The serendipitous disruption of the klotho gene promoter in 1997 by a cardiologist in Japan begot a phenotype of early multiorgan failure mimicking premature aging [1]. The gene was aptly named after the Greek Goddess who spins the threads of life. In 2005, the same investigator did the opposite experiment and showed that transgenic overexpression of klotho in mice extends life, placing Klotho once again in the spotlight and generated volumes of antiaging research [2]. Several findings that followed changed the landscape. Klotho is a single-pass transmembrane protein, primarily expressed in the kidney, but its extracellular domain is secreted into circulation as a soluble protein after being cleaved by proteases [3]; thus, the kidney supplies the body with soluble Klotho [3]. In multiple preclinical studies with diverse models, both acute and chronic kidney diseases are states of renal and systemic klotho deficiency [3], including human CKD. The relationship between Klotho and kidney disease is more than just a biomarker because restoration of Klotho ameliorated renal dysfunction and extrarenal complications in both acute [4] and chronic settings bringing Klotho supplementation into the therapeutic realm. However, how should Klotho be given?
In rodents, several methods have been used successfully to raise systemic Klotho levels (Fig. 1). The transgenic insertion of klotho into the genome of a mouse was the first attempt [2]. While this has been enormously useful as proof-of-concept in experimental animals, this technique is not applicable to patients currently. Recombinant Klotho protein was used successfully in the laboratory in both acute and chronic [5] settings that prevented AKI, accelerated AKI recovery, presented and retarded AKI-to-CKD transition, and ameliorated extrarenal complications [4]. Recombinant Klotho protein administration is a method where translation to human therapeutics is much more practical and proximal. Challenges in optimization of preparation, achieving adequate bioactivity, and prolongation of its short half-life notwithstanding, recombinant Klotho is still the only method that allows dosing and precise control of therapeutic levels. Klotho driven by strong universal promoter in conventional cDNA plasmids has also been either directly injected [6] or delivered while encapsulated by adeno-associated virus which is not pathogenic [7]. Although only selective studies are cited, these methods have been used by multiple laboratories and Klotho expression and biologic effects were all verified. Pharmacologic means to increase endogenous Klotho levels include phosphate restriction, vitamin D, angiotensin-converting enzyme inhibitor, peroxisome proliferator-activated receptor-gamma agonists (thiazolidinediones), 3-hydroxy-3-methylglutaryl CoA reductase inhibitors; all currently approved for clinical use for other indications [4]. The increase in Klotho with these agents has not been uniformly proven in humans, may be limited in magnitude and consistency, and likely will not work in advanced CKD when the capacity of the kidney to increase Klotho output is limited.
Fig. 1.
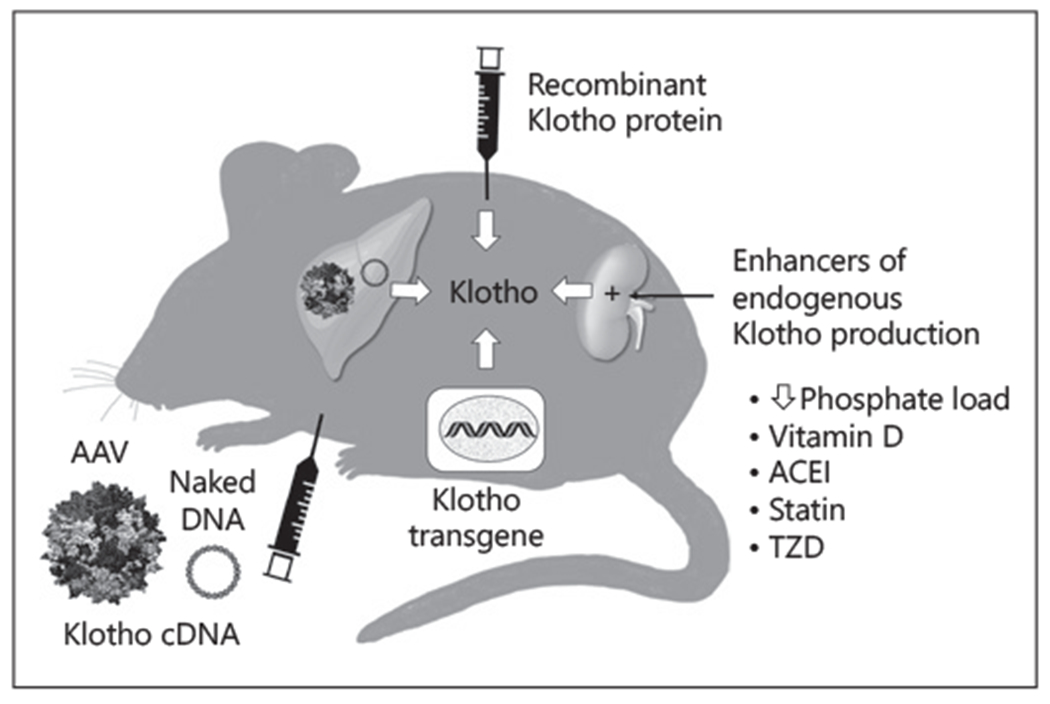
Existent methods to increase circulating Klotho levels. Recombinant Klotho protein can be given parentally to achieve adequate circulating levels. Klotho cDNA can be delivered. either as naked cDNA (full-length plasmid or minicircles) or encapsulated by adeno-associated virus. The majority of these reside in the liver and Klotho is released. The Klotho gene can be inserted into the genotype as a transgene with a strong promoter driving Klotho protein production. Finally, there are pharmacologic means to increase endogenous Klotho production. Statin: 3-hydroxy-3-methylglutaryl CoA reductase inhibitors. TZD or peroxisome proliferator-activated receptor-gamma agonists. The minicircle technology represents another version of Klotho cDNA. AAV, adeno-associated; PPAR-γ, peroxisome proliferator-activated receptor-gamma; ACEI, angiotensin-converting enzyme inhibitors; TZD, thiazolidinediones.
The current study by Yoo et al. [8] utilized yet another method for in vivo delivery of the Klotho plasmid using the minicircle vector technology. The minicircle DNA is generated from a special host bacterial strain harboring an inducible system that produces the minicircles from its full-size parental DNA plasmids. Minicircle DNA vectors allow sustained gene expression in quiescent cells and tissues with long-term expression in vivo and in vitro without the risk of immunogenicity that can potentially be instigated by the bacterial backbone in standard plasmids. Minicircles are now commercially available, require only minor bench-work and preparation time, do not replicate within the host cell, and their small size facilitates efficient cell uptake.
In this study by Yoo et al. [8], the minicircle cDNA produced modest elevation of systemic Klotho levels based on the ELISA reading with Klotho secretion lasted a reported 10 days in vivo, while the fluorescent marker with a much longer half-life remained in the liver for up to 30 days as expected. As was performed by the other investigators who used transgenes, recombinant protein, adeno-associated virus, and conventional plasmid, Yoo et al. [8] also confirmed the acute rise in serum Klotho conferred protective effect on 2 models: ischemia–reperfusion-injury and unilateral ureteral obstruction. They showed biologic benefits of reduction of creatinine and histology for ischemia-reperfusion injury and renal fibrosis for unilateral ureteral obstruction, thus confirming equivalence of efficacy of the minicircle delivery with the previous methods.
The current study did not demonstrate that finding, but consecutive repeated administration of the minicircles containing Klotho cDNA may provide a way to chronically increase Klotho levels, which are useful, as many long-term studies of renal and extrarenal pathology of CKD are needed to be performed. Since it is the circulating Klotho that is efficacious, organ-specific targeted delivery is less critical. The ability to control the dose and subsequent serum levels will also be desirable and needs to be demonstrated. Dual or multiple minicircles encoding Klotho and another deficit protein in CKD will also be a useful strategy. In summary, minicircles provide yet another way to perform experimental Klotho replacement. Significant hurdles still need to be surmounted on the trajectory to translational applications.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997. November;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005. September;309(5742):1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75(1):503–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone. 2017. July;100:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017. May;91(5):1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J Am Soc Nephrol. 2015. May;26(5):1150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J Am Soc Nephrol. 2017. April;28(4):1162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin YJ, Luo K, Quan Y, Ko EJ, Chung BH, Lim SW, Yang CW. Therapeutic challenge of minicircle vector encoding klotho in animal model. Am J Nephrol. 2019;49:413–23. [DOI] [PubMed] [Google Scholar]


