Abstract
Background
Even with antiretroviral therapy (ART), persons with HIV (PWH) experience increased morbidity/mortality. Cytomegalovirus (CMV) and Epstein-Barr-Virus (EBV) co-infections likely exacerbate inflammatory-related diseases.
Objective
To determine if presence of detectable CMV or EBV DNA in peripheral blood mononuclear cells (PBMC) is associated with non-AIDS events among PWH receiving modern ART.
Design
We performed a case-control study of PWH starting ART and HIV-suppressed at year 1 and thereafter, 140 cases who experienced non-AIDS events and 305 matched controls. Events included myocardial infarction, stroke, malignancy, serious bacterial infection or death.
Methods
Blood samples were studied pre-ART, 1-year post-ART and pre-event. Controls had an event-free follow-up equal or greater than cases. CMV and EBV levels were measured in PBMC. Conditional logistic regression analysis assessed associations and adjusted for relevant covariates; Spearman’s correlations compared CMV and EBV levels with other biomarkers.
Results
CMV was detected in PBMC of 25% of participants, EBV was detected in >90%. Higher EBV levels were associated with increased risk of events at all time points (odds ratio (OR) per one IQR = 1.5–1.7, all p<0.009). At year 1, detectable CMV was associated with increased risk of events in most adjusted models (OR =1.4–1.8, p-values ranging 0.03–0.17). Higher levels of CMV and EBV correlated with multiple inflammatory markers and lower CD4/CD8 ratio.
Conclusions
In PWH starting ART, CMV and EBV in PBMC were associated with development of non-AIDS events. Clinical trials will be needed to understand causal mechanisms and ways to interrupt them.
Keywords: HIV, Inflammation, Non-AIDS event, CMV, EBV, Non-AIDS mortality, Viral suppression, CMV and EBV DNA
Introduction
Antiretroviral therapy (ART) improves health, prolongs survival and reduces HIV transmission [1]. Nevertheless, even when successfully treated, HIV infection remains associated with higher morbidity and mortality due to non-AIDS defining events, such as cardiovascular disease (CVD) and non–AIDS-defining malignancies [2]. This increased morbidity and mortality is also associated with inflammation and immune dysfunction, which persists in most people with HIV (PWH) despite modern ART [3]. Potential contributors to such inflammation include: direct effect of HIV expression, translocation of bacterial and fungal products from the gut into the systemic circulation, and chronic viral co-infections like cytomegalovirus (CMV) or Epstein-Barr Virus (EBV) [2, 4].
Nearly all PWH are co-infected with CMV and EBV, and most PWH experience intermittent bursts of CMV and EBV replication, even during ART [5]. Such replication is linked to repeated stimulation of the CD8+ T-cell population [6, 7], and presence of CMV DNA is associated with lower CD4/CD8 ratio during ART [6, 8, 9], and increased activation, cycling, and exhaustion of T-cells [10, 11]. Further, magnitude of humoral and cellular CMV-specific immune response among PWH is associated with cardiovascular disease and neurocognitive dysfunction [12–16]. During advanced stages of HIV infection, the immune system is often damaged to the point that it can no longer contain CMV replication, thus causing significant morbidity and mortality. Older studies among PWH not receiving modern ART found that CMV DNA in plasma consistently predicted mortality, mostly with AIDS associated events [17, 18]. However, the vast majority of PWH on modern ART are undetectable for CMV DNA in plasma, and yet continue to have residual inflammation and are at increased risk for adverse clinical outcomes [2]. Co-infection with EBV has also been associated with inflammation, end-organ diseases, and cancer [19, 20] among both PWH and HIV-uninfected persons, although less consistently than CMV.
Most previous studies evaluating the effect of CMV and EBV on end-organ diseases in the setting of HIV, have considered antibody responses and not viral replication [15, 16, 21, 22]. Thus, it is still unclear if identified end-organ diseases are directly associated with viral activity itself or are a consequence of the immune response towards CMV and EBV. The present study aimed to determine if presence of detectable CMV or EBV DNA in peripheral blood mononuclear cells (PBMC) is associated with non-AIDS events among PWH receiving modern ART.
Methods
Study Cohort and Samples
The study population includes treatment-naïve PWH who received initial treatment as part of a prior ACTG study (i.e. A388, A384, A5014, A5095, A5142, A5202) [23–29], see Supplementary Table 1 details. All 445 participants (140 cases who experienced non-AIDS events and 305 matched controls) were ART naïve at the time of enrollment. They all had a plasma HIV-1 RNA load of <400 copies/mL at week 48 after ART initiation and maintained a plasma HIV RNA load of < 400 copies/mL at subsequent visits (isolated values ≥400 copies/mL were allowed if preceding and subsequent values were <400 copies/mL). All participants provided written informed consent, and institutional review board approval was obtained by each study site.
Cases and controls were originally identified as part of a prior case-control study [30]. Briefly, cases were defined as people who had a myocardial infarction (MI), stroke, non–AIDS-defining malignancy, serious bacterial infection (i.e. bacterial pneumonia/bronchitis, sepsis, or bacterial meningitis) or died from a non-accidental non–AIDS-related event after week 48 [30, 31]. For each case, 2 or 3 controls were identified who had an endpoint-free follow-up time equal or greater than that of the case and were matched on the basis of age (within 10 years; median 45 years), sex (84% male), baseline CD4+ T cell count (within 50 cells/mm3; median 219 cells/mm3), ART regimen at week 48 (whether it contained a protease inhibitor or abacavir), and parent study [30, 31]. In our current study, we included all cases and controls who had available PBMC specimens for testing. Controls without a corresponding case, and cases without at least one corresponding control were excluded.
Stored PBMC specimens were evaluated at the following 3 time points: before ART initiation (baseline); 48–64 weeks after starting ART (year 1); and the ACTG study visit immediately preceding the event for cases (median of 10.5 weeks [IQR: 6–19]) and a similar time point for corresponding controls (pre-event). PBMC samples were available for 232 participants at baseline (85 cases and 147 controls), 404 participants at year 1 (128 cases and 276 controls), and 347 participants pre-event (110 cases and 237 controls).
CMV and EBV Antibody (IgG) and DNA Levels
Anti-CMV and EBV IgG antibody levels were measured in blood plasma at year 1 using GenWay Biotech ELISA kits [22, 32, 33]. DNA was extracted from 5 million PBMCs at each time point using Qiagen QIAmp DNA Mini Kit. Total CMV and EBV DNA were quantified by droplet digital PCR (ddPCR) [22, 34, 35]. Copy numbers were calculated as the mean of replicate PCR measurements and normalized to one million cells, determined by the housekeeping gene Ribonuclease P/MRP Subunit P30 (RPP30) [36]. CMV and EBV IgG level < 1.2 IU/mL were considered as seronegative.
Inflammation Associated Soluble and Cellular Biomarkers
Cellular and soluble markers of inflammation, immune activation, and gut damage were generated as part of previous studies at baseline, year 1, and pre-event [30, 31]. These included plasma interleukin (IL)-6, Interferon gamma-induced protein (IP)-10, soluble tumor necrosis factor receptors (sTNFR) -I, sTNFR-II, soluble cluster of differentiation (sCD)14, D-Dimer, Intestinal fatty-acid binding protein (I-FABP), Lipopolysaccharide binding protein (LBP), sCD163, soluble urokinase-type plasminogen activator receptor (suPAR), (1–3)-β-D-Glucan (BDG), and cellular %CD4+:CD28−CD57+, %CD8+:CD28−CD57+, %CD4+:CD38+HLADR+, %CD8+:CD38+HLADR+, %CD4+:PD1+, %CD8+:PD1+, and CD4/CD8 ratio.
Statistical Analysis
Non-normal data were log-transformed to the log10 scale; values of zero were imputed to 0.1 prior to log-10 transformations. Descriptive summaries of continuous variables included the frequency, median, and interquartile range (IQR), while frequency and percentage were used for categorical variables. All statistical tests used a two-sided 5% significance level, without adjustment for multiple comparisons. We emphasized magnitudes of effect sizes, consistency of observed effects, and robustness to our adjusted models.
First, we assessed associations between CMV DNA, EBV DNA, antibody levels and previously generated soluble and cellular biomarkers using Spearman’s correlations at concurrent time points. Only the controls were included in these analyses to provide a more generalizable estimate of the true association in the general population, as cases were enriched for Non-AIDS events.
Subsequently, we used conditional logistic regression analysis to determine if higher levels of EBV DNA or presence of CMV DNA were associated with increased risk of having subsequent non-AIDS events in a cross-sectional analysis at each time point. For this analysis, EBV DNA was analyzed continuously on the log10 scale and was categorized by the pooled (combined cases and controls) IQR such that the levels of the biomarkers were grouped as <25th percentile, 25th-49th percentile, 50th-74th percentile, and ≥ 75th percentile. Due to the large proportion of undetectable by lab assay, CMV DNA was analyzed as a dichotomous variable: detectable (>0 copies/million) versus undetectable (0 copies/million). Models were individually adjusted for each covariate (i.e., HIV RNA level, CD4 cell count, Hepatitis B/C status, smoking status, history of injection drug use, diabetes, hypertension, use of any cardiac medications, waist-to-hip ratio, family history of MI); additional adjustments for previously analyzed biomarkers (as mentioned above) were also evaluated [30, 31]. Separate unadjusted conditional logistic regression models further examined four event subgroups: death, malignancy, cardiovascular (MI/stroke), and serious bacterial infections. For the death subgroup analysis of CMV DNA, due to the small number of deaths, we examined the association using the Cochran-Mantel-Haenszel test for stratified categorical data.
Sensitivity analysis was performed to assess the potential impact of false negatives in CMV and EBV DNA detection due to small low template input (in samples with low DNA concentration). Conditional logistic regression analysis was repeated excluding samples with the lowest template input (RPP30 levels <10th percentile).
Results
Cohort and Samples
All participants were seropositive for CMV and EBV IgG. Study participants who did and did not have non-AIDS events (i.e. cases and controls) had overall similar demographic characteristics at baseline, but differences were observed in the percentage with chronic Hepatitis B/C, history of hypertension, and smoking status (Table 1); models include adjustments for these characteristics. Among 140 cases, non–AIDS-defining events occurred at a median of 2.9 years (IQR: 1.8–4.7) after ART initiation. Among the cases, 15% were deaths, 29% were MI/strokes, 35% were malignancies, and 24% were bacterial infections; eight cases died from non-AIDS events (3 MI/strokes, 2 malignancies, 3 bacterial infections) and are included twice in these summaries.
Table 1:
Baseline Characteristics
| Characteristic | Case (N=140) | Control (N=305) | Total (N=445) | |
|---|---|---|---|---|
| Age (at parent study entry) | Years, Median (IQR) | 46 (40–53) | 44 (39–50) | 45 (39–50) |
| Regimens (by parent study) | ACTG 384 / ACTG 388 | 39 (28%) | 84 (28%) | 123 (28%) |
| A5014 / A5095 / A5142 | 69 (49%) | 158 (52%) | 227 (51%) | |
| A5202 | 32 (23%) | 63 (21%) | 95 (21%) | |
| Sex | Male, N (%) | 118 (84%) | 258 (85%) | 376 (84%) |
| Female, N (%) | 22 (16%) | 47 (15%) | 69 (16%) | |
| Race/ethnicity | White Non-Hispanic, N (%) | 72 (51%) | 148 (49%) | 220 (49%) |
| Black Non-Hispanic, N (%) | 52 (37%) | 87 (29%) | 139 (31%) | |
| Hispanic, N (%) | 15 (11%) | 59 (19%) | 74 (17%) | |
| Others, N (%) | 1 (1%) | 11 (3%) | 12 (3%) | |
| Baseline CD4 | Cells/ul, Median (IQR) | 211 (89–341) | 222 (76–335) | 219 (77–336) |
| Baseline log10 HIV RNA | Copies/mL, Median (IQR) | 4.8 (4.4–5.3) | 4.8 (4.4–5.4) | 4.8 (4.4–5.4) |
| Chronic Hepatitis B/C | Yes, N (%) | 36 (26%) | 34 (11%) | 70 (16%) |
| Injection Drug Use | Yes, N (%) | 20 (14%) | 30 (10%) | 50 (11%) |
| Waist-to-hip Ratio | Median (IQR) | 0.92 (0.89–0.96) | 0.92 (0.88–0.97) | 0.92 (0.89–0.97) |
| History of Diabetes | Yes, N (%) | 11 (8%) | 15 (5%) | 26 (6%) |
| History of Hypertension | Yes, N (%) | 46 (33%) | 55 (18%) | 101 (23%) |
| Use of antihypertensive or lipid lowering agents | Yes, N (%) | 32 (23%) | 39 (13%) | 71 (16%) |
| Current or Past Smoker | Yes, N (%) | 105 (75%) | 168 (55%) | 273 (61%) |
| Family history of MI | Yes, N (%) | 30 (21%) | 45 (15%) | 75 (17%) |
IQR: interquartile range
See supplement for ACTG study details and regimens.
Frequency and Levels of CMV and EBV DNA
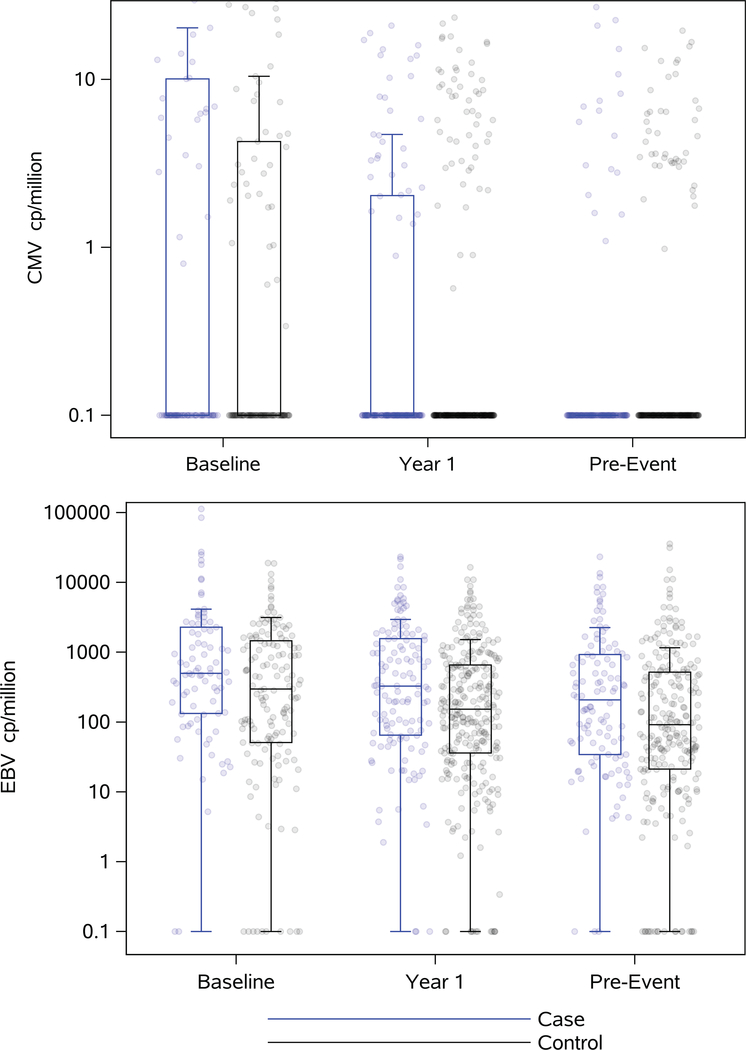
CMV DNA was detected in 40% of PBMC samples at baseline, 24% at year 1 and 18% at pre-event. In contrast, >90% of all samples contained detectable EBV DNA at all time points (Figure 1 and Supplementary Table 2). At baseline, 36% of PBMC samples were detectable for both viruses (CMV and EBV), while 23% and 18% were detectable for both viruses at year 1 and pre-event, respectively.
Figure 1:
Levels of CMV and EBV DNA per million of mononuclear cells at each time point (baseline (case: N=85, control: N=147), year 1 (case: N=128, control: N=276) and pre-event (case: N=110, control: N=237)) for cases (in blue) and controls (black). Boxplots show medians and interquartile ranges. Lower limit of detection is 0 copies/million, represented as 0.1 copies/million due to log-transformation.
CMV and EBV DNA Associations with Inflammation Among Controls
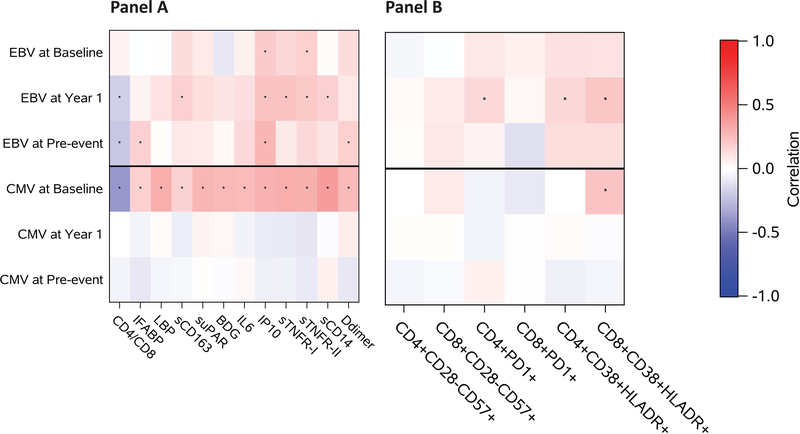
In analyzing associations between CMV DNA, EBV DNA, and antibody levels, there was a weak association between CMV DNA and EBV DNA at the pre-event time point (r=0.18, p=0.005), but no associations were seen at year 1 or baseline. At year 1, we observed a modest correlation between EBV DNA and EBV IgG antibody levels (r=0.43, p<0.0001), but not between CMV DNA and CMV IgG level. Figure 2 summarizes correlations between CMV and EBV DNA and inflammation associated biomarkers. Overall, levels of CMV DNA at baseline had modest positive correlations with all soluble inflammatory markers (r ranging between 0.18 and 0.35, p<0.036) and was negatively correlated with the CD4/CD8 ratio (r=−0.39, p<0.0001). These associations for CMV were not observed at year 1 or pre-event. On the other hand, EBV DNA level was only associated with IP-10 at baseline (r= 0.16, p=0.046), which remained at year 1 (r =0.18, p=0.003). At year 1, levels of EBV DNA were also positively associated with sCD163, sTNFR-I, sTNFR-II, %CD8+:CD38+HLADR+, %CD4+:CD38+HLADR+, and %CD4+:PD1+ (r ranging between 0.14 and 0.22, p<0.023) and negatively associated with the CD4/CD8 ratio (r = −0.17, p=0.005). At pre-event, EBV DNA levels were also associated with IP-10 (r=0.20, p=0.002) and with the CD4/CD8 ratio (r = −0.21, p=0.001), and new associations were observed with IL-6 and D-dimers (r=0.16, p<=0.015). Scatterplots of the raw data are included in Supplementary Figures 5A–F.
Figure 2.
Heatmap showing correlation coefficients between EBV and CMV DNA at each time point with various soluble (A) and cellular (B) markers of inflammation, immune activation, and gut damage among controls at baseline (A: N=125–147, B: N=110), year 1 (A: N=254–276, B: N=267), and pre-event (A: N=215–237, B: N=184). * indicated statistical significance at p<0.05.
EBV DNA and CMV DNA as Predictors of Non-AIDS Events
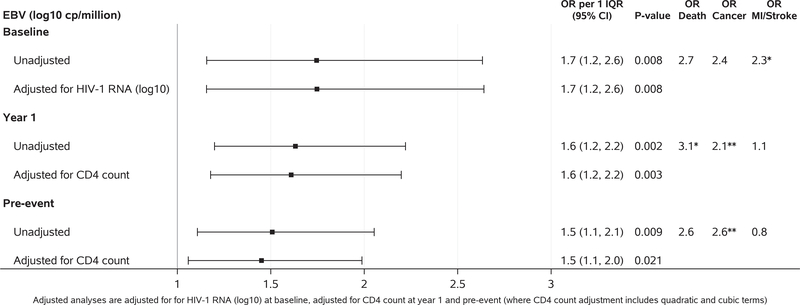
Higher levels of EBV DNA were associated with increased risk of subsequent non-AIDS events at all time points (odd ratios per one IQR ranging 1.5–1.7, p<0.009). The strongest associations were observed at baseline. The effect persisted after adjusting for each of the clinical factors and inflammatory biomarkers (odds ratios per one IQR ranging 1.3 to 2.0) (Figure 3 and Supplementary tables 3 A–F). Examining event sub-groups, higher levels of EBV DNA at baseline were associated with increased risk of MI/stroke (odds ratio per one IQR (95% CI) = 2.3 (1.1, 4.7)), and marginally with elevated risk of malignancy (odd ratio per one IQR (95% CI) = 2.4 (1.0, 6.0)). Although not significant, higher odds of death were observed for higher baseline EBV DNA levels (odds ratio per one IQR (95% CI) = 2.7 (0.5, 13.2)). At year 1 and pre-event, higher EBV DNA levels were associated with increased risk of death (year 1: odds ratio per one IQR (95% CI) =3.1 (1.2, 8.1); pre-event: odds ratio per one IQR (95% CI) = 2.6 (1.0, 6.8), malignancy (year 1: odds ratio per one IQR (95% CI) = 2.1 (1.2, 3.6); pre-event: odds ratio per one IQR (95% CI) = 2.6 (1.4, 4.9)), and serious bacterial infection (year 1: odds ratio per one IQR (95% CI) = 1.9 (1.0, 3.4); pre-event: odds ratio per one IQR (95% CI) = 1.8 (0.9, 3.5)). Increased risk of MI/stroke was not seen for EBV DNA at year 1 or pre-event.
Figure 3.
Associations between levels of EBV DNA and subsequent non-AIDS event at baseline (case: N=85, control: N=147), year 1 (case: N=128, control: N=276) and pre-event (case: N=110, control: N=237). Figure shows unadjusted analysis and analysis adjusted for HIV RNA (log10) at baseline and for CD4 count at year 1 and pre-event (where CD4 count adjustment includes quadratic and cubic terms). * indicated statistical significance at p<0.05 while ** indicated p<0.01.
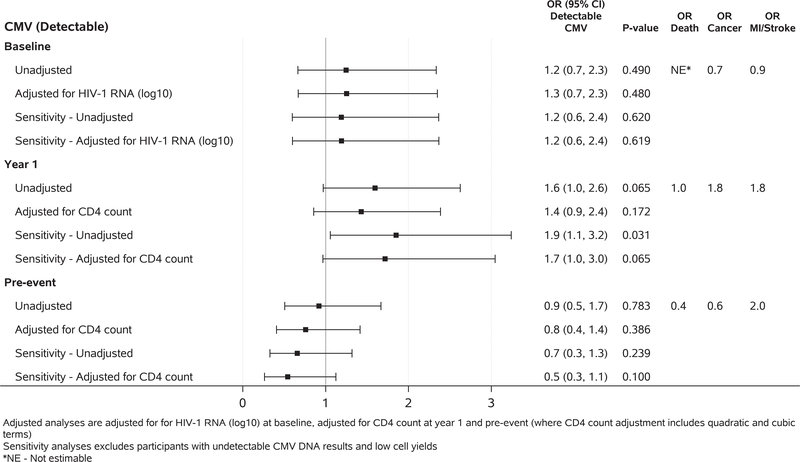
Similar to the EBV analysis, we observed a trend between having detectable CMV DNA in PBMC at year 1 and increased risk of non-AIDS events (odds ratio (95% CI) = 1.6 (1.0, 2.6), p=0.07); this effect persisted after adjustment for clinical factors (odds ratios ranging 1.4 to 1.7) (Figure 4 and Supplementary tables 4 A–C). The analyses were repeated after excluding participants with low template input (i.e. those in the lowest 10th percentile of RPP30). The results were unchanged at baseline and at the pre-event time points, but the results at year 1 showed stronger associations with significantly increased risk of non-AIDS events (odds ratio (95% CI) = 1.9 (1.1, 3.2), p=0.017) with the presence of CMV DNA. In event sub-group analyses, marginal associations were observed between CMV DNA and risk of malignancy and MI/Stroke at year 1 (malignancy: odds ratio (95% CI) = 1.8 (0.8, 4.1); MI/Stroke: odds ratio (95% CI) = 1.8 (0.7, 4.6)). The Cochran-Mantel-Haenszel test was used to evaluate the risk of death due to the small number of deaths and analytic convergence issues. There was an increased risk of death for those with detectable CMV DNA at baseline (odds ratio (95% CI) = 9.0 (1.9, 42.6)).
Figure 4.
Associations between presence of detectable CMV DNA and subsequent non-AIDS event at baseline (case: N=85, control: N=147), year 1 (case: N=128, control: N=276) and pre-event (case: N=110, control: N=237). Analysis adjusted for HIV RNA (log10) at baseline, adjusted for CD4 count at year 1 and pre-event (where CD4 count adjustment includes quadratic and cubic terms). Sensitivity analyses exclude participants with low cell yield. *NE – Not estimable. Using a post-hoc Cochran-Mantel-Haenszel test, there was an increased risk of death for those with detectable CMV DNA at baseline [odds ratio (95% CI) = 9.0 (1.9, 42.6), p=0.002, not shown in the figure].
Discussion
Despite ART, HIV infection remains associated with higher morbidity and mortality, driven by increased inflammation [2], and chronic CMV and EBV co-infections may exacerbate this inflammation [5]. To better understand the impact of chronic EBV and CMV co-infection in PWH on modern ART, we examined associations between viral DNA in peripheral blood cells and non-AIDS events in a case-control study of 445 PWH who did and did not have non-AIDS events over time. The analysis evaluated associations with a composite non-AIDS endpoint, as chronic inflammation driven by CMV and EBV co-infections is a plausible mechanism underlying each of the non-AIDS events; event-specific analyses were also performed.
Overall, cellular CMV DNA was detected in 25% of all samples, while EBV DNA was detected in >90%. Higher levels of EBV DNA in blood cells were consistently associated with an increased risk of developing subsequent non-AIDS events, with the strongest associations between EBV DNA at baseline and subsequent non-AIDS events. Detectable CMV DNA at year 1 was weakly associated with increased risk of sub-sequent non-AIDS events in adjusted analysis, but this was not observed for CMV DNA in blood cells at other time points. These results are consistent with a study that showed an approximately 50% higher risk of severe non–AIDS-defining events in PWH who were seropositive for CMV antibody [15], and other studies that described associations between CMV IgG or cellular immune response and non-AIDS events [22], in particular cardiovascular [12, 14] and neurological complications [16]. However, it is not clear what the correlations are between EBV and CMV replication and immune responses, especially as a previous study showed that higher CMV IgG levels were associated with lower CMV shedding in the setting of HIV [33]. Interestingly, in our study we did not see a correlation between CMV DNA in PBMC and CMV IgG levels at year 1, but we did see a positive correlation between EBV DNA and EBV IgG. This discordance could be due to the differences in EBV and CMV DNA detectability in blood cells or preferred sites of replication between viruses. Alternative explanations are that T cell immunity might reflect the burden of infection rather than humoral response, or that measurement of latent infection does not reflect the burden of active, lytic infection.
In people with intact immune system, CMV does not frequently replicate in blood but at other mucosal sites (e.g. salivary glands, genital tract, but, endothelial cells), while EBV commonly replicates in the circulating B cells [5]. Thus, the modest association observed between CMV DNA in PBMC and non-AIDS events and the lack of association between CMV and inflammation during ART (year 1 and pre-event) was likely because we did not collect and evaluate mucosal specimens. The presence of CMV DNA in blood cells (usually monocytes) may reflect low level viral replication but may also simply reflect latent CMV infection and not necessarily CMV replication. Also, the clinical significance of detectable CMV DNA in blood cells is unknown. Therefore, future studies of CMV replication and clinical outcomes should assess mucosal specimens, as well. In contrast, the frequency of EBV DNA in circulating blood cells (mostly B-cells [5]) was consistently associated with non-AIDS events before and after ART. The link between EBV levels and clinical outcomes merit further study to help determine if these are linked to each other through systemic inflammation or more directly.
We also explored associations between CMV and EBV replication and various inflammatory markers. As in earlier studies [4, 37], levels of CMV DNA in PBMC were positively correlated with several soluble inflammatory markers at baseline, but not at subsequent time points. EBV DNA levels in PBMC were correlated with a few biomarkers at baseline but with more markers at year 1 of therapy. Both CMV and EBV DNA were associated with lower CD4/CD8 ratio, compatible with expansion of virus specific CD8+ T cells[6]. These diminished ratios are also important predictors of morbidity and mortality in PWH[38], yet the determinants of this relationship are unclear.
This study had a number of limitations. Because this was an observational study, we cannot establish a causal relationship between EBV and CMV DNA in PBMC and non-AIDS events. Also, some unmeasured variables might have confounded our analysis. For example, immune dysfunction from HIV infection alone could be driving both inflammation-related clinical events and CMV or EBV replication independently. The case-control design also makes it impossible to generate direct estimates of incident morbidity risk related to each assessed variable. In addition, the number of cases and controls with available samples varied between time points and the small sample size within each event subgroup limited our power to observe associations between CMV DNA levels and specific non-AIDS events. Nevertheless, this study provides insights into the association between CMV and EBV DNA and non-AIDS clinical outcomes. Carefully designed clinical trials aimed at suppressing CMV and EBV replication among PWH are needed to confirm our findings and may help clarify the mechanisms between inflammation and related clinical events and might be important in improving the health of PWH who are co-infected with CMV and EBV.
Supplementary Material
Acknowledgments / Funding
This work was supported by the Department of Veterans Affairs and grants from the National Institutes of Health: M1 AI068634, AI68636, AI68634, AI036214, MH062512, AI134295, HD094646, AI131385, AI100665, MH107345, AI027763, ID15-SD-063 (California HIV Research Program).
Conflicts of Interest
MH and MML have received grant funding from Gilead. DMS has received consulting or grants funds from ViiV and AIDS Healthcare Foundation. All other authors did not report conflicts.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Current opinion in immunology 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ML, Lederman MM, Gianella S. Partners in Crime: The Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr HIV/AIDS Rep 2016; 13:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianella S, Massanella M, Wertheim JO, Smith DM. The Sordid Affair between Human Herpesvirus and Human Immunodeficiency Virus J Infect Dis 2015; 212:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman ML, Mudd JC, Shive CL, et al. CD8 T cell expansion and inflammation linked to CMV co-infection in ART-treated HIV infection,. Clin Infect Dis 2015; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. [DOI] [PubMed] [Google Scholar]
- 8.Caby F, Guihot A, Lambert-Niclot S, et al. Determinants of a Low CD4/CD8 Ratio in HIV-1-Infected Individuals Despite Long-term Viral Suppression. Clin Infect Dis 2016; 62:1297–303. [DOI] [PubMed] [Google Scholar]
- 9.Smith DM, Nakazawa M, Freeman ML, et al. Asymptomatic CMV Replication During Early Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower CD4/CD8 Ratio During HIV Treatment. Clin Infect Dis 2016; 63:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianella S, Massanella M, Richman DD, et al. Cytomegalovirus Replication in Semen is Associated with Higher Levels of Proviral HIV DNA and CD4+ T cell Activation during Antiretroviral Treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dan JM, Massanella M, Smith DM, et al. Brief Report: Effect of CMV and HIV Transcription on CD57 and PD-1 T-Cell Expression During Suppressive ART. J Acquir Immune Defic Syndr 2016; 72:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 13.Sacre K, Hunt PW, Hsue PY, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 2012; 26:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrinello CM, Sinclair E, Landay AL, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 2012; 205:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus Coinfection Is Associated With an Increased Risk of Severe Non-AIDS-Defining Events in a Large Cohort of HIV-Infected Patients. J Infect Dis 2015; 211:178–86. [DOI] [PubMed] [Google Scholar]
- 16.Letendre S, Bharti A, Perez-Valero I, et al. Higher Anti-CMV IgG Concentrations are Associated with Worse Neurocognitive Performance During Suppressive Antiretroviral Therapy. Clin Infect Dis 2018; 67:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest 1998; 101:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding K, Koba A, Grant AD, et al. Cytomegalovirus viremia as a risk factor for mortality prior to antiretroviral therapy among HIV-infected gold miners in South Africa. PLoS One 2011; 6:e25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawa K Epstein-Barr virus--associated diseases in humans. International journal of hematology 2000; 71:108–17. [PubMed] [Google Scholar]
- 20.Co-infections Rickinson A., inflammation and oncogenesis: future directions for EBV research. Seminars in cancer biology 2014; 26:99–115. [DOI] [PubMed] [Google Scholar]
- 21.Appay V, Fastenackels S, Katlama C, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25:1813–22. [DOI] [PubMed] [Google Scholar]
- 22.Hodowanec A, Lurain N, Krishnan S, Bosch R, Landay A. Increased CMV IgG Antibody Titer is Associated with Non-AIDS Events among Virologically Suppressed HIV-Positive Persons. Pathogens and Immunity 2019; In pressa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. New England Journal of Medicine 2003; 349:2304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. New England Journal of Medicine 2003; 349:2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl MA, Ribaudo HJ, Collier AC, et al. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. The Journal of infectious diseases 2003; 188:625–34. [DOI] [PubMed] [Google Scholar]
- 26.Landay AL, Spritzler J, Kessler H, et al. Immune reconstitution is comparable in antiretroviral-naive subjects after 1 year of successful therapy with a nucleoside reverse-transcriptase inhibitor–or protease inhibitor–containing antiretroviral regimen. The Journal of infectious diseases 2003; 188:1444–54. [DOI] [PubMed] [Google Scholar]
- 27.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. New England Journal of Medicine 2004; 350:1850–61. [DOI] [PubMed] [Google Scholar]
- 28.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. New England Journal of Medicine 2008; 358:2095–106.18480202 [Google Scholar]
- 29.Sax PE, Tierney C, Collier AC, et al. Abacavir–lamivudine versus tenofovir–emtricitabine for initial HIV-1 therapy. New England Journal of Medicine 2009; 361:2230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoenigl M, Moser C, Funderburg N, et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) is predictive of Non-AIDS Events during Antiretroviral Therapy-mediated Viral Suppression. Clinical Infectious Diseases 2018; doi: 10.1093/cid/ciy966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrinello CM, Sinclair E, Landay AL, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. The Journal of infectious diseases 2012; 205:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianella S, Morris SR, Tatro E, et al. Virologic Correlates of Anti-CMV IgG Levels in HIV-1 Infected Men. J Infect Dis 2014; 209:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strain MC, Lada SM, Luong T, et al. Highly Precise Measurement of HIV DNA by Droplet Digital PCR. PLoS One 2013; 8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianella S, Anderson CM, Var SR, et al. Replication of Human Herpesviruses Is Associated with Higher HIV DNA Levels during Antiretroviral Therapy Started at Early Phases of HIV Infection. J Virol 2016; 90:3944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massanella M, Gianella S, Lada SM, Richman DD, Strain MC. Quantification of Total and 2-LTR (Long terminal repeat) HIV DNA, HIV RNA and Herpesvirus DNA in PBMCs. Bio Protoc 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman ML, Mudd JC, Shive CL, et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.