---|---|---|---|---|
| Prolonged post-procedural antibiotics (>24 hrs) | 0.65 | (0.41, 1.05) | 0.079 | ||
| Age | 0.98 | (0.96, 1.00) | 0.025 | ||
| Antibiotic solution | 2.04 | (0.81, 5.11) | 0.129 | ||
| Chlorhexidine skin cleaning | 0.41 | (0.22, 0.76) | 0.004 | ||
| Elixhauser Risk Score | 1.05 | (0.97, 1.13) | 0.232 | ||
| MRSA pre-procedure | 6.56 | (1.55, 27.77) | 0.011 | ||
| Revision | 2.40 | (1.59, 3.63) | <.001 | ||
| Wound complication | 8.74 | (3.16, 24.20) | <.001 | ||
| INR | 1.56 | (1.12, 2.18) | 0.009 | ||
| Type of pre-procedure antibiotics (vs Vancomycin) | Beta-lactam | 0.60 | (0.37, 0.96) | 0.034 | |
| None/Other | 0.29 | (0.10, 0.88) | 0.028 |
Discussion
Using this large, multi-center cohort with detailed manual review, we were able to robustly evaluate the effectiveness of the individual infection prevention strategies commonly used in clinical practice to reduce CIED infections. Our study found that many of these frequently applied interventions are ineffective and that the simplest strategies—those designed to limit bleeding risk and avoid implantations in patients with active infections—have the strongest potential to improve CIED infection outcomes. We also identified several strategies, such as prolonged use of antimicrobials after skin closure, that are both ineffective and increase harms, such as acute kidney injury and C. difficile infections.48 Taken together and in combination with other high-quality studies42 these findings can be used to drive development of evidence-based, cardiac-device specific infection prevention protocols and guidelines.
Chlorhexidine skin cleaning is an intervention directly adopted from traditional surgical settings; this was also associated with reduced risk of post-procedural CIED infections. Chlorhexidine was likely effective in both settings because skin commensals introduced through incisions constitute the major microbiological culprits in both CIED procedures and conventional surgical settings.49,50 Similarly, the association we found between pre-procedure MRSA status and CIED infection underscores the importance of skin commensals as drivers of procedure-related infections in this setting.
Pre-incisional antimicrobial prophylaxis is a mainstay of infection prevention in procedural settings. Although available evidence and surgical site infection prevention guidelines support only the use of a single pre-incisional dose,6,8,51 continuation of antibiotics for greater than one day is common following electrophysiology procedures.16 Prior studies demonstrate that prolonged post-procedural antibiotics following device placement are considered the standard of care by clinical electrophysiologists.52 In our cohort, there was no benefit to prolonging post-procedural antibiotics beyond a single pre-incisional dose; this finding is aligned with other studies in many surgical settings. Reducing post-procedural prescribing is an important stewardship effort with the potential to improve care by reducing preventable harms.21,25 Thus, implementing standardized protocols and guidelines to limit their use, or instituting a national quality metric, similar to the highly effective Surgical Care Improvement Project INF-3 measure, may be necessary to change this pervasive and potentially harmful practice.12,53 Furthermore, identifying non-modifiable risk factors that may trigger the application of ineffective interventions may be an area of focus for provider feedback. For example, revision procedures and generator changes are oft recognized as risk factors for infection. During unpublished qualitative interviews with electrophysiologists, we found that processes for revisions and generator replacements are more aggressive than prevention strategies applied to initial procedures. For example, during interviews, one provider reported “Infections are a lot more common with generator changes…that’s why I extended the antibiotics for those patients but I stop with the new devices.”
Beta-lactam antimicrobials appeared to be more effective for preventing CIED infections than vancomycin. Studies in other settings have generally favored beta-lactam prophylaxis9 as superior agents for Staphylococcal species, the most common cause of CIED infections.54 Another factor impacting vancomycin’s effectiveness as a prophylactic agent in this setting may be resource and environmental constraints that are present in the EP laboratory but not traditional operating rooms; several providers reported during unpublished interviews that limited space and beds in the pre-procedural area cause administration challenges with vancomycin, due to the need for prolonged infusion times with this antibiotic. These restraints prevent infusion of the antimicrobial prior to incision, thereby potentially limiting the effectiveness of the prophylaxis.
We also found that vancomycin prophylaxis also did not reduce CIED infection risk even after accounting for MRSA status; this finding is of particular importance given the strong association between pre-procedure MRSA colonization status and post-procedural CIED infections. Our ability to explore the question of whether vancomycin was superior in the MRSA-colonized population was limited by the small number of patients tested for colonization at the time of their CIED procedure, however, infusion challenges described above would apply to all patients undergoing cardiac device procedures. In addition, supporting our findings is a recently published randomized controlled crossover trial measuring the impact of an antimicrobial prophylaxis bundle on CIED infection risk. 42 This randomized controlled trial found no additional benefit to adding vancomycin as part of a pre-incisional prophylaxis bundle.42 Our work expands upon this study by evaluating a broader variety of antibiotic regimens and also teasing out the individual impact of different non-antibiotic bundle elements.42 An additional factor that must be considered when evaluating the clinical utility of vancomycin as a prophylactic agent is its toxicity: prior work demonstrates a higher rate of acute kidney injury among patients who received vancomycin-based prophylaxis regimens compared to regimens without vancomcyin.48 Taken together, these studies suggest that the most impactful way to minimize risk among MRSA colonized patients might be an evidence-based skin antisepsis and decolonization bundle, rather than a shift toward more wide-spread use of vancomycin as the primary agent for pre-incisional prophylaxis.
Some measures specifically targeted for CIED implantations, such as the use of topical antibiotic washes for pocket irrigation and antibiotic-impregnated device envelopes, are widely used despite limited evidence supporting their effectiveness. Our analysis suggests that these topical administration strategies do not reduce CIED infections. In fact, there was a trend toward an increased risk of infection among the population of patients who received antimicrobial washes; this finding is congruent with prior studies evaluating this intervention that have demonstrated a lack of efficacy.42,55,56 Potential reasons why this strategy may not reduce infections include the risk of direct tissue toxicity from high doses of antimicrobials, increased risk of wound dehiscence, and the unpredictable pharmacodynamics of the diffusion of antimicrobials away from the device pocket.57 Another topical strategy, antimicrobial-impregnated TYRX™ envelopes, is specifically designed to reduce CIED infections and approved for use in 2008. These absorbable and non-absorbable mesh pockets release minocycline and rifampin in the generator pocket and are associated with reduced infection risk in some observational studies.40,58,59 However, in our large, multicenter, national sample, the use of mesh pockets was not associated with reduced infection risk. This observation is in contrast to a recently published randomized controlled trial supporting the efficacy of absorbable antibacterial envelopes.60 Reasons for this difference may include type of pocket evaluated, as during the time frame of our study both absorbable and non-absorbable pockets may have been used, and the newer pockets may be more clinically effective than older designs. Another important difference is the 365-day infection outcome applied in the randomized controlled trial.
The high rate of wound complications preceding the development of CIED infections suggests that interventions that reduce the risk of wound complications—such as those aimed at reducing bleeding—may lead to additional risk reduction. Consideration should be given to measures that reduce risk of hematoma, such as procedural checkpoints38 to institute an INR cut off of <1.5 with consideration to delay procedures if elevated, particularly in high-risk patients. The use of compression vests and compression dressings are other interventions that may be beneficial in patients who cannot safely stop their anticoagulants and/or antiplatelet agents.61,62 In addition, delaying procedures if a patient is found be febrile pre-procedure may be a simple and effective risk reduction strategy.
There are several limitations to this study. As the CART-EP application only captures a subset of voluntarily-reporting VA electrophysiology procedures, it is possible that the sample may not be representative of all CIED procedures. In addition, the VA population may not be generalizable to non-VA populations, particularly to those with a higher proportion of female patients. Given the reliance on data available in VA records, adverse events occurring outside the VA system may not have been captured. However, prior studies have demonstrated that the majority of patients return to the closed VA healthcare system for subsequent care, somewhat mitigating this limitation.37 In addition, we conducted a detailed manual review of scanned-in paper records including of outside facility records, so the vast majority of non-VA care was captured and reviewed. Although our cohort was strengthened by manual review of all patients, infection prevention efforts that were not documented would not have been measured; this would tend to bias our findings toward the null and was most likely to impact variables typically not captured in any medical record, such as type of skin antiseptic and receipt of shaving or clipping prior to the procedure. Furthermore, some variables may have only been intermittently documented (e.g. chlorhexidine use) therefore underestimating the true scope of practice and the clinical effectiveness of this intervention.
Conclusions
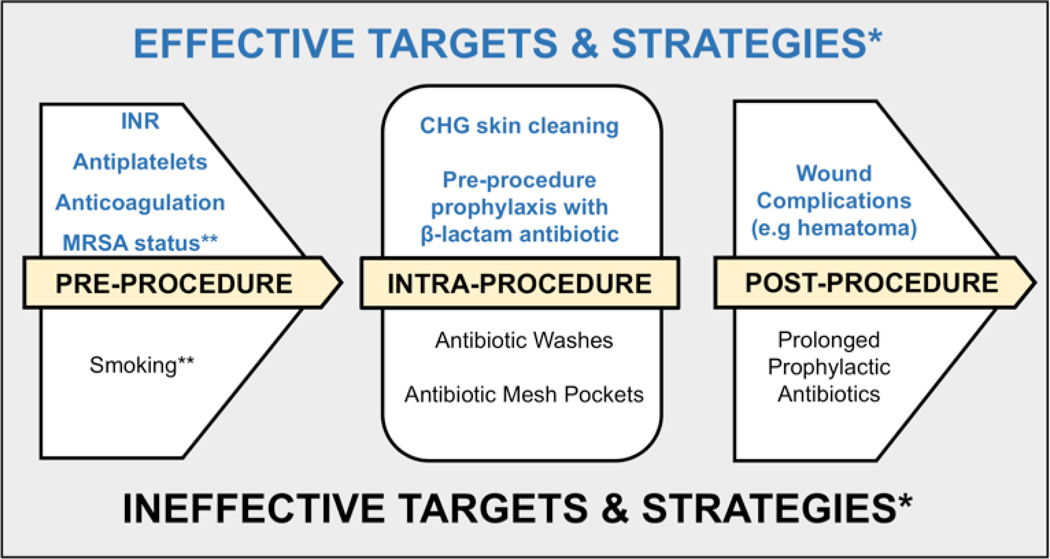
Straight-forward and easy-to-implement interventions should form the basis of standardized protocols and bundles (Figure 3) to improve infection prevention in the electrophysiology laboratory. Although a variety of activities are commonly used in clinical practice, our findings highlight the principle that “less is more.”
Figure 3:
Proposed Infection prevention bundle for CIED infections
*Non-modifiable factors (e.g. age, co-morbidities, device type and lead dislodgement) were omitted from this schematic as they are not often included in infection prevention initiatives
**These variables are potentially modifiable but may be difficult to target in the immediate pre-procedural setting
Supplementary Material
Acknowledgements:
This research would not have been possible without the help and support of the VA Clinical Assessment, Reporting, and Tracking Program.
Funding:
The work was supported by American Heart Association Institute for Precision Cardiovascular Medicine Award # 17IG33630052. WBE is supported by a Veterans Integrated Service Network (VISN)-1 Career Development Award.
Conflicts: AA is an investigator for studies funded by Gilead Sciences since the completion of this study. PZ has received speaking honoraria from Abbott Laboratories. WBE provides consulting services to DLA Piper, LLC. All other authors have no conflicts to disclose.
References
- 1.Bradshaw PJ, Stobie P, Knuiman MW, Briffa TG, Hobbs MS. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart 2014;1:e000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 2012;60:1540–1545. [DOI] [PubMed] [Google Scholar]
- 3.Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 4.Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006;48:590–591. [DOI] [PubMed] [Google Scholar]
- 5.Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol 2009;2:129–134. [DOI] [PubMed] [Google Scholar]
- 6.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458–477. [DOI] [PubMed] [Google Scholar]
- 7.Darouiche R, Mosier M, Voigt J. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. Pacing Clin Electrophysiol 2012;35:1348–1360. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 9.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- 10.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 11.McDonald M, Grabsch E, Marshall C, Forbes A. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. Aust N Z J Surg 1998;68:388–396. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt) 2011;12:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branch-Elliman W, Ripollone J, Strymish J, Itani K, Gupta K. Unintended Consequences of Double Versus Single Antimicrobial Prophylaxis in Patients Undergoing Cardiac Surgery. Paper presented at: Open Forum Infectious Diseases 2016. [Google Scholar]
- 14.Branch-Elliman W, Ripollone JE, O’Brien WJ, et al. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study. PLoS Med 2017;14:e1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branch-Elliman W, Stanislawski M, Strymish J, et al. Cardiac Electrophysiology Laboratories: A Potential Target for Antimicrobial Stewardship and Quality Improvement? Infect Control Hosp Epidemiol 2016;37:1005–1011. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra P, Gupta K, Strymish J, et al. Implementation of Infection Prevention and Antimicrobial Stewardship in Cardiac Electrophysiology Laboratories: Results from the SHEA Research Network. Infect Control Hosp Epidemiol 2017;38:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol 2014;114:1750–1757. [DOI] [PubMed] [Google Scholar]
- 18.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–748. [DOI] [PubMed] [Google Scholar]
- 19.Kuntz JL, Smith DH, Petrik AF, et al. Predicting the Risk of Clostridium difficile Infection upon Admission: A Score to Identify Patients for Antimicrobial Stewardship Efforts. Perm J 2016;20:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bratzler DW. Surgical care improvement project performance measures: good but not perfect. Clin Infect Dis 2013;56:428–429. [DOI] [PubMed] [Google Scholar]
- 22.Bratzler DW, Houck PM, Surgical Infection Prevention Guidelines Writers W, et al. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004;38:1706–1715. [DOI] [PubMed] [Google Scholar]
- 23.Epitools: Epidemiology Tools. R package [computer program]. Version 0..5–92017. [Google Scholar]
- 24.R: A language and environment for statistical computing. [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 25.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000;101:2916–2921. [DOI] [PubMed] [Google Scholar]
- 26.Poeran J, Mazumdar M, Rasul R, et al. Antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2016;151:589–597 e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamgbola O Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 2016;7:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriaras I, Michalopoulos A, Turina M, Geroulanos S. Evolution of antimicrobial prophylaxis in cardiovascular surgery. Eur J Cardiothorac Surg 2000;18:440–446. [DOI] [PubMed] [Google Scholar]
- 29.Remmelts HH, Meine M, Loh P, et al. Infection after ICD implantation: operating room versus cardiac catheterisation laboratory. Neth Heart J 2009;17:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011;53:42–48. [DOI] [PubMed] [Google Scholar]
- 31.Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents 2016;48:1–10. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013;68:1951–1961. [DOI] [PubMed] [Google Scholar]
- 33.Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 2015;29:123–134. [DOI] [PubMed] [Google Scholar]
- 34.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821–1828. [DOI] [PubMed] [Google Scholar]
- 35.Mull HJ, Gellad ZF, Gupta RT, et al. Factors Associated With Emergency Department Visits and Hospital Admissions After Invasive Outpatient Procedures in the Veterans Health Administration. JAMA Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mull HJ, Rosen AK, O’Brien WJ, et al. Factors Associated with Hospital Admission after Outpatient Surgery in the Veterans Health Administration. Health Serv Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pindyck T, Gupta K, Strymish J, et al. Validation of an electronic tool for flagging surgical site infections based on clinical practice patterns for triaging surveillance: Operational successes and barriers. Am J Infect Control 2017. [DOI] [PubMed] [Google Scholar]
- 38.Strymish J, Welch B, Peralta A, Hoffmeister P, Branch-Elliman W, Gupta K. Implementation of a surgical site infection prevention bundle in the cardiac electrophysiology laboratory for management of a cluster of device infections. Open forum infectious diseases 2016;3. [Google Scholar]
- 39.Ali S, Kanjwal Y, Bruhl SR, et al. A meta-analysis of antibacterial envelope use in prevention of cardiovascular implantable electronic device infection. Therapeutic advances in infectious disease 2017;4:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahsan SY, Saberwal B, Lambiase PD, et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace 2014;16:1482–1489. [DOI] [PubMed] [Google Scholar]
- 41.Manolis AS, Melita H. Prevention of Cardiac Implantable Electronic Device Infections: Single Operator Technique with Use of Povidone-Iodine, Double Gloving, Meticulous Aseptic/Antiseptic Measures and Antibiotic Prophylaxis. Pacing Clin Electrophysiol 2017;40:26–34. [DOI] [PubMed] [Google Scholar]
- 42.Krahn AD, Longtin Y, Philippon F, et al. Prevention of Arrhythmia Device Infection Trial: The PADIT Trial. J Am Coll Cardiol 2018;72:3098–3109. [DOI] [PubMed] [Google Scholar]
- 43.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–777. [DOI] [PubMed] [Google Scholar]
- 44.Nichols CI, Vose JG. Incidence of Bleeding-Related Complications During Primary Implantation and Replacement of Cardiac Implantable Electronic Devices. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325–359. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen JC, Gerdes JC, Varma N. Infected cardiac-implantable electronic devices: prevention, diagnosis, and treatment. European heart journal 2015;36:2484–2490. [DOI] [PubMed] [Google Scholar]
- 47.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 48.Asundi A, Stanislawski M, Mehta P, et al. Prolonged antimicrobial prophylaxis following cardiac device procedures increases preventable harm: insights from the VA CART program. Infect Control Hosp Epidemiol 2018;39:1030–1036. [DOI] [PubMed] [Google Scholar]
- 49.Chen HC, Chen MC, Chen YL, Tsai TH, Pan KL, Lin YS. Bundled preparation of skin antisepsis decreases the risk of cardiac implantable electronic device-related infection. Europace 2016;18:858–867. [DOI] [PubMed] [Google Scholar]
- 50.George S, Leasure AR, Horstmanshof D. Effectiveness of Decolonization With Chlorhexidine and Mupirocin in Reducing Surgical Site Infections: A Systematic Review. Dimensions of critical care nursing : DCCN 2016;35:204–222. [DOI] [PubMed] [Google Scholar]
- 51.Lee WH, Huang TC, Lin LJ, et al. Efficacy of postoperative prophylactic antibiotics in reducing permanent pacemaker infections. Clinical cardiology 2017;40:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basil A, Lubitz SA, Noseworthy PA, et al. Periprocedural Antibiotic Prophylaxis for Cardiac Implantable Electrical Device Procedures: Results From a Heart Rhythm Society Survey. JACC. Clinical electrophysiology 2017;3:632–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bratzler DW, Houck PM, Richards C, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg 2005;140:174–182. [DOI] [PubMed] [Google Scholar]
- 54.Bolon MK, Morlote M, Weber SG, Koplan B, Carmeli Y, Wright SB. Glycopeptides are no more effective than beta-lactam agents for prevention of surgical site infection after cardiac surgery: a meta-analysis. Clin Infect Dis 2004;38:1357–1363. [DOI] [PubMed] [Google Scholar]
- 55.Kang FG, Liu PJ, Liang LY, et al. Effect of pocket irrigation with antimicrobial on prevention of pacemaker pocket infection: a meta-analysis. BMC cardiovascular disorders 2017;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakshmanadoss U, Nuanez B, Kutinsky I, Khalid R, Haines DE, Wong WS. Incidence of Pocket Infection Postcardiac Device Implantation Using Antibiotic versus Saline Solution for Pocket Irrigation. Pacing Clin Electrophysiol 2016;39:978–984. [DOI] [PubMed] [Google Scholar]
- 57.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res 1996:245–251. [PubMed] [Google Scholar]
- 58.Henrikson CA, Sohail MR, Acosta H, et al. Antibacterial Envelope Is Associated With Low Infection Rates After Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Device Replacement: Results of the Citadel and Centurion Studies. JACC. Clinical electrophysiology 2017;3:1158–1167. [DOI] [PubMed] [Google Scholar]
- 59.Koerber SM, Turagam MK, Winterfield J, Gautam S, Gold MR. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: A meta-analysis. Journal of cardiovascular electrophysiology 2018;29:609–615. [DOI] [PubMed] [Google Scholar]
- 60.Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 61.Turagam MK, Nagarajan DV, Bartus K, Makkar A, Swarup V. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (STOP-HEMATOMA-I). Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing 2017;49:197–204. [DOI] [PubMed] [Google Scholar]
- 62.Douketis JD. Perioperative management of patients who are receiving warfarin therapy: an evidence-based and practical approach. Blood 2011;117:5044–5049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.