Abstract
Pulsed low intensity focused ultrasound (PLIFUS) has shown promise inducing neuromodulation in several animal and human studies. Therefore, it is of clinical interest to develop experimental platforms to test repetitive PLIFUS as a therapeutic modality in humans with neurological disorders. Here, we aimed to develop a laboratory-built experimental device platform intended to deliver repetitive-PLIFUS to the hippocampus in Seizure-Onset-Zones (SOZ) of patients with drug-resistant temporal lobe epilepsy (TLE). The system uses neuronavigation targeting over multiple therapeutic sessions. PLIFUS (548 kHz) was emitted across multiple hippocampal targets in a human subject with TLE using a mechanically-steered piezoelectric transducer. Stimulation was delivered up to 2.25-W/cm2 spatial peak temporal average intensity (free-field equivalent), with 36-50% duty-cycle, 500-ms sonications, and 7-second interstimulation-intervals lasting 140-seconds per target and repeated for multiple sessions. A first-inhuman PLIFUS course of treatment was successfully delivered using the device platform resulting in no adverse events.
Keywords: Low intensity focused ultrasound, Therapeutic neuromodulation, Neuronavigation, Human hippocampus, Multi-target
INTRODUCTION
Laboratory-built devices have been used to study brain responses in several healthy human subjects during pulsed low intensity focused ultrasound (PLIFUS) neuromodulation. Targets have included the somatosensory, motor, and visual cortices, as well as thalamus (Biase et al. 2019; Fomenko et al. 2018). Investigations demonstrated the ability of PLIFUS to suppress evoked potentials in the brain recorded by electroencephalography (EEG) or subjects reporting PLIFUS-induced tactile sensations (Lee et al. 2016a; Lee et al. 2016b; Legon et al. 2014; Legon et al. 2018). In a recent study, PLIFUS of healthy rat brain yielded evoked potential suppression for 35 minutes post-stimulation (Yoo et al. 2018) and low intensity ultrasound also suppressed visual evoked potentials in cats for up to 30 minutes (Fry et al. 1958), suggesting that PLIFUS induces prolonged neuroplasticity--setting the stage for a promising therapeutic modality. Although the mechanisms of PLIFUS neuromodulation are still under investigation, multiple preclinical epilepsy rodent model studies have shown suppression of epileptic activity via PLIFUS in the temporal lobe and hippocampus (Chen et al. 2019; Hakimova et al. 2015; Min et al. 2011). However, exposing human in-vivo epileptogenic tissue to repetitive PLIFUS experimentally over multiple sessions to study the effects of therapeutic neuromodulation to our knowledge has not been reported. Here we present a laboratory-built device platform designed to investigate PLIFUS neuromodulation of the human hippocampus in adults with drug-resistant temporal lobe epilepsy (TLE) (NCT03868293). We present our techniques and methods.
MATERIALS AND METHODS
Human use and regulatory status:
A protocol for device usage on human subjects was approved by the Brigham and Women’s Hospital Institutional Review Board and granted Investigational Device Exemption by the USA Food and Drug Administration. A subject (Subject 1, female, age 26) provided written informed consent to participate in the ongoing epilepsy device study.
Ultrasound emitting device
The custom device was designed and constructed in the Focused Ultrasound Laboratory at Brigham and Women’s Hospital, Boston, MA (STB). PLIFUS was emitted using a spherically-focused air-backed piezoceramic transducer (Piezo Kinetics, Bellfonte, Pennsylvania, PZT-4, 548 kHz, 5 cm diameter, focal length ≈ 6.7 cm) mounted by silicone adhesive in an acrylic housing that maintains air backing under water. Hydrophone mapping and an acoustic radiation balance were used to spatially characterize the transducer pressure field and calibrate acoustic power, respectively, within a 50-ohm matching circuit. Measurements were taken in a degassed-deionized water tank with no skull or membrane obstructions. Two function generators (33220A, Agilent Technologies, Santa Clara, CA) and an RF-amplifier (240L, Electronics & Innovation Ltd, Rochester, NY) powered the transducer while operating in degassed-deionized water within a windowed vessel that is coupled to the human temporal acoustic window through a 0.01-mm thick polyethylene membrane and ultrasound gel (Fig. 1). Transducer steering is achieved via a cantilever and manual 3D-positioning system fixed to the windowed vessel housing to keep the PLIFUS beam orientation perpendicular to the temporal acoustic window. The windowed vessel is comprised of several acrylic ring spacers and aluminum mounting plates sealed together with threaded rods and silicone sealer. A passive acoustic detector is mounted in the windowed vessel for verifying the device is powered on during operation.
Fig. 1:
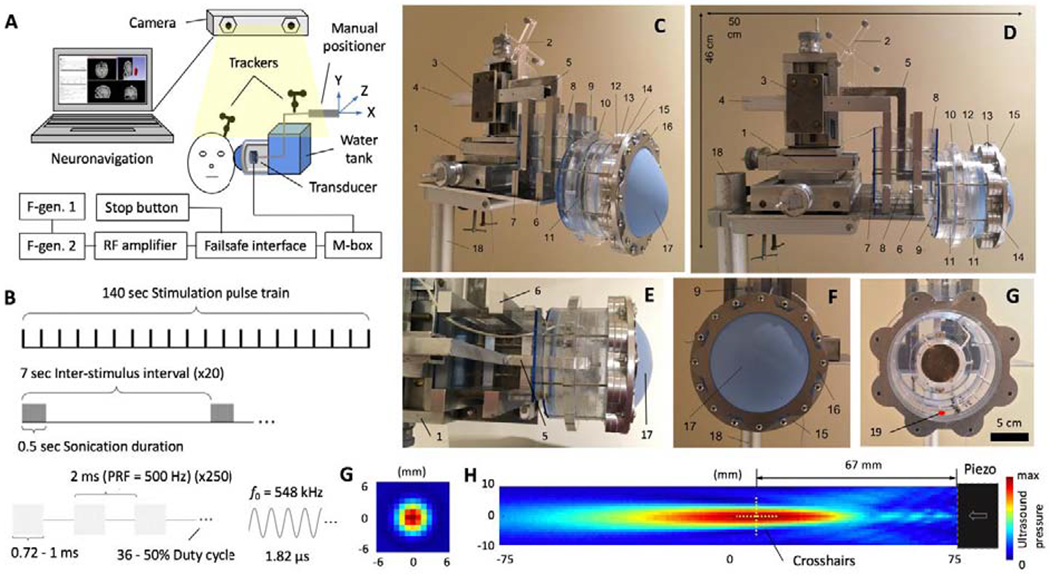
Focused ultrasound platform. (A) System schematic for control system, failsafe interface, neuronavigation, F-gen: function generator, M-box: matching circuit, and device. The trackers attached to the transducer and strapped to the subject’s head must be in view of the camera so the PLIFUS beam focus can be displayed in the subjects MRI on the neuronavigation computer display. (B) Stimulation parameters of 140-s sonications are emitted to each hippocampal target with: fundamental frequency (f0) = 548 kHz, Pulse Repetition Frequency (PRF) = 500 Hz, duty cycle = 36-50%, sonication duration = 500 ms, inter-stimulus interval = 7 sec, stimulation pulse train = 140 sec, ISPTA = 0.5-2.25 W/cm2 (peak negative pressure = 0.14-0.36 MPa, free-field equivalent). (C, D, E, & F) Photos of the device and water membrane for coupling the device to the subject’s head at the temple acoustic window. Photographs include, 1: positioner platform, 2: transducer tracker, 3-5: transducer cantilever, 6-11: water tank assembly, 12-17: membrane assembly, 18: device stand, and 19: red dot representing the location of the mounted passive acoustic detector oriented in the direction of the membrane. (G) Membrane cover removed to show the emitting face of the steerable piezoelectric transducer. (G & H) Ultrasound intensity map from hydrophone scanning of the piezoelectric transducer in the axial and longitudinal planes.
Failsafe system
A custom failsafe system is integrated into the transducer power system to limit the maximum PLIFUS intensity to Spatial Peak Temporal Average (ISPTA) = 3.0 W/cm2 (free-field equivalent) during device operation. The RF-amplifier power is triggered off by a voltage limiter circuit installed between the function generators and RF-amplifier input when voltages associated with greater than ISPTA = 3.0 W/cm2 are detected. An emergency stop button is also incorporated into the failsafe system for immediate device shutdown and all electronic equipment is routed through an isolation transformer. failsafe system circuit drawings are included in Supplementary Figure 1.
Navigating the hippocampus
Targeting the hippocampus was achieved using landmarked T2-weighted MRI images with an open-source neuronavigation package using 3D-slicer we previously designed for custom PLIFUS systems (Preiswerk et al. 2019). Optically reflective spheres mounted to the transducer cantilever and affixed to the volunteer’s head are tracked by a stereoscopic camera. A virtual crosshair is assigned to the focal center via a reference stylus during beam characterization and projected over T2-weighted images for viewing on the device operator screen (detailed instructions included in (Preiswerk et al. 2019)). Hippocampal target markers are assigned to the MRI images before treatment begins by the epileptologist and ultrasound physicists together (EJB, PJW). The operator then positions the crosshair over a target marker prior to ultrasound stimulation and then repeats for each hippocampus target while the subject is seated.
RESULTS
A schematic and photos of the final platform design are shown in Fig. 1. Stimulation pulse trains lasting for 140 seconds (Fig. 1B) were successfully delivered to multiple hippocampal targets and repeated in biweekly therapy sessions for 3 weeks to Subject 1 with TLE. Ultrasound sonication was set to 548 kHz sign wave, pulse repetition frequency = 500 Hz, sonication duration = 0.5 sec, inter-stimulus interval = 7 sec, and duty cycle = 36-50%. The intensity level did not exceed ISPTA = 2.25 W/cm2 (peak negative pressure = 0.36 MPa free-field equivalent). The transducer pressure field (Fig. 1.G & H) resulting from hydrophone mapping was used to assign virtual PLIFUS crosshairs at the beam spatial peak pressure. Fig. 2 depicts the estimated stimulation regions of four assigned hippocampus targets for Subject 1.
Fig. 2:
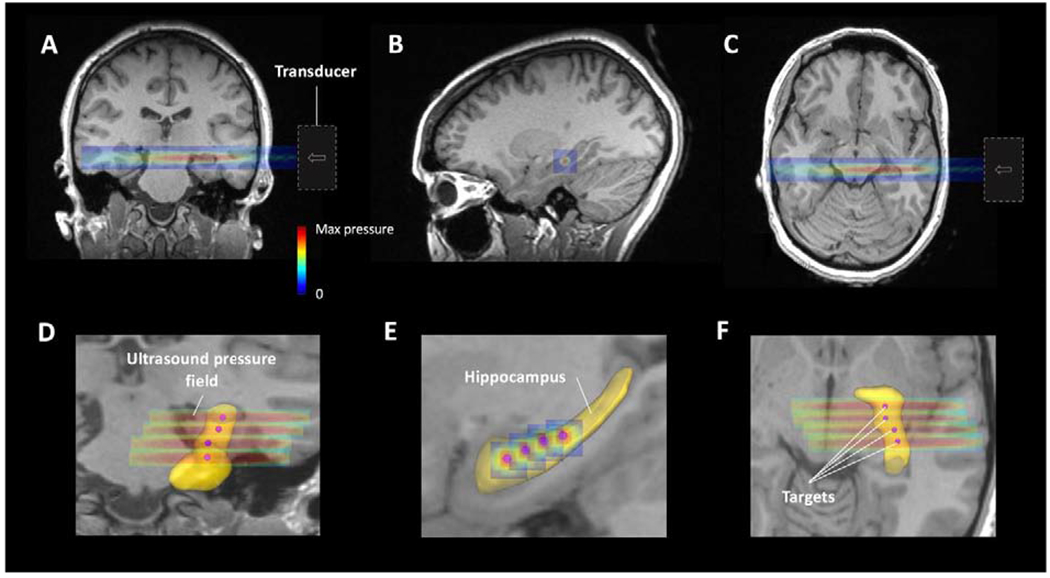
Predicted FUS beam intensity maps projected onto the left hippocampus of Subject 1 in the (A) coronal, (B) sagittal, and (C) axial planes, respectively, for one target used during a PLIFUS session. (D, E, & F) Zoomed in views of the TLE volunteers’ masked hippocampus (yellow) with four PLIFUS target locations and associated predicted ultrasound beam pressures emitted across the hippocampus during the full course of treatment.
DISCUSSION
Device development
The technique presented offers a means to investigate therapeutic neuromodulation with repetitive PLIFUS treatment Stimulation Parameters (SPs), multiple targets, multiple sessions, and a new strategy to deliver the energy across large volumes of brain tissue with one coupling location using laboratory-built equipment. We chose this design and SPs based on successful design characteristics of past transcranial ultrasound systems and human studies using SPs which resulted in no adverse effects. The primary goal was to investigate PLIFUS therapeutic neuromodulation in subjects with hippocampal SOZs. Producing a compact device prototype for manufacturing production was not a design goal of this prototype. Recent FDA-cleared hemispherical phased-array systems used for brain-ablation require real-time MRI and a stereotactic head frame (Elias et al. 2016), which may not be ideal for multi-session therapeutic neuromodulation. Considering the recent success and design simplicity of neuronavigation-guided single-element piezoelectric transducers and their SPs (Lee et al. 2016a; Lee et al. 2016b; Legon et al. 2014; Legon et al. 2018), we chose a similar design. Other PLIFUS investigations incorporated a large and static coupling water bag or housing to the temporal acoustic window while mechanically steering a transducer internally (Gavrilov 1984; Monti 2015). One advantage of our design is not having to recouple the device to the temporal acoustic window for every target as is the case with transducer cone-shaped gel or water bag coupling connected to a positioning arm (Lin et al. 2016; Wu et al. 2018). The windowed vessel was sized to allow transducer translational steering to all regions of the hippocampus using one coupling location. However, it may be more beneficial to use small non-stereotactic phased-arrays in future studies to decrease session time using electronic beam steering and also the ability to correct skull aberration (Liu et al. 2014; Pernot et al. 2003; Rosnitskiy et al. 2019; Smiley et al. 2019). There is work being done to use robotic-assisted positioning of transducers (Kim and Lee 2016), and with the semi-real-time computational skull-aberration correction (Yoon et al. 2018), this may also decrease session time and increase transducer targeting accuracy.
Irradiated tissue regions
The overlaid beam projections shown in Fig. 2 illustrate where the ultrasound beam spatial peak was estimated to reach the left hippocampus of Subject 1. It also shows that the longitudinal Full-Width-Half-Maximum (FWHM) extends approximately 19 mm lateral and/or medial to the hippocampus. Past studies have determined the spatial error with neuronavigation-guided single-element piezoelectric transducers without skull-aberration correction using bench top testing in animals, yielding as much as 5 mm error in all directions (Brinker et al. 2019; Kim et al. 2012; Yoon et al. 2018). The longitudinal-FWHM might be advantageous because it will most likely cover the extents of the hippocampus even during the maximum predicted skull-induced beam distortions. Here, the intention is to introduce the majority of mechanical disruption energy to the SOZ (hippocampus). The PLIFUS target locations for Subject 1 were chosen so that the treatment area would be similar to what tissue regions would conventionally be resected in hippocampectomy for TLE (the anterior two-thirds of the hippocampus). Four targets were required for the radial cross-section of the ultrasound beam to span the length of the anterior hippocampus as depicted in Fig. 2E. These target locations were also limited from the beam having to enter through the acoustic temporal window. Future subjects may require more or fewer targets based on their head size and SOZ.
The intensity levels used for this device are calibrated only to yield functionally modulatory effects in the beam focus previously shown to be safe in human brain (Lee et al. 2016a; Legon et al. 2018). Effects induced beyond the beam focus are assumed to be vanishingly small or negligible.
Stimulation strategy
Extensive research has been conducted to correlate SPs and acoustic intensity levels with brain tissue heating, cavitation, blood-brain-barrier disruption, and ultrasound beam transmission through the scalp-skull-meninges media to ensure safe preservation of tissue integrity during PLIFUS neuromodulation. The SPs for therapeutic neuromodulation used for this platform were developed by adapting safe human PLIFUS SPs used in brain mapping studies into a therapeutic train of repetitive stimulation for multiple targets over multiple sessions. No more than 140 seconds of continuous PLIFUS was emitted to one brain target per session reported in most previous single-element piezoelectric transducer PLIFUS human studies (Izadifar et al. 2017; Krishna et al. 2017; Monti et al. 2016; Yoo 2018) and acoustic intensity was estimated to reach ISPTA = 4.4 W/cm2 at the highest reported level in the cortex after crossing the skull (Lee et al. 2016a). Hippocampal targets stimulated using our platform were limited to 140 sec each and ISPTA = 3 W/cm2 was set as the maximum allowable beam intensity before it entered the skull to satisfy the International Electrotechnical Commission (60601 part 2) standard limit for therapeutic ultrasound (Duck 2007).
CONCLUSION
The presented technique and methods offer a means to investigate PLIFUS therapeutic neuromodulation across the human hippocampus and lays the groundwork for how future hardware in platforms can be designed to study PLIFUS neuromodulation for neurological disorders. Future directions may incorporate EEG and acoustic emission acquisition methods (Brinker et al. 2018; Wu et al. 2018) for providing real-time feedback measurements during PLIFUS therapeutic neuromodulation to quantify and verify the therapeutic dosage during treatment. The use of this platform is still ongoing in a study to investigate the effects of PLIFUS therapeutic neuromodulation in the SOZ of patients with TLE.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jim MacArthur from the John A. Paulson School of Engineering and Applied Sciences of Harvard University for help designing and building the failsafe circuit. The authors also thank Wonhye Lee, PhD, Kyungho Yoon, PhD, and Rees Cosgrove, MD from Brigham and Women’s Hospital and Harvard Medical School for their insight regarding device and stimulation protocol design. This work was supported by the National Institutes of Health under Grants P01 CA174645 and R25CA089017 and the Brigham Research Institute’s 2017 Bright Futures Prize. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
TYM has previously consulted for Janssen Pharmaceuticals Inc and Ad Scientiam SAS, and he is currently employed by Sage Therapeutics, Inc. None of these supported or influenced the present work. All other authors have nothing to declare.
REFERENCES
- Biase L, Falato E, Lazzaro V Di. Transcranial Focused Ultrasound ( tFUS ) and Transcranial Unfocused Ultrasound ( tUS ) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front Neurol 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker S, Preiswerk F, McDannold N, Parker K, Mariano T. Virtual brain projection for evaluating trans-skull beam behavior of transcranial ultrasound devices. Ultrasound Med Bio 2019;45:1850–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker ST, Crake C, Ives JR, Bubrick EJ, Mcdannold NJ. Scalp sensor for simultaneous acoustic emission detection and electroencephalography during transcranial ultrasound. Phys Med Biol IOP Publishing, 2018;63:11pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tsai C, Lin C, Lee C, Yu H, Hsieh T, Liu H. Transcranial Focused Ultrasound Pulsation Suppresses Pentylenetetrazol Induced Epilepsy In Vivo. Brain Stimul 2019;BRS 1569. [DOI] [PubMed] [Google Scholar]
- Duck FA. Medical and non-medical protection standards for ultrasound and infrasound. Pog Biophys Mol Biol 2007;93:176–191. [DOI] [PubMed] [Google Scholar]
- Elias WJ, Lipsman N, Ondo WG, Ghanouni P. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med , al 2016;375:730–739. [DOI] [PubMed] [Google Scholar]
- Fomenko A, Neudorfer C, Dallapiazza RF, Kalia SK, Lozano A. Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications. Brain Stimul Elsevier Ltd, 2018;11:1–9. [DOI] [PubMed] [Google Scholar]
- Fry F, Ades H, Fry W. Production of Reversible Changes in the Central Nervous System by Ultrasound. Science (80- ) 1958;127:83–84. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR. Use of focused ultrasound for stimulation of nerve structures. Ultrasoincs 1984; 132–138. [DOI] [PubMed] [Google Scholar]
- Hakimova H, Kim S, Chu K, Kun S, Jeong B, Jeon D. Epilepsy & Behavior Ultrasound stimulation inhibits recurrent seizures and improves behavioral outcome in an experimental model of mesial temporal lobe epilepsy. Epilepsy Behav 2015;49:26–32. [DOI] [PubMed] [Google Scholar]
- Izadifar Z, Babyn P, Chapman D. Review mechanical and biological effects of ultrasound: A review of present knowledge. Ultrasound Med Bio 2017;43:1085–1104. [DOI] [PubMed] [Google Scholar]
- Kim H, Chiu A, Park S, Yoo S-S. Image-guided Navigation of Single-element Focused Ultrasound Transducer. Int J Imaging Syst Technol 2012;22:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S. Development of a Wearable Robotic Positioning System for Noninvasive Transcranial Focused Ultrasound Stimulation. IEEE/ASME Trans Mechatronics IEEE, 2016;21:2284–2293. [Google Scholar]
- Krishna V, Sammartino F, Rezai A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology Advances in Diagnosis and Treatment. JAMANeurology 2017;75:246–54. [DOI] [PubMed] [Google Scholar]
- Lee W, Chung YA, Jung Y, Song I-U, Yoo S-S. Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused. BMC Neurosci BioMed Central, 2016a;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim H, Jung Y, Chung YA, Song I, Lee J. Transcranial focused ultrasound stimulation of human primary visual cortex. Nat Publ Gr Nature Publishing Group, 2016b;: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Ai L, Bansal P, Mueller JK. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp Wiley Online Library, 2018;39:1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Publ Gr Nature Publishing Group, 2014;17:322–329. [DOI] [PubMed] [Google Scholar]
- Lin Y, Chen K, Huang C, Wei K. Non-invasive focused ultrasound-based synergistic treatment of brain tumors. J Cancer Res Pract 2016;3:63–68. [Google Scholar]
- Liu H, Jan C, Chu P, Hong J, Lee P, Hsu J, Lin C, Huang C, Chen P, Wei K. Design and Experimental Evaluation of a Phased-Array System for Transcranial Blood - Brain Barrier Opening and Brain Drug Delivery. IEEE Trans Biomed Eng IEEE, 2014;61:1350–1360. [DOI] [PubMed] [Google Scholar]
- Min B, Bystritsky A, Jung K, Fischer K, Zhang Y, Maeng L, Park SI, Chung Y, Jolesz FA, Yoo S. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci BioMed Central Ltd, 2011;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM. Thalamic Low Intensity Focused Ultrasound in Acute Brain Injury (LIFUP). FDA # NCT02522429. 2015. [Google Scholar]
- Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM. Brain Stimulation Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury : A First-in-Man Report. Brain Stimul Elsevier Inc., 2016;9:940–941. [DOI] [PubMed] [Google Scholar]
- Pernot M, Aubry J, Tanter M, Thomas J, Fink M. Physics in Medicine & Biology High power transcranial beam steering for ultrasonic brain therapy High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol 2003;48:2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiswerk F, Brinker S, McDannold N, Mariano T. Open-source neuronavigation for multimodal non-invasive brain stimulation using 3D Slicer. arXiv:190912458 [physics.med-ph] 2019;https://github.com/fpreiswerk/neuronav. [Google Scholar]
- Rosnitskiy PB, Yuldashev PV, Sapozhnikov OA, Gavrilov LR, Khokhlova VA, Rosnitskiy PB, Yuldashev PV, Sapozhnikov OA. fully populated ultrasound array with aberration correction Simulation of nonlinear trans-skull focusing and formation of shocks in brain using a fully populated ultrasound array with aberration correction. J Acoust Soc Am 2019;146:1786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley A, Howell M, Clement GT, Fleischman AJ. Toward transcranial ultrasound tomography : design of a 456-element low profile conformal array. Biomed Phys Eng Express 5 IOP Publishing, 2019;5:025025. [Google Scholar]
- Wu SY, Aurup C, Sanchez CS, Grondin J, Zheng W, Kamimura H, Ferrera VP, Konofagou EE. Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping. Sci Rep 2018;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Kyungho Y, Croce P, Cammalleri A, Margolin RW, Lee W. Focused ultrasound brain stimulation to anesthetized rats induces long-term changes in somatosensory evoked potentials. Int J Imaging Syst Technol 2018;28:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S Technical Review and Perspectives of Transcranial Focused Ultrasound Brain Stimulation for Neurorehabilitation Technical Review and Perspectives of Transcranial Focused Ultrasound Brain Stimulation for Neurorehabilitation. Brain & NeuroRehabilitation 2018;11:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Lee W, Croce P, Cammalleri A, Yoo SS. Multi-resolution simulation of focused ultrasound propagation through ovine skull from a single-element transducer. Phys Med Biol IOP Publishing, 2018;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


