SUMMARY
The key sites within the gastrointestinal (GI) tract where T cells mediate effector responses and the impact of these responses on intestinal stem cells (ISCs) remain unclear. Using experimental bone marrow transplantation to model immune-mediated GI damage and 3-D imaging to analyze T cell localization, we found that the ISC compartment is the primary intestinal site targeted by T cells post-transplant. Recruitment to the crypt base region resulted in direct T cell engagement with the stem cell compartment and loss of crypt base columnar ISCs, which expressed both MHC class I and II. Vasculature expressing the adhesion molecule MAdCAM-1 clustered near the crypt base, preferentially regulating crypt compartment invasion and ISC elimination without impacting T cell migration to villi. These findings indicate that allogeneic T cells rapidly access the stem cell niche post-transplant, and this targeted recruitment to the stem cell compartment results in ISC elimination during immune-mediated GI damage.
Keywords: Intestinal stem cells, Paneth cell, transplantation, allogeneic BMT, mucosal immunology, imaging of immunity, graft vs. host disease, beta7 integrin, LPAM, MAdCAM-1
ETOC blurb
The key sites within the gastrointestinal tract where T cells mediate effector responses and their impact on intestinal stem cells remain unclear. Using 3-D microscopy, Fu et al. visualize T cell spatial localization within the gastrointestinal tract, revealing the stem cell compartment as the primary intestinal target of allogeneic T cells during immune-mediated tissue damage occurring after bone marrow transplantation.
Graphical Abstract
INTRODUCTION
Intestinal tissues are routinely exposed to potential enteric pathogens. Naive T lymphocytes patrol the intestines, circulating between the blood and gut-associated lymphoid tissues (GALT), including the mesenteric lymph nodes (MLNs), Peyer’s patches, and isolated lymphoid follicles (Mempel et al., 2004; Miller et al., 2002; Stoll et al., 2002). After stimulation in the GALT, antigen-experienced T cells migrate to parenchymal areas of the intestinal mucosa via blood vessels (Jeurissen et al., 1987; Masopust et al., 2010; Masopust and Schenkel, 2013). Within the mucosa, lymphocytes are subdivided among the intraepithelial and lamina propria effector compartments (Cheroutre and Madakamutil, 2004). These immunologic classifications focus on the lymphocytes themselves and emphasize the histologic location of the tissue in which the lymphocytes reside, but fail to address the epithelial biology of the tissue and thus fail to communicate the functional implications for immune responses occurring in different compartments within the GI tract.
T cell homing to the intestines is an important component of intestinal immunity and a promising target for treatment of immune-mediated GI damage. It requires expression of gut-specific homing molecules on the T cells and adhesion ligands on the vasculature (Berlin et al., 1995; Butcher and Picker, 1996; Johansson-Lindbom and Agace, 2007; Kunkel et al., 2003; Masopust and Schenkel, 2013; Mora et al., 2003; Mora and von Andrian, 2006; Nakache et al., 1989; Salmi and Jalkanen, 2005). This process is involved in physiologic T cell recruitment to the GI tract and is also critical for T cell-mediated intestinal pathology. Donor T cells migrate to the intestines early after allogeneic hematopoietic transplantation (Beilhack et al., 2005), inhibition of T cell homing to the intestines can prevent immune-mediated damage to the GI tract in the form of graft vs. host disease (GVHD) (Floisand et al., 2015; Petrovic et al., 2004; Reshef et al., 2012; Waldman et al., 2006; Wysocki et al., 2005), and inhibition of this homing has recently been approved for treatment of inflammatory bowel diseases (IBD) (Feagan et al., 2013; Neurath, 2017; Podolsky et al., 1993; Sandborn et al., 2013) and is currently under investigation in GVHD (NCT02728895, ClinicalTrials.gov). However, clinical GVHD biopsies frequently fail to identify lymphocytic infiltrates in the GI tract. It is thus unclear where disease-causing effector T cells invade the target tissue and mediate the responses resulting in GI damage, and it is also unclear why sampling separate intestinal sections in a given host undergoing a single systemic process can reveal local variability in tissue injury.
ISCs located at the crypt base maintain the epithelial lining of the GI tract, generating all distinct cell types of the intestinal epithelium (Barker et al., 2012). While hypothesized for some time (Sale, 1996), advances in stem cell biology have helped to identify that immune-mediated GI damage can be associated with loss or dysregulation of ISCs (Davidson et al., 2012; Hanash et al., 2012; Lindemans et al., 2015; Takashima et al., 2011). Despite the importance of inflammation and immunity in GI pathology and even malignant transformation (Asquith and Powrie, 2010; He et al., 2007), it is not understood how pathogenic T cells access the ISC compartment to interact with ISCs and mediate injury. Moreover, it remains unclear if pathologic T cell responses primarily occur in the luminal areas of the tissue such as the villi where mature enterocytes mediate barrier function and nutrient absorption, the deeper regions of the crypt compartment where ISCs and progenitors are located, or all regions of the tissue non-selectively. Given the inability of traditional two-dimensional imaging to fully elucidate in situ mucosal T cell behavior, we sought to develop an approach using three-dimensional (3-D) microscopy of intact intestinal tissue following experimental allogeneic bone marrow transplantation (BMT) to define the specific locations of disease-causing T cells within the intestines, their relationship to the ISC compartment, and the functional significance of this relationship for immune-mediated GI damage. Using this approach, we found that the ISC compartment is the primary target of donor T cells invading the small intestine after allogeneic BMT. Both CD4+ and CD8+ T cells had the potential to mediate injury to the ISC compartment, as the initial crypt base region infiltration was due to CD4+ T cells, subsequent invasion resulted in a mixed infiltrate of CD4s and CD8s, and ISCs expressed both MHC class I and MHC class II. The β7-Integrin:MAdCAM-1 axis was a key regulator of T cell infiltration within the ISC compartment, and inhibition of this cell adhesion pathway resulted in improved ISC numbers following transplantation.
RESULTS
3-D imaging precisely identifies quantifiable T cell positioning within the intestinal mucosa
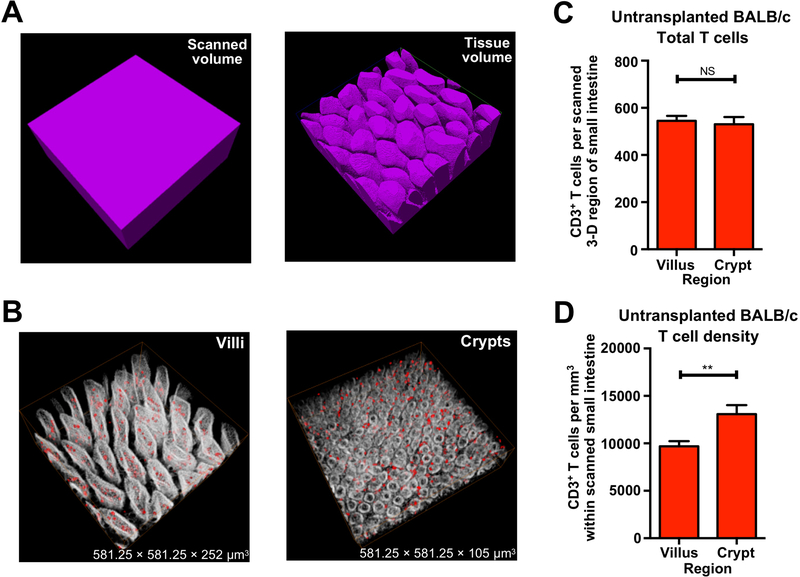
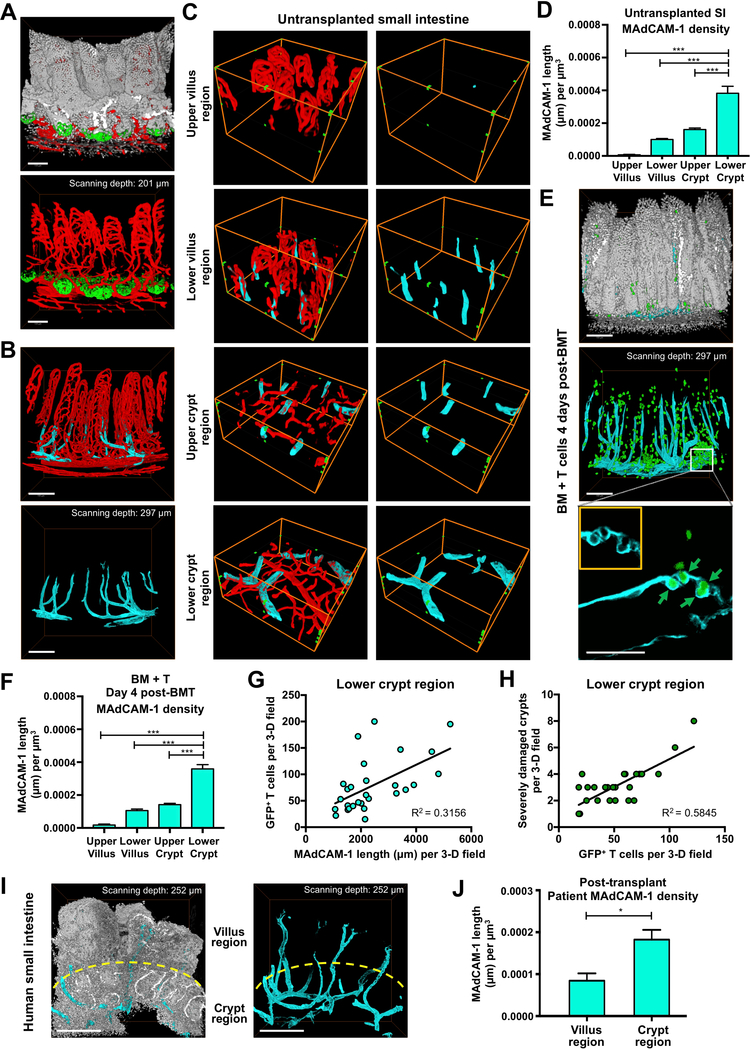
Given the lack of understanding of how T cells damage the ISC compartment, we sought to identify where pathologic T cells migrate to within the intestines when they mediate disease. We first performed 3-D microscopy with whole-mount immunofluorescent staining for CD3 to establish an approach for imaging and quantifying T cell localization within the full-depth of the small intestine (SI) during homeostasis. Because intestinal villi are finger-like projections with a complex 3-D structure that is not accurately represented by the overall scanning volume, tissue volume was determined by processing the 3-D images to quantify the tissue present within the full scanned field (Figure 1A). 3-D scans of full-depth SI were divided into villus and crypt regions for analysis of T cells within the two compartments (Figure 1B and Movie S1) for analysis of T cells within the two compartments. We observed similar total CD3+ T cell numbers within the crypt and villus compartments in BALB/c mice at baseline (Figure 1C). However, the size of the villus region was substantially larger than the crypt region (Figure 1B and Movie S1), and after normalizing CD3+ T cell numbers to the tissue volume, CD3+ T cell density in the crypt region was significantly higher than that in the villi of BALB/c mice (Figure 1D). Given the challenges in comparing absolute T cell numbers in 3-D fields with different sizes, subsequent analyses of T cell localization within different tissue compartments during damage focused on T cell density, normalized to the tissue volume.
Figure 1. 3-D imaging approach provides accurate tissue volume and cell localization.
(A) 3-D images of scanned volume and processed tissue volume in ileum. (B) 3-D reconstruction of villi and crypts from full-thickness BALB/c ileum; red, CD3+ T cells; white, nuclei. Note that villus tissue volume is larger than crypt tissue volume: mean villus Z-depth is approximately 250 μm; mean crypt Z-depth is approximately 100 μm. (C) Quantification of CD3+ T cell number per full-thickness 3-D field in BALB/c ileum. (D) Quantification of CD3+ T cell density in BALB/c ileum per full-thickness 3-D field; n = 17 (villus region) and n = 16 (crypt region) independent 3-D views (with 4–5 independent 3-D views per mouse and 4 mice per group). Bar graphs represent mean and SEM; **p < 0.01. Data combined from two independent experiments.
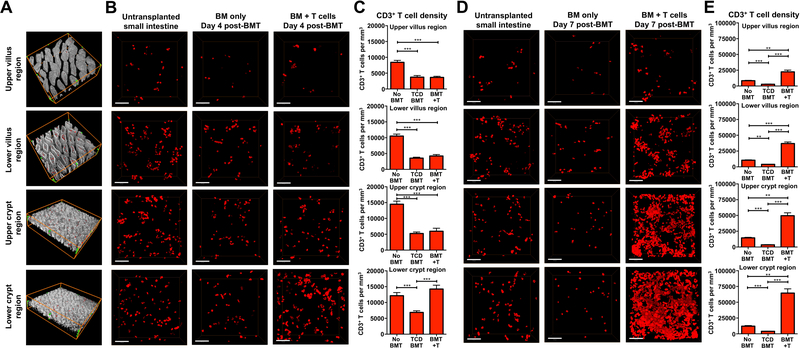
We next evaluated T cell localization during intestinal injury in a mouse model of GVHD, where T cells from the transplant donor migrate to the GI tract, resulting in recipient weight loss and mortality. Following transplantation, donor T cells migrate to the GALT before arriving at the intestinal mucosa, and there is little migration of donor T cells to the intestinal mucosa during the first three days after BMT (Beilhack et al., 2005; Panoskaltsis-Mortari et al., 2004). We thus examined SI T cell infiltration on day 4 after transplant to determine where pathologic T cells mediate the initial phases of tissue damage. Transplantation of C57BL/6 (B6) bone marrow (BM) into irradiated allogeneic BALB/c recipients (H-2b→H-2d, 850 cGy) was performed with T-cell-depleted (TCD) marrow alone or with a mixed allograft of TCD marrow and purified donor T cells for comparison of T cell tissue infiltration. Villus and crypt regions were divided into upper and lower halves to further compartmentalize T cell localization during tissue damage (Figure 2A). In comparison to normal untransplanted mice, allogeneic BMT recipients transplanted with only TCD BM demonstrated reduced CD3+ T cell density in all regions (Figures 2B and 2C). These likely represented residual host T cells whose numbers were reduced due to the pre-transplant conditioning. In comparison to TCD BMT, only the lower crypt region demonstrated an increase in T cell density after BMT with T cells (Figures 2B and 2C and Movie S2), indicating that the crypt base region where the ISC compartment is located is an initial site of T cell accumulation within the intestines after allogeneic BMT.
Figure 2. T cell density within the intestinal mucosa during immune-mediated GI damage is greatest in the crypt base region where the stem cell compartment is located.
Full-thickness SI (ileum) was imaged by performing immunofluorescent confocal microscopy on clarified whole-mount intestinal tissue. 3-D images were divided into upper and lower halves of the villus and crypt regions for quantification of T cell density after B6-into-BALB/c allogeneic BMT. Transplantation into irradiated recipients was performed with TCD marrow +/− 1 × 10 6 purified donor T cells, and T cells were identified by anti-CD3 immunofluorescence. (A) Representative images of full-thickness SI tissue divided into upper and lower halves of the villus and crypt regions; red, CD3+ T cells; white, nuclei. Green dots outline the scanned volume dimensions: 581.25 × 581.25 × 123 μm 3 (both in upper and lower villus regions); 581.25 × 581.25 × 62.5 μm 3 (both in upper and lower crypt regions). (B) 3-D projections of CD3+ T cells present at different ileal depths in normal untransplanted BALB/c controls and in BALB/c recipient mice four days after BMT; scale bars, 100 μm. (C) Quantification of CD3+ T cell densities four days after BMT; n = 21 (no BMT), n =18 (TCD BMT), and n = 13 (BMT + T) independent views in upper and lower villus regions; n = 20 (no BMT), n = 27 (TCD BMT), and n = 17 (BMT + T) independent views in upper and lower crypt regions. Data represent 3–6 independent 3-D views per mouse and 4–6 mice per group, combined from two independent experiments. (D) 3-D projections of CD3+ T cells present at different ileal depths in normal untransplanted BALB/c controls and in BALB/c recipient mice seven days after BMT; scale bars, 100 μm. (E) Quantification of CD3+ T cell densities seven days after BMT. n = 21 (no BMT), n =24 (TCD BMT), and n = 21 (BMT + T) independent views in upper and lower villus regions; n = 20 (no BMT), n = 23 (TCD BMT), and n = 21 (BMT + T) independent views in upper and lower crypt regions. Data represent 3–6 independent 3-D views per mouse and 4–6 mice per group combined from two independent experiments. Bar graphs represent mean and SEM; **p < 0.01; ***p < 0.001.
To evaluate the progression of the immune response and determine the durability of crypt base infiltration, we next examined transplant recipients three days later on day 7 after BMT. Once again, all regions of SI demonstrated reduced T cell density after TCD BMT (Figures 1D and 1E). In contrast, T cell infiltration increased substantially at this timepoint after transplant with T cells, and increased T cell density was now apparent in all regions (Figures 2D and 2E). Despite the increase in T cell infiltration throughout the tissue, T cell density remained greatest in the lower crypt region, and thus in close proximity to the ISC compartment (Figures 2D and 2E and Movie S3). The disproportionate densities of CD3+ T cells in the intestinal mucosa were detected both day 4 and day 7 after transplants, suggesting that the crypt base region was the primary site targeted by T cells.
Loss of intestinal stem cells precedes damage to the stem cell niche early after bone marrow transplantation
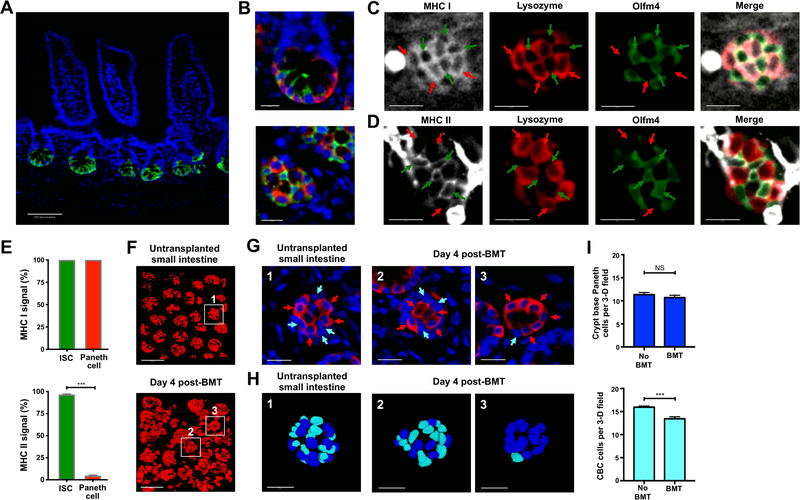
We next sought to characterize the impact of this early T cell infiltration on the ISC compartment. Intestinal crypts contain the stem cells and progenitors maintaining the epithelium, with the ISCs localizing to the crypt base as visualized by performing full-thickness SI imaging using Olfm4-GFP reporter mice (Schuijers et al., 2014) during homeostasis (Figure 3A). Paneth cells, in addition to secreting bactericidal products such as lysozyme (Sonnenberg et al., 2011), provide an epithelial stem cell niche by producing ISC growth factors (Sato et al., 2011). 3-D imaging after staining for lysozyme identified Paneth cells and Olfm4+ crypt base columnar (CBC) ISCs interspersed with one another and in direct contact, thus composing the ISC compartment (Barker, 2014) (Figure 3B and Movie S4).
Figure 3. Crypt injury and loss of intestinal stem cells occur early after allogeneic bone marrow transplantation.
(A and B) 2-D optical slices from immunofluorescent 3-D imaging of Olfm4+ ISCs (GFP staining, green) and nuclei (DAPI staining, blue) in SI (ileum) from Olfm4-GFP mice after staining and scanning of intact clarified whole-mount ileal tissue. (A) Transverse view of Olfm4+ ISCs; scale bar, 100 μm. (B) 2-D optical slices from 3-D imaging of the ISC compartment with staining for both Olfm4+ ISCs and Paneth cells (lysozyme staining, red). Upper panel shows a high magnification view of the ISC compartment at transverse orientation. Lower panel shows a high magnification view of the ISC compartment with a viewing orientation from the serosal intestinal surface; scale bars, 20 μm. (C-E) Whole-mount staining of MHC (white) along with lysozyme to identify Paneth cells (red) and GFP to identify ISCs (green) in SI from Olfm4-GFP mice. (C) 2-D optical slice from 3-D imaging of MHC class I (white) with Olfm4+ ISCs and lysozyme+ Paneth cells at the crypt base; red arrows indicate lysozyme+ Paneth cells; green arrows indicate Olfm4+ ISCs; scale bars, 20 μm. (D) 2-D optical slice from 3-D imaging of MHC class II (white) with Olfm4+ ISCs and lysozyme+ Paneth cells at the crypt base; red arrows indicate lysozyme+ Paneth cells; green arrows indicate Olfm4+ ISCs; scale bars, 20 μm. (E) Upper panel, quantification of cell frequency with MHC class I signal detected on Olfm4+ ISCs and lysozyme+ Paneth cells; n = 6 independent 3-D views from three mice. Lower panel, quantification of the cell frequency with MHC class II signal detected on Olfm4+ ISCs and lysozyme+ Paneth cells; n = 6 independent 3-D views from three mice. (F-I) 3-D imaging and quantification of the SI (ileum) ISC compartment after B6-into-BALB/c allogeneic BMT with 1 × 10 6 donor T cells and assessment of lysozyme+ Paneth cells and adjacent CBC ISCs. (F) 3-D projections of lysozyme+ Paneth cells (red) indicating crypt base architecture in SI from normal untransplanted BALB/c mice and from mice four days after BMT; scale bars, 100 μm. (G) High magnification 2-D images of BALB/c crypt bases shown in (F) from a normal untransplanted mouse (left panel) and four days after BMT (middle and right panels), with lysozyme staining in red and DAPI nuclear signal in blue; red arrows indicate lysozyme+ Paneth cells, and light blue (cyan) arrows indicate nuclei from lysozyme− CBC cells; scale bars, 25 μm. (H) 3-D projections of DAPI nuclear signal from the crypt bases shown in (G); dark blue nuclei indicate Paneth cells; light blue (cyan) nuclei indicate CBC cells; scale bars, 25 μm. (I) Quantification of Paneth cells and CBC cells from 3-D images. Data combined from two independent experiments; n = 13 independent views per group (with 3–4 independent 3-D views per mouse and four mice per group). Graphs indicate mean and SEM; ***p < 0.001.
To assess the potential for ISC compartment interactions with T cells post-transplant, we evaluated expression of major histocompatibility complex (MHC) class I and class II on ISCs and Paneth cells (Figures 3C–3E). The intestinal epithelium has long been known to express both MHC class I and II, with some more recent efforts focused on characterizing this within the ISC compartment (Agudo et al., 2018; Biton et al., 2018; Groh et al., 1996; Mayer et al., 1991; Perera et al., 2007; Wosen et al., 2018). After whole-mount staining and 3-D imaging, MHC class I was clearly expressed on all nucleated cells in the lamina propria and the epithelial layer. Within the crypt epithelium, expression of MHC class I was identified both on Olfm4+ ISCs and lysozyme+ Paneth cells (Figures 3C, 3E, and S1A). Evaluation of MHC class II demonstrated intense staining outside the crypt, likely representing macrophages or dendritic cells in the lamina propria (Cerovic et al., 2014; Gabanyi et al., 2016), while evaluation within the ISC compartment indicated clear expression of MHC class II primarily on ISCs (Figures 3D, 3E, and S1B).
We next evaluated the ISC compartment in GVHD, during the early phases of T cell localization to the crypt base region following B6-into-BALB/c BMT. 3-D images of lysozyme+ Paneth cells four days post-BMT showed altered morphology of the crypt base compartment after transplant, with disordered positioning and frequencies of Paneth cells within the tissue (Figure 3F). DAPI+ nuclear signals identified lysozyme− CBC cells at the crypt base in contact with lysozyme+ Paneth cells during homeostasis and after BMT (Figure 3G). 3-D visualization provided a detailed portrait of varying degrees of injury to the ISC compartment early post-transplant (Figure 3H), with some crypts still containing several CBC ISCs and other crypts demonstrating profound ISC loss (Figures 3H and 3I and Movie S5). During homeostasis, the ISC compartment contained approximately 15 lysozyme−DAPI+ CBC cells (Figure 3I), which was consistent with the number of ISCs quantified using Lgr5-GFP ISC reporter mice (Snippert et al., 2010). However, quantification of CBC cells early post-BMT indicated significant loss of ISCs (Figure 3I). In contrast, although Paneth cells are reduced in experimental BMT models (Eriguchi et al., 2012; Jenq et al., 2012; Lindemans et al., 2015) and in patients with GVHD (Levine et al., 2013), the frequency of Paneth cells at the crypt base was not significantly altered at this early timepoint four days after BMT (Figure 3I), suggesting that the loss of ISCs in GVHD precedes damage to the stem cell niche.
The crypt base stem cell compartment is the primary target of donor T cell infiltration within the intestinal mucosa
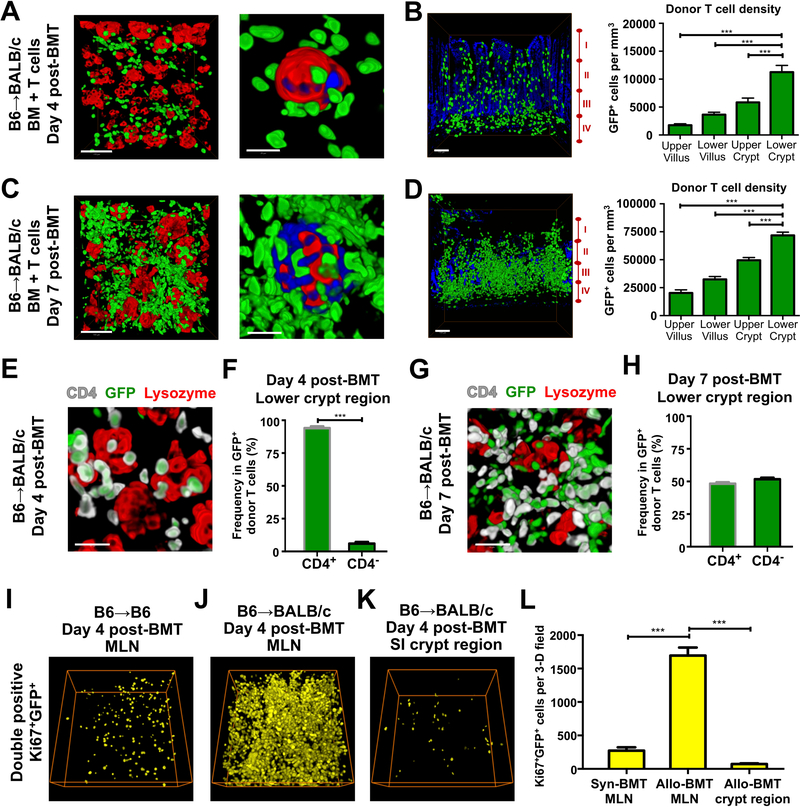
To specifically track the behavior of donor T cells and determine their contribution to the T cell infiltration of the ISC compartment post-transplant, we next performed B6-into-BALB/c allogeneic BMT with wild type (WT) B6 marrow and GFP+ B6 T cells. Notably, 2-D images were generally far less revealing than the full-thickness 3-D imaging of T cell localization throughout the mucosa (Figure S2A). Immunofluorescent staining of lysozyme to identify Paneth cells and the stem cell niche four days after transplant indicated GFP+ donor T cell infiltration of the crypt base region and at times direct interaction between donor T cells and damaged crypts during tissue injury (Figure 4A). Furthermore, 3-D imaging of full-thickness SI demonstrated that the greatest area of donor T cell infiltration during the early phases of GVHD occurred in the crypt base region (Figure 4B). Evaluation seven days after BMT with GFP+ donor T cells also demonstrated massive infiltration of the crypt base compartment along with disproportionate T cell invasion of the lower crypt region (Figure 4C and 4D). Despite the large increase in donor T cell infiltration of the intestinal mucosa between days 4 and 7 after transplant, the lower crypt region remained the greatest area of donor T cell invasion.
Figure 4. Allogeneic donor T cells preferentially invade the stem cell compartment within the intestines.
(A-H) Whole-mount 3-D imaging of SI (ileum) after B6-into-BALB/c allogeneic BMT with 1 × 10 6 GFP+ purified donor T cells. (A) GFP+ donor T cell infiltration of the crypt base region in relation to lysozyme+ Paneth cells four days after BMT. Left panel, low magnification image shows donor T cells in relation to several crypts; scale bar, 100 μm. Right panel, close-up image shows GFP+ donor T cells in contact with a damaged crypt base lacking CBC cells. Green, GFP+ donor T cells; red, lysozyme+ Paneth cells; blue, nuclei of crypt base epithelium; scale bar, 25 μm. (B) Image and quantification of GFP+ donor T cell infiltration in full-thickness SI, greatest in lower crypt region four days after BMT. Image shows 3-D projection of GFP+ donor T cells (green), with 2-D nuclear background (blue) indicating the surrounding intestinal structure in transverse orientation; I, upper villus; II, lower villus; III, upper crypt; IV, lower crypt; scale bar, 100 μm. Graph shows quantification of donor T cell density in the different regions; n = 12 (upper and lower villus) and n = 10 (upper and lower crypt) independent views per region from 5–7 independent 3-D views per mouse and two mice per group; ***p < 0.001. (C) GFP+ donor T cell infiltration of the crypt base region in relation to lysozyme+ Paneth cells seven days after BMT. Left panel, low magnification image of the entire field shows donor T cells in relation to several crypts; scale bar, 100 μm. Right panel, high magnification image shows GFP+ donor cells in contact with a damaged crypt base. Green, GFP+ donor T cells; red, lysozyme+ Paneth cells; blue, nuclei of crypt base epithelium; scale bar, 25 μm. (D) Image and quantification of GFP+ donor T cell infiltration in full-thickness SI, greatest in lower crypt region seven days after BMT. Image shows 3-D projection of GFP+ donor T cells (green), with 2-D nuclear background (blue) indicating the surrounding intestinal structure in transverse orientation; I, upper villus; II, lower villus; III, upper crypt; IV, lower crypt; scale bar, 100 μm. Graph shows quantification of donor T cell density in the different regions; n = 12 independent views per region from 3–9 independent 3-D views per mouse and two mice per group. Data are mean and SEM; lower crypt region vs. other regions; ***p < 0.001. (E) High magnification 3-D projection of GFP+ donor T cells (green) with anti-CD4 (white) and anti-lysozyme (red) immunostaining shows CD4+GFP+ donor T cells infiltrating the crypt base compartment four days after BMT; scale bar, 50 μm. (F) CD4+ and CD4− frequencies in GFP+ donor T cells in the lower crypt region four days after BMT; n = 16 independent 3-D views from 4–8 views per mouse and four mice per group. Data are mean and SEM; ***p < 0.001. (G) High magnification 3-D projection of GFP+ donor T cells (green) with anti-CD4 (white) and anti-lysozyme (red) immunostaining shows both CD4+GFP+ and CD4−GFP+ (i.e. CD8) donor T cells infiltrating the crypt base compartment seven days after BMT; scale bar, 50 μm. (H) CD4+ and CD4− frequencies in GFP+ donor T cells in the lower crypt region seven days after BMT; n = 16 independent 3-D views from 8 views per mouse and two mice per group. (I-L) 3-D images and quantification of proliferating GFP+ donor T cells (yellow) after B6-into-B6 syngeneic BMT or B6-into-BALB/c allogeneic BMT with 1 × 10 6 GFP+ purified donor T cells; dimensions of 3-D fields: 303.34 × 303.34 × 99 μm 3. (I) 3-D projection of double positive Ki67+GFP+ cells in MLN four days after syngeneic BMT. (J) 3-D projection of double positive Ki67+GFP+ cells in MLN four days after allogeneic BMT. (K) 3-D projection of double positive Ki67+GFP+ cells in SI crypt region four days after allogeneic BMT. (L) Quantification of Ki67+GFP+ cells in MLNs and SI crypt region four days after syngeneic (syn) or allogeneic (allo) BMT; ***p < 0.001.
Consistent with the results of CD3 staining after BMT, transplantation with GFP+ donor T cells thus indicated that the crypt base region where the ISC compartment is located is an initial target of donor T cell invasion within the intestines after allogeneic BMT. T cell phenotyping combined with lysozyme staining demonstrated that the donor T cell infiltrate in the crypt base compartment was largely composed of CD4 cells on day 4 post-transplant (Figures 4E–4F and S2B), indicating that CD4+ T cells were responsible for the initial invasion during early loss of ISCs (Figures 3G–3I). By day 7, there was a mixed infiltrate of CD4s and CD8s (Figures 4G–4H and S2C) capable of targeting all crypt cells, and thus the expansion of donor T cells within the intestinal mucosa between day 4 and day 7 occurred with recruitment of new cells to the GI tract, and not simply local T cell activation and expansion.
The B6-into-BALB/c BMT model results in aggressive GVHD due to the full MHC mismatch between donor and host. To assess the role of alloreactivity in crypt base invasion and determine if B6 donor T cells targeted the recipient BALB/c ISC compartment because of a specific strain-dependent antigenicity, we evaluated GFP+ donor T cell infiltration of the intestinal mucosa using two additional transplant models: a syngeneic BMT model (B6→B6, H-2b→H-2b) lacking antigenic disparity and a minor-histocompatibility-antigen (minor)-mismatched BMT model (B6→LP, H-2b→H-2b) resulting in less severe GVHD than transplants with a MHC mismatch. We observed very little donor T cell migration to the intestinal mucosa after syngeneic BMT, although the small proportion of donor T cells present in recipient SI were identified in the crypt compartment (Figures S3A and S3B). Following minor-mismatched (MHC-matched) allogeneic BMT, the lower crypt region once again was the major site of donor T cell infiltration, both four days (Figures S3C and S3D) and seven days (Figures S3E and S3F) post-transplant. Similar to the MHC mismatched-model, there was substantial expansion of the donor T cell infiltrate between day 4 and day 7, but in both cases the greatest density of T cell invasion was present at the crypt base compartment, although overall donor T cell numbers were lower in the minor mismatch setting. Syngeneic, minor-mismatched, and MHC-mismatched BMT thus demonstrated that the degree of antigenic disparity affected the magnitude of T cell invasion in the intestinal mucosa, but not its location.
To assess T cell activation and proliferation more directly, Ki67 was evaluated in lymph nodes and within the GI tract after BMT (Figures 4I–4L). Donor T cells could be readily identified in recipient MLNs early after syngeneic BMT (Figures 4I and S3G), even though relatively few managed to reach the intestinal mucosa (Figures S3A and S3B). However, consistent with the reduced T cell activation expected after syngeneic BMT, there were many fewer Ki67+ proliferating donor T cells in the MLNs after syngeneic BMT than after allogeneic BMT (Figures 4I, 4J, 4L, S3G, and S3H), suggesting that the lack of alloactivation led to the absence of donor T cell infiltration in the gut after syngeneic BMT. Additionally, there were many fewer Ki67+ proliferating donor T cells in the crypt region intestinal mucosa than in the MLNs early after allogeneic BMT (Figures 4J–4L, S3H, and S3I), indicating that there was substantial T cell activation in secondary lymphoid tissue early post-BMT prior to the subsequent expansion in mucosal T cell invasion occurring by day 7 post-transplant. Along with the shift in the infiltrate from predominantly CD4+ T cells to a mix of CD4s and CD8s (Figures 4E–4H), the reduced frequency of proliferating donor T cells in the mucosa compared to the MLNs (Figure 4L) also supported the interpretation that the expansion in T cell invasion of the crypt base occurred with recruitment of additional T cells after their activation in lymphoid tissue.
To evaluate the role of pre-transplant conditioning in T cell migration to the gut stem cell niche, we evaluated GFP+ donor T cells in a previously described conditioning-free parent-into-F1 BMT model (B6→unirradiated BDF1, H-2b→H-2b×d) without pre-transplant irradiation (Shimoji et al., 2017). For comparison, seven days after B6-into-B6 syngeneic BMT without pre-transplant irradiation, there was virtually no infiltration of donor T cells into the syngeneic recipient intestinal mucosa (Figures S3J and S3K). Only one donor T cell was identified among ten independent 3-D fields on day 7 after syngeneic transplant without preconditioning. As in the allogeneic setting, that one T cell was identified in the lower crypt region. In contrast, while overall donor T cell infiltration of the intestinal mucosa remained relatively low even after allogeneic BMT without pre-transplant irradiation, transplantation of GFP+ B6 donor T cells into unirradiated BDF1 recipients again demonstrated the greatest density of donor T cell infiltration in the lower crypt region (Figures S3L and S3M). Therefore, similar to decreasing donor/host antigenic disparity, elimination of pre-transplant conditioning reduced the magnitude of the allogeneic mucosal infiltrate, but did not change its emphasis on the crypt base region of the mucosa.
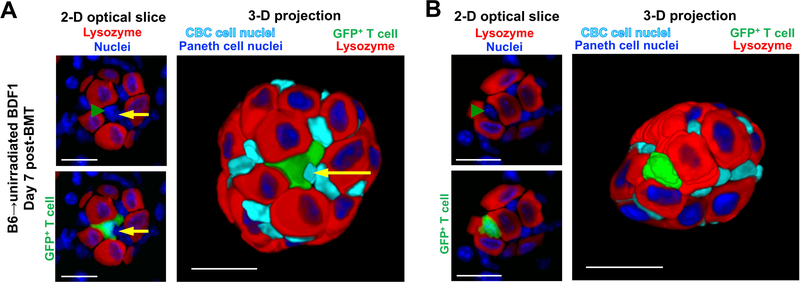
The reduced frequency of donor T cell infiltration in the unirradiated BMT model, as well as the absence of radiation injury, facilitated visualization of specific cell-to-cell interactions within the ISC compartment. While no donor T cells could be identified within recipient crypts after unirradiated syngeneic BMT (Figures S3N), following unirradiated allogeneic BMT donor T cells were observed embedded within the recipient ISC compartment (Figures 5A and 5B and S3O and S3P and Movie S6). Allogeneic donor T cells were identified adjacent to crypt base ISCs (Figures 5A and S3O and Movie S6) and at times donor T cells appeared to have replaced ISCs between lysozyme+ Paneth cells where ISCs should have been located (Figures 5B and S3P). Donor T cells thus invaded the ISC compartment and intimately interacted with stem cells during immune-mediated GI damage. Furthermore, in total, across MHC-matched, MHC-mismatched, and even unirradiated parent-into-F1 allogeneic BMT models, the greatest area of donor T cell invasion was the crypt base compartment.
Figure 5. Donor T cells invaded the ISC compartment adjacent to or replacing CBC ISCs after unirradiated BMT.
SI crypt base images of CBC stem cell nuclei next to Paneth cells and infiltrating T cells, day 7 after B6-into-unirradiated-BDF1 allogeneic BMT with 30 × 10 6 GFP+ purified donor T cells. DAPI+ nuclei are shown in blue, lysozyme+ Paneth cells are shown in red, GFP+ donor T cells are shown in green. Left panels show 2-D slices of nuclei and anti-lysozyme signal, with T cell nuclei highlighted by green arrowheads (left upper panels) and T cell shape demonstrated with GFP signal (left lower panels). Right panels show 3-D projections of the entire crypt base with ISC nuclei highlighted in light blue. (A) Images of the crypt base region with an invading GFP+ donor T cell adjacent to a stem cell and directly interacting with it. Yellow arrows indicate the nucleus of the ISC surrounded by the donor T cell; scale bars, 25 μm. (B) Images of the crypt base region with a GFP+ donor T cell between several Paneth cells in a position that would normally be occupied by an ISC, which appears to be absent; scale bars, 20 μm.
T cell invasion near the crypt base does not correlate with overall density of the vasculature
The GI tract contains intricate vascular networks facilitating nutrient absorption and immunosurveillance (Bernier-Latmani and Petrova, 2016; Fu and Tang, 2010b). We investigated the relationship of the intestinal vasculature to the ISC compartment by performing vessel painting in Olfm4-GFP ISC reporter mice. 3-D imaging showed that vascular density in the SI villus region was much higher than in the crypt region (Figure 6A and Movie S7). The configuration of the mucosal vasculature thus appeared compatible with reported findings of tissue-resident T cell recirculating to the villi during homeostasis (Fujimori et al., 2002; Koseki et al., 2001). However, this vascular architecture contrasted with the pattern of T cell recruitment to the crypt base compartment that we identified in GVHD (Figures 2, 4A–4D, and S3).
Figure 6. MAdCAM-1+ vessels primarily localize to the crypt regions of the intestinal mucosa.
(A-D) Vessel painting and 3-D immunofluorescent imaging of ileum in normal (untransplanted) mice. (A) 3-D imaging of small intestine vasculature (red) after vessel painting in Olfm4-GFP mice shows greater vascular density in the villus region compared to the crypt region; white, nuclei (DAPI); green, ISCs (anti-GFP); scale bars, 100 μm. (B) 3-D immunofluorescent imaging of MAdCAM-1 staining in light blue (cyan), with MAdCAM-1−vasculature shown in red, reveals that MAdCAM-1+ vessels are predominantly located around the crypt compartment; scale bars, 150 μm. (C) Representative 3-D imaging of MAdCAM-1−vasculature (red) and MAdCAM-1+ vasculature (light blue) in different regions within ileum of BALB/c mice. Green dots outline the scanned volume dimensions: 303.34 × 303.34 × 105 μm 3 (both in upper and lower villus regions); 303.34 × 303.34 × 42 μm 3 (both in upper and lower crypt regions). (D) Quantification of MAdCAM-1 density in different regions as shown in (C); n = 18 (upper and lower villus regions) and n = 23 (upper and lower crypt regions) independent views per region combined from 3–7 independent 3-D views per mouse and five mice per group from two independent experiments. (E-H) Analyses of ileum four days after B6-into-BALB/c BMT with 1 × 10 6 GFP+ donor T cells. (E) 3-D imaging of MAdCAM-1+ vessels (light blue) and donor T cells (green) in recipient SI post-transplant. Upper and middle panels: upper panel provides architectural orientation from DAPI nuclear staining (white); in the middle panel, GFP+ donor T cells appear to localize near MAdCAM-1+ vessels; scale bars, 150 μm. Lower panel: close-up 2-D image of a MAdCAM-1+ vessel in the crypt base region from the 3-D projection image shown in middle panel. Green arrows indicate transendothelial migration of 4 GFP+ donor T cells. A MAdCAM-1+ vessel encircles the donor T cells (inset) while they are migrating out of the vessel to parenchymal areas; scale bar, 50 μm. (F) Quantification of MAdCAM-1 density in different regions four days after BMT as shown in (E); n = 11 (upper and lower villus regions) and n = 28 (upper and lower crypt regions) independent views per region combined from 2–6 independent 3-D views per mouse and 3–5 mice per group from two independent experiments. (G) Comparison of GFP+ donor T cells and MAdCAM-1 density in the lower crypt region; n = 28 independent 3-D views combined from two independent experiments. (H) Comparison of severely damaged crypts (<5 CBCs per crypt) and GFP+ donor T cells in the lower crypt region; n = 29 independent 3-D views combined from two independent experiments. (I) 3-D imaging of MAdCAM-1+ vessels in human duodenal biopsy specimen after clinical allogeneic hematopoietic transplantation; light blue, anti-MAdCAM-1; white, nuclei (DAPI); scale bar, 250 μm. (J) Quantification of patient duodenal MAdCAM-1 density in the villus and crypt regions after transplantation as shown in (I); n = 3 independent 3-D views. Bar graphs represent mean and SEM; lower crypt region vs. other regions; *p < 0.05; ***p < 0.001.
To investigate how T cells gain access to the ISC compartment and resolve the discrepancy between vascular density and cellular migration during immune-mediated GI damage, we evaluated expression of the integrin ligand MAdCAM-1 on the intestinal vasculature. MAdCAM-1 is known to contribute to T cell recruitment to the GI tract as a whole in GVHD and IBD, and targeting its receptor α4β7 on donor T cells can reduce GVHD pathology and improve survival after experimental BMT (Adams and Eksteen, 2006; Petrovic et al., 2004; Waldman et al., 2006). However, there is little understanding of the tissue compartments to which MAdCAM-1 recruits immune cells within the intestines, and its relationship to the ISC compartment has not been defined. Immunofluorescent staining and vessel painting indicated MAdCAM-1 expression on only a small proportion of SI vessels, which appeared to cluster in close proximity to the crypt compartment (Figure 6B and Movie S8). 3-D imaging and quantification of MAdCAM-1+ vessel location revealed that vessels expressing MAdCAM-1 predominately localized to the lower crypt region (Figures 6C and 6D), similar to T cells invading the intestines to mediate GVHD (Figures 4B and 4D).
To gain further insight into the expression pattern of MAdCAM-1 and its implications for T cell recruitment during tissue damage, we evaluated MAdCAM-1 localization after BMT. Endothelial quantification post-transplant indicated that the expression was stable, and the greatest density of MAdCAM-1+ vessels remained in the lower crypt region (Figures 6E and 6F). Additionally, MAdCAM-1 staining and 3-D SI imaging after BMT with GFP+ T cells indicated that donor T cell localization and crypt region infiltration appeared to correlate with vessels expressing MAdCAM-1 (Figure 6E and Movie S9). Indeed, comparison of MAdCAM-1 vessels and adjacent T cell infiltrates from independent 3-D fields demonstrated that the degree of donor T cell invasion within a given tissue segment post-transplant correlated with the local MAdCAM-1 density (Figure 6G). There was also a direct correlation between the size of the local T cell infiltrate and stem cell loss in neighboring crypts (Figure 6H). Furthermore, assessment of MAdCAM-1 distribution after clinical allogeneic transplantation indicated predominant localization of MAdCAM-1+ vessels to the crypt region in human intestinal mucosa as well (Figures 6I and 6J). Taken together, these results indicated that MAdCAM-1 expression was greatest on blood vessels near the ISC compartment, this expression pattern was stable post-transplant, and it correlated directly with local recruitment of allogeneic T cells that could mediate tissue damage.
Inhibition of the integrin-β7/MAdCAM-1 axis specifically reduces T cell invasion of the crypt compartment and protects the stem cells after transplant
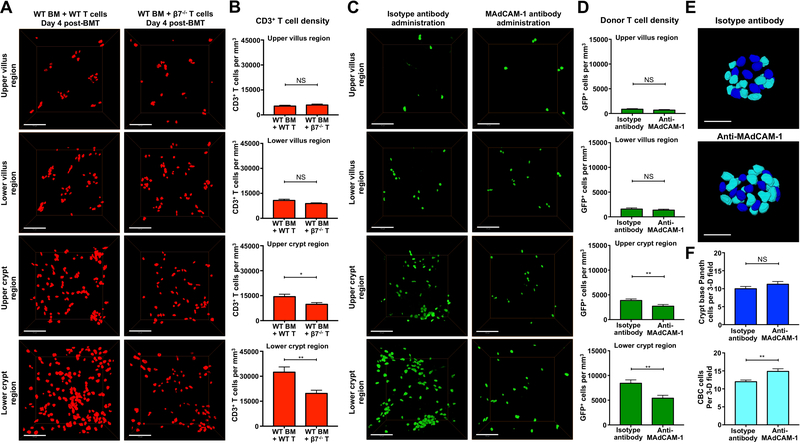
Given that the pattern of MAdCAM-1 expression in SI vasculature (Figure 6) appeared to mirror the pattern of post-transplant T cell localization to the crypt base region (Figures 2 and 4), we next sought to determine if T cell invasion of the ISC compartment was indeed regulated by the α4β7/MAdCAM-1 pathway. We first transplanted BALB/c recipients with WT or β7−/− T cells and evaluated the densities of CD3+ T cells within the full-thickness SI four days after BMT. Consistent with a primary role in regulation of T cell recruitment to the epithelial precursor compartment, genetic deletion of β7 in donor T cells led to a decrease in CD3+ T cell density in crypt region without impacting the T cell frequency in the villi (Figures 7A and 7B). Furthermore, the greatest difference in T cell density after transplant with β7−/− T cells was detected in the lower crypt region containing the stem cell compartment (Figures 7A and 7B).
Figure 7. β7 integrin and MAdCAM-1 regulate T cell recruitment to the stem cell compartment and loss of intestinal stem cells during immune-mediated GI damage.
(A and B) Full-thickness SI tissue (ileum) was divided into upper and lower halves of the villus and crypt regions for quantification of T cell density after B6-into-BALB/c allogeneic BMT. BMT was performed with TCD marrow and 1 × 10 6 purified WT or β7−/− T cells. T cells were identified by anti-CD3 immunofluorescence. β7 deficiency specifically affected T cell density in the crypt compartment, not in the villi. (A) Representative 3-D images of CD3+ T cells in ileal crypt and villus compartments; scale bars, 100 μm. (B) Quantification of CD3+ T cell densities; n = 24 independent views from six mice per group and two independent experiments. (C-F) BMT recipients were treated with 150 μg anti-MAdCAM-1 or isotype antibodies on days 2 and 3 after BMT, then 3-D imaging was performed in the ileum on day 4 post-transplant. B6-into-BALB/c BMT was performed with TCD marrow and 1 × 10 6 purified GFP+ donor T cells. Treatment with anti-MAdCAM-1 neutralizing antibodies specifically reduced donor T cell infiltration in the crypt region without altering donor T cell migration to the villi and prevented loss of ISCs post-transplant. (C) Representative 3-D images of GFP+ donor T cells in ileal crypt and villus compartments; scale bars, 100 μm. (D) Quantification of GFP+ donor T cell densities; n = 24 (isotype) and n = 23 (anti-MAdCAM-1) independent views in upper and lower villus regions; n = 32 (isotype) and n = 33 (anti-MAdCAM-1) independent views in upper and lower crypt regions from 6–12 independent 3-D views per mouse and 3–4 mice per group from two independent experiments. (E) Representative nuclear images of CBC cells (light blue) and Paneth cells (dark blue) after treatment with anti-MAdCAM-1 or isotype; scale bars, 25 μm. (F) Quantification of CBC cells and Paneth cells from 3-D images after treatment with anti-MAdCAM-1 or isotype antibody. n = 9 independent views per group from 2–3 independent 3-D views per mouse and 4 mice per group from two independent experiments. Bar graphs represent mean and SEM; *p < 0.05; **p < 0.01.
In addition to the genetic deletion of β7 in donor T cells, the function of MAdCAM-1, the endothelial ligand for α4β7 integrin, in regulation of donor T cell recruitment to the ISC compartment was investigated by performing allogeneic BMT with GFP+ donor T cells and treating BMT recipients with anti-MAdCAM-1 blocking antibodies. MAdCAM-1 expression on high endothelial venules contributes to T cell recruitment to Peyer’s patches and MLNs, which precedes T cell migration to the intestinal mucosa in GVHD (Beilhack et al., 2008; Beilhack et al., 2005; Sackstein, 1995; Wysocki et al., 2005). We thus blocked α4β7:MAdCAM-1 interactions with anti-MAdCAM-1 antibody treatment only on days 2 and 3 after BMT in order to permit initial T cell circulation to the GALT. Similar to the results of allogeneic transplantation with β7−/− T cells (Figures 7A and 7B), treatment with anti-MAdCAM-1 had no effect on donor T cell infiltration of SI villi (Figures 7C and 7D and Movie S10). However, anti-MAdCAM-1 treatment, initiated two days post-transplant, reduced donor T cell invasion specifically in the crypt compartment, with the greatest reduction again occurring in the lower crypt region of the mucosa (Figures 7C and 7D and Movie S10).
While T cell invasion primarily occurred near the ISC compartment, and recruitment to the area appeared to be regulated by MAdCAM-1, it remained possible that loss of ISCs could have been happening concurrently with these processes and not necessarily due to the T cell invasion occurring there. We thus investigated the impact of inhibiting T cell migration to the crypt compartment by assessing damage to the stem cells and their epithelial niche after treatment with anti-MAdCAM-1. Indeed, treatment with anti-MAdCAM-1 and reduction of donor T cell crypt invasion protected the ISC compartment, increasing CBC cell numbers in BMT recipients while Paneth cell frequencies again remained stable (Figures 7E and 7F). Loss of ISCs after BMT was thus directly related to T cell invasion of the stem cell niche, which was orchestrated by MAdCAM-1-mediated recruitment of activated T cells to the crypts and the stem cell compartment.
DISCUSSION
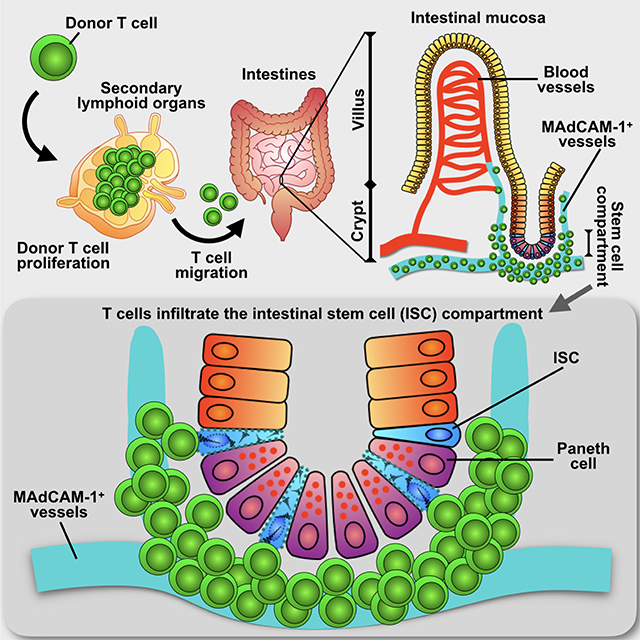
Recent studies have made significant progress in understanding the microenvironment of ISCs, particularly in identifying the niche factors that regulate the ISCs during homeostasis as well as epithelial and stromal cells that contribute to ISC maintenance (Akcora et al., 2013; Bjerknes and Cheng, 2001; Sato et al., 2009; Shoshkes-Carmel et al., 2018). While the GI tract harbors an abundance of lymphocytes, which mediate a diverse range of physiologic and pathologic immune responses (Cheroutre and Madakamutil, 2004; Nagler-Anderson, 2001), and impairment of ISC compartment has been identified in immune-mediated diseases in the GI tract such as IBD and GVHD (Dotti et al., 2016; Hanash et al., 2012; Nakanishi et al., 2016; Takashima et al., 2011), in vivo interactions between T lymphocytes and crypt base epithelial cells during homeostasis and tissue damage remain unclear. This deficiency is due perhaps at least in part to the limitations of standard 2-D histology. To improve the evaluation of T cell positioning in the GI tract and interactions with the stem cell compartment, we developed a 3-D microscopy approach using intact intestinal tissue to accurately preserve spatial relationships and dissect T cell localization within the GI tract. Applying this approach to five different BMT models, including models with varying degrees of antigenic disparity and models with and without pre-transplant conditioning, we found that the ISC compartment is a primary target and an early site of T cell invasion during immune-mediated GI damage post-transplant. Varying the degree of antigenic disparity and forgoing pre-conditioning affected the magnitude of T cell infiltration within the intestinal mucosa, but not the location of the infiltrate. In all models there was disproportionate invasion of the ISC compartment.
While elegant and important work has investigated pathways involved in recruiting T cells to the GI tract (Berlin et al., 1993; Svensson et al., 2002), there remains limited understanding of where within the mucosa these pathways regulate trafficking, how the specific localization within the mucosa might impact the effects of T cells recruited to the tissue, or the relevance of specific recruitment pathways to stem cell compartment. In this study, the ISC compartment was the first site of T cell infiltration within the intestinal mucosa after allogeneic BMT. As the pathologic immune response progressed, the areas of T cell involvement increased, but the lower crypt region remained the most densely infiltrated with donor T cells. Although the villus compartment has a high density of vasculature, MAdCAM-1 was expressed in only a portion of vessels in the intestinal mucosa, clustering primarily around the crypt compartment. The process of pathologic T cell infiltration of the intestinal mucosa during immune-mediated GI damage in GVHD thus likely includes several steps: (1) naive T cells undergo activation in GALT; (2) activated T cells access the ISC compartment via MAdCAM-1; (3) pathogenic allo-activated T cells invade the stem cell compartment and directly engage the ISCs; and (4) pathologic T cell invasion extends throughout the intestinal mucosa. Given that T cells can upregulate α4β7 integrin after activation, and given the finding that MAdCAM-1 is primarily expressed on vessels near the crypt base, the process of T cell activation and migration to the GI tract thus functioned to recruit all-activated T cells preferentially to the stem cell compartment, resulting in loss of ISCs.
Abnormal epithelial barrier function is a prominent feature of immune-mediated GI damage (Nalle and Turner, 2015). However, there has been little direct evidence demonstrating the process of epithelial injury occurring with pathologic T cell invasion of the intestines. Injury to the GI tract and loss of barrier function could occur in a number of distinct sequences. It is possible that: (1) T cells initially infiltrate the ISC niche and compromise the ISCs, and the damaged ISCs thus fail to regenerate and maintain the epithelial barrier; and (2) T cells primarily target the mature enterocytes via delivery to the mucosa through villus vasculature, and pathologic T cell responses directed at the surface epithelium cause loss of barrier function allowing for translocation of luminal pathogens or toxins into the intestinal mucosa; or (3) some combination of the two. In the second scenario, bacterial translocation could extend to the ISC niche, and impact the ISCs, or the ISCs could be lost or exhausted due to the increased turnover necessary to restore the damage occurring at the surface. However, in this study, T cell invasion of the mucosa more closely resembled the first scenario: the ISC compartment was an early site of pathologic T cell invasion during immune-mediated GI damage and remained the most densely infiltrated region even after widespread T cell infiltration throughout the mucosa. While either the stem cells or niche cells could be targeted in this scenario, and loss of both ISCs and Paneth cells has been identified in GVHD (Eriguchi et al., 2012; Jenq et al., 2012; Levine et al., 2013; Lindemans et al., 2015), we found that the ISCs were diminished early in GI GVHD, prior to the loss of Paneth cells. Along with the donor T cell invasion identified primarily in the lower crypt region, these findings suggested that GVHD-causing T cells primarily target the ISC compartment during immune-mediated GI damage after BMT, the stem cells are the initial target within the ISC compartment, and they may be directly engaged by infiltrating donor T cells. Indeed, post-transplant imaging here appeared to demonstrate direct contact between donor T cells and crypt base ISCs.
Although epithelial apoptosis is a hallmark of GI GVHD pathology, lymphocytic infiltration is frequently not apparent in clinical GI GVHD biopsies, and it has remained unclear where donor T cells are mediating tissue damage within the intestinal tract. Additionally, GVHD can be a patchy disease, and random sampling of the mucosa for GVHD diagnosis may not reflect the full extent of involvement. Direct correlations between the size of the local T cell infiltrate, the density of adjacent MAdCAM-1+ vessels, and associated crypt injury as observed here provide a potential explanation for such variations in histopathology. Furthermore, the findings that transplantation with β7−/− T cells led to a major reduction in T cell invasion of the lower crypt region and that MAdCAM-1 blockade specifically reduced crypt base infiltration by donor T cells and protected the stem cell compartment highlight the physiologic and pathologic functions of the α4β7/MAdCAM-1 axis and provide insight into the therapeutic mechanisms of action for homing inhibitors that target this pathway clinically. Rather than simply reducing T cell migration to the GI tract overall, therapeutic efficacy of such inhibitors may actually result from limiting T cell infiltration of the stem cell compartment.
In summary, we found that the crypt base region where ISCs and their niche reside is a primary site of T cell invasion within the intestinal mucosa during immune-mediated GI damage after BMT. Loss of ISCs preceded damage to the stem cell niche, and even as pathologic T cell infiltration of the intestines intensified, the lower crypt region remained the primary site of T cell activity. T cell recruitment to the ISC compartment was regulated by MAdCAM-1, which was found to be an important regulator of crypt region T cell migration during GVHD. We interpret these findings to indicate that the physiologic process of T cell homing to the intestinal mucosa during active immunity may emphasize recruitment to the crypt base in order to regulate tolerance at the level of the ISC compartment and protect the compartment from enteric pathogens or other toxic insults. However, during dysregulated pathologic immune responses such as GVHD, disease-causing T cells co-opt this first responder system to access the ISC compartment, resulting in a pathophysiology of primary ISC impairment. These findings provide insight into the basic biology of a critical pathway for both physiologic and pathologic mucosal immunity. Furthermore, given the potential of targeting the α4β7:MAdCAM-1 axis to treat immune-mediated GI damage, these findings reveal a fundamental mechanism of action for this class of therapeutics.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Alan Hanash (hanasha@mskcc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6 (B6, H-2b), C57BL/6-EGFP (B6-GFP, H-2b), BALB/c (H-2d), LP (H-2b), B6D2F1 (BDF1, H-2b×d), and β7−/− B6 mice were obtained from Jackson Laboratory (Bar Harbor, USA). Olfm4-IRES-eGFPCreERT2 mice were kindly provided by H. Clevers (Schuijers et al., 2014). Male and female mice, typically at an age of 8 to 10 weeks were analyzed in all experiments. Mouse maintenance and procedures were done in accordance with the institutional protocol guideline of the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Animal Care and Use Committee. Mice were housed in micro-isolator cages, up to five-per-cage, in MSKCC pathogen-free facilities and received standard chow and autoclaved sterile drinking water.
Human samples
Collection and analysis of human patient samples was performed under MSKCC IRB-approved protocols number 09–141 and 17–092, which included informed consent for sample collection and IRB approval for sample use. Adult human duodenal tissue was obtained from a 56 year old female approximately one month after umbilical cord blood transplantation for AML arising from MDS. Clinical GVHD assessment for the patient was determined to be overall GVHD Stage 1 and upper GI GVHD Grade 2. Clinical pathology evaluation was not diagnostic for histologic GVHD.
METHOD DETAILS
Bone marrow transplantation
BMT procedures were performed as previously described (Hanash et al., 2012). A major-histocompatibility-antigen-mismatched BMT model (B6→BALB/c, H-2b→H-2d), a minor-histocompatibility-antigen-mismatched BMT model (B6→LP, H-2b→H-2b), an unirradiated major-histocompatibility-antigen-mismatched BMT model (B6→BDF1, H-2b→H-2b×d) were utilized. BALB/c, LP, BDF1, and B6 mice were used as recipients for transplantation at an age of 8 to 10 weeks. Recipient mice received split-dosed irradiation (425 cGy × 2 for BALB/c or 550 cGy × 2 for B6 and LP) 3–4 hours apart to reduc e gastrointestinal toxicity.
To obtain B6 bone marrow cells from euthanized donor mice, the femur and tibia were harvested aseptically and the bone marrow canals washed out with sterile medium. Bone marrow cells were depleted of T cells by incubation with anti-Thy 1.2 and low-TOX-M rabbit complement (Cedarlane Laboratories). The T-cell-depleted (TCD) bone marrow was analyzed for purity by quantification of the remaining contaminating T cells. The WT or β7−/− B6 and B6-GFP donor T cells were prepared by harvesting splenocytes aseptically from euthanized donor mice. T cells were purified using positive selection with CD5 magnetic Microbeads with the MACS system (Miltenyi Biotec). Irradiated BALB/c, LP, and B6 recipients were transplanted with 5 × 10 6 TCD bone marrow cells with or without 1 × 10 6 CD5-selected WT or β7−/− T cells (or 3 × 10 7 CD5-selected T cells for unirradiated B6 and BDF1 recipients) per mouse via tail vein injection.
In vivo antibody administration
Recipient BALB/c mice were treated with either 150 μg anti-MAdCAM-1 (MECA-367, Bio X Cell) or IgG2a isotype control MAbs (Bio X Cell) via intraperitoneal injection on day 2 and day 3 after BMT. Mice were harvested on day 4 after BMT.
Immunofluorescent staining and vessel painting
Immunofluorescent staining and vessel painting were performed as previously described (Fu et al., 2009; Fu et al., 2013; Fu and Tang, 2010a, b). Mouse small intestines were fixed by perfusion with paraformaldehyde (4%). For vessel painting, perfusion was first performed with wheat germ agglutinin (WGA) Alexa Fluor 555 conjugate (30 μg/g of body weight, Invitrogen) followed by the fixation perfusion with paraformaldehyde prior to being harvested for examination. Once harvested, small intestines were flushed with PBS to remove the luminal contents and were fixed in paraformaldehyde (4%) overnight. For human endoscopic biopsies, duodenal samples were fixed in formalin (10%). The fixed tissues were immersed in 2% Triton-X 100 solution for permeabilization.
Nine different primary antibodies were used to immunolabel the tissues according to the protocol outlined below. The antibodies used were polyclonal rabbit-anti-human lysozyme 3.2.1.17 (Dako, A009902-2), polyclonal chicken anti-mouse GFP (Abcam, ab13970), monoclonal rat-anti-mouse CD3 (R&D systems, MAB4841), polyclonal goat-anti-mouse MAdCAM-1 (R&D systems, AF993), monoclonal rat-anti-mouse MHC class I (Abcam, ab15681), monoclonal rat-anti-mouse MHC class II (Millipore, MABF33), monoclonal rat-anti-mouse CD4 (eBioscience, 11-0042-82), polyclonal rabbit-anti-mouse Ki67 (Abcam, ab15580), and polyclonal rabbit-anti-human MAdCAM-1 (Proteintech, 21917-1-AP). Before the staining steps, tissues were blocked with the blocking buffer (2% Triton X-100 and 10% normal goat serum or normal donkey serum in PBS). The primary antibody was then diluted (1:100) in the dilution buffer (0.25% Triton X-100 and 1% normal goat serum or normal donkey serum in PBS). The whole-mount small intestine tissues were incubated with the primary antibody for one day. An Alexa Fluor 647 conjugated goat-anti-rabbit or goat-anti-rat or donkey-anti-goat secondary antibody or Alexa Fluor 488 conjugated goat-anti-chicken secondary antibody (1:250, Invitrogen) was then used to reveal the immunopositive structure. Afterward, tissues were incubated with DAPI (4’,6-Diamidino-2-Phenylindole, 20 μg/ml, Invitrogen) for one hour to label the nuclei. Finally, the labeled specimens were immersed in the FocusClear solution (CelExplorer, Hsinchu, Taiwan) for optical clearing before being imaged via confocal microscopy.
Microscopic imaging and 3-D image processing
The optical-cleared whole-mount tissues were imaged by a Zeiss LSM 880 confocal microscope equipped with 25× long working distance and 40× lon g working distance multi-immersion objective lenses or a Leica TCS SP8 confocal microscope equipped with 20× multi-immersion and 40× water immersion objective lenses. Amira 6.0.1 image reconstruction software (FEI) was used for 3-D processing and projection of the confocal images. The Amira software was executed by an HP workstation with an 8 core Xeon processor, 64 GB RAM, and Nvidia Quadro K4200 graphics card.
QUANTIFICATION AND STATISTICAL ANALYSIS
CBC cell, Paneth cell, T cell, and MAdCAM-1 density quantifications
Whole-mount immunostaining for the Paneth cell marker lysozyme and DAPI nuclear staining were used to quantify the Paneth cells and CBC cells at the crypt base. The crypt base region was defined as extending luminally from the base of the crypt through all crypt depths containing at least 2 Paneth cells. CBC cells were quantified as lysozyme−DAPI+ cells with at least 1 point of contact with Paneth cells at the crypt base. The full depth small intestine images from untransplanted mice and from mice day 4 or day 7 after BMT were divided into upper and lower half villus regions and upper and lower half crypt regions. T cell numbers were normalized with the actual tissue volume within the scanned tissue for determination of T cell densities. MAdCAM-1 density was derived from MAdCAM-1 length normalized with tissue volume in different regions. 6 to 27 3-D fields in each group were analyzed.
Statistics and software
All bars and error bars represent the means and standard error of the mean (SEM) for the various groups. For the comparisons of two groups, a t-test or nonparametric U test was performed. ANOVA was utilized for comparisons of more than two groups. All statistics were calculated and display graphs were generated using Graphpad Prism. All experiments were performed at least twice with at least 2–4 mice and 6–27 independent tissue segments imaged in each group.
Supplementary Material
Highlights.
The crypt base region is the primary intestinal location invaded by T cells after BMT
T cell infiltration does not correlate with overall intestinal vascular architecture
MAdCAM-1 localizes to crypt region vessels and directs T cells to the ISC compartment
Allo donor T cells rapidly access the ISC compartment and directly interact with ISCs
ACKNOWLEDGEMENTS
We thank Jennifer Tsai for her helpful advice, and we thank Jarrod Dudakov for his expert review of our manuscript. We gratefully acknowledge the MSKCC Gastroenterology and Nutrition Service and the endoscopy unit staff for their collection of patient biopsies. We also gratefully acknowledge the technical assistance of the MSKCC Molecular Cytology Core Facility and the MSKCC Research Animal Resource Center. This research was supported by National Institutes of Health award numbers K08-HL115355 (A.M.H.), R01-HL125571 (A.M.H), R01-HL146338 (A.M.H), and P30-CA008748 (MSKCC Core Grant). Support was also received from the Susan and Peter Solomon Divisional Genomics Program, the Parker Institute for Cancer Immunotherapy, the Ludwig Center for Cancer Immunotherapy, and the Anna Fuller Fund (A.M.H.). A.M.H. was also supported by the Amy Strelzer Manasevit Research Program and a Scholar Award from the American Society of Hematology. Y.Y.F. was supported by a New Investigator Award from the American Society for Blood and Marrow Transplantation.
Footnotes
DECLARATION OF INTERESTS
H.C. is inventor on several patents related to organoids. He is a board member of Roche and co-founder/stockholder of Surrozen. A.M.H. holds intellectual property related to Interleukin-22 and in the last three years has performed consulting for Ziopharm and Nexus Global Group.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams DH, and Eksteen B (2006). Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 6, 244–251. [DOI] [PubMed] [Google Scholar]
- Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, and Brown BD (2018). Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 48, 271–285 e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcora D, Huynh D, Lightowler S, Germann M, Robine S, de May JR, Pollard JW, Stanley ER, Malaterre J, and Ramsay RG (2013). The CSF-1 receptor fashions the intestinal stem cell niche. Stem Cell Res 10, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith M, and Powrie F (2010). An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med 207, 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15, 19–33. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, and Clevers H (2012). Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 11, 452–460. [DOI] [PubMed] [Google Scholar]
- Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, Landan G, Herman EI, Butcher EC, Contag CH, and Negrin RS (2008). Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood 111, 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, and Negrin RS (2005). In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 106, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, and Butcher EC (1995). alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422. [DOI] [PubMed] [Google Scholar]
- Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, and Butcher EC (1993). Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195. [DOI] [PubMed] [Google Scholar]
- Bernier-Latmani J, and Petrova TV (2016). High-resolution 3D analysis of mouse small-intestinal stroma. Nat Protoc 11, 1617–1629. [DOI] [PubMed] [Google Scholar]
- Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, et al. (2018). T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 175, 1307–1320 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, and Cheng H (2001). Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 98, 12497–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, and Picker LJ (1996). Lymphocyte homing and homeostasis. Science 272, 60–66. [DOI] [PubMed] [Google Scholar]
- Cerovic V, Bain CC, Mowat AM, and Milling SW (2014). Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol 35, 270–277. [DOI] [PubMed] [Google Scholar]
- Cheroutre H, and Madakamutil L (2004). Acquired and natural memory T cells join forces at the mucosal front line. Nature Reviews Immunology 4, 290–300. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Goldsby JS, Callaway ES, Shah MS, Barker N, and Chapkin RS (2012). Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta 1822, 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti I, Mora-Buch R, Ferrer-Picon E, Planell N, Jung P, Masamunt MC, Leal RF, Martin de Carpi J, Llach J, Ordas I, et al. (2016). Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriguchi Y, Takashima S, Oka H, Shimoji S, Nakamura K, Uryu H, Shimoda S, Iwasaki H, Shimono N, Ayabe T, et al. (2012). Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood 120, 223–231. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, et al. (2013). Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369, 699–710. [DOI] [PubMed] [Google Scholar]
- Floisand Y, Lundin K, Lazarevic V, Kristiansen JD, Osnes LTN, Tjonnfjord GE, Reims HM, and Gedde-Dahl T (2015). Targeting Integrin a4b7-Expressing T-Cells in Steroid Refractory Intestinal GvHD. Blood 126. [Google Scholar]
- Fu YY, Lin CW, Enikolopov G, Sibley E, Chiang AS, and Tang SC (2009). Microtome-Free 3-Dimensional Confocal Imaging Method for Visualization of Mouse Intestine With Subcellular-Level Resolution. Gastroenterology 137, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YY, Peng SJ, Lin HY, Pasricha PJ, and Tang SC (2013). 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 304, G1–11. [DOI] [PubMed] [Google Scholar]
- Fu YY, and Tang SC (2010a). At the Movies: 3-Dimensional Technology and Gastrointestinal Histology. Gastroenterology 139, 1100-+. [DOI] [PubMed] [Google Scholar]
- Fu YY, and Tang SC (2010b). Optical clearing facilitates integrated 3D visualization of mouse ileal microstructure and vascular network with high definition. Microvasc Res 80, 512–521. [DOI] [PubMed] [Google Scholar]
- Fujimori H, Miura S, Koseki S, Hokari R, Komoto S, Hara Y, Hachimura S, Kaminogawa S, and Ishii H (2002). Intravital observation of adhesion of lamina propria lymphocytes to microvessels of small intestine in mice. Gastroenterology 122, 734–744. [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, and Mucida D (2016). Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 164, 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, and Spies T (1996). Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A 93, 12445–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. (2012). Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. (2007). PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. (2012). Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 209, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen SHM, Duijvestijn AM, Sontag Y, and Kraal G (1987). Lymphocyte Migration into the Lamina Propria of the Gut Is Mediated by Specialized Hev-Like Blood-Vessels. Immunology 62, 273–277. [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, and Agace WW (2007). Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunological Reviews 215, 226–242. [DOI] [PubMed] [Google Scholar]
- Koseki S, Miura S, Fujimori H, Hokari R, Komoto S, Hara Y, Ogino T, Nagata H, Goto M, Hachimura S, et al. (2001). In situ demonstration of intraepithelial lymphocyte adhesion to villus microvessels of the small intestine. Int Immunol 13, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, and Butcher EC (2003). CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest 111, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Huber E, Hammer ST, Harris AC, Greenson JK, Braun TM, Ferrara JL, and Holler E (2013). Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood 122, 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. (2015). Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. (2010). Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, and Schenkel JM (2013). The integration of T cell migration, differentiation and function. Nature Reviews Immunology 13, 309–320. [DOI] [PubMed] [Google Scholar]
- Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, and Piccinini L (1991). Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology 100, 3–12. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, and von Andrian UH (2004). T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, and Cahalan MD (2002). Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, and von Andrian UH (2003). Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93. [DOI] [PubMed] [Google Scholar]
- Mora JR, and von Andrian UH (2006). T-cell homing specificity and plasticity: new concepts and future challenges. Trends in Immunology 27, 235–243. [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C (2001). Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol 1, 59–67. [DOI] [PubMed] [Google Scholar]
- Nakache M, Berg EL, Streeter PR, and Butcher EC (1989). The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 337, 179–181. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Reina-Campos M, Nakanishi N, Llado V, Elmen L, Peterson S, Campos A, De SK, Leitges M, Ikeuchi H, et al. (2016). Control of Paneth Cell Fate, Intestinal Inflammation, and Tumorigenesis by PKClambda/iota. Cell Rep 16, 3297–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalle SC, and Turner JR (2015). Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol 8, 720–730. [DOI] [PubMed] [Google Scholar]
- Neurath MF (2017). Current and emerging therapeutic targets for IBD. Nature Reviews Gastroenterology & Hepatology 14, 269–278. [DOI] [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A, Price A, Hermanson JR, Taras E, Lees C, Serody JS, and Blazar BR (2004). In vivo imaging of graft-versus-host-disease in mice. Blood 103, 3590–3598. [DOI] [PubMed] [Google Scholar]
- Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, Geraghty D, Groh V, Spies T, Jabri B, and Mayer L (2007). Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis 13, 298–307. [DOI] [PubMed] [Google Scholar]
- Petrovic A, Alpdogan O, Willis LM, Eng JM, Greenberg AS, Kappel BJ, Liu C, Murphy GJ, Heller G, and van den Brink MRM (2004). LPAM(alpha(4)beta(7) integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood 103, 1542–1547. [DOI] [PubMed] [Google Scholar]
- Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, and deBeaumont M (1993). Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest 92, 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, Goldstein SC, Stadtmauer EA, Smith J, Bailey S, et al. (2012). Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med 367, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackstein R (1995). Lymphocyte migration following bone marrow transplantation. Ann N Y Acad Sci 770, 177–188. [DOI] [PubMed] [Google Scholar]
- Sale GE (1996). Does graft-versus-host disease attack epithelial stem cells? Mol Med Today 2, 114–119. [DOI] [PubMed] [Google Scholar]
- Salmi M, and Jalkanen S (2005). Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev 206, 100–113. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, et al. (2013). Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 369, 711–721. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, and Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, and Clevers H (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schuijers J, van der Flier LG, van Es J, and Clevers H (2014). Robust Cre-Mediated Recombination in Small Intestinal Stem Cells Utilizing the Olfm4 Locus. Stem Cell Reports 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji S, Hashimoto D, Tsujigiwa H, Miyawaki K, Kato K, Takahashi S, Ogasawara R, Jiromaru T, Iwasaki H, Miyamoto T, et al. (2017). Graft-versus-host disease targets ovary and causes female infertility in mice. Blood 129, 1216–1225. [DOI] [PubMed] [Google Scholar]
- Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Toth B, Kondo A, Massasa EE, Itzkovitz S, and Kaestner KH (2018). Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, and Clevers H (2010). Intestinal Crypt Homeostasis Results from Neutral Competition between Symmetrically Dividing Lgr5 Stem Cells. Cell 143, 134–144. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, and Artis D (2011). Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature Immunology 12, 383–390. [DOI] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, and Germain RN (2002). Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876. [DOI] [PubMed] [Google Scholar]
- Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, and Agace WW (2002). CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest 110, 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K, Akashi K, and Teshima T (2011). The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med 208, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, Muriglan SJ, Kim TD, Heller G, Murphy GF, et al. (2006). Absence of beta 7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood 107, 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosen JE, Mukhopadhyay D, Macaubas C, and Mellins ED (2018). Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front Immunol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, and Serody JS (2005). Leukocyte migration and graft-versus-host disease. Blood 105, 4191–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.