Abstract
Diarylamines are among the most common chemotypes in modern drug discovery. While diarylamines can potentially possess two axes of chirality, there are no studies on their catalytic enantioselective synthesis as these axes typically possess lower stereochemical stabilities. Herein we report a chiral phosphoric acid catalyzed atroposelective electrophilic halogenation of N-aryl quinoids, a class of compounds that are analogous to diarylamines. This chemistry yields a large range of stereochemically stable N-aryl quinoids in excellent yields and atroposelectivity. This work represents the first example of the atroposelective synthesis of a diarylamine-like scaffold and will serve as a gateway to fundamental and applied studies on the scarcely studied chirality of these ubiquitous chiral scaffolds.
Graphical Abstract
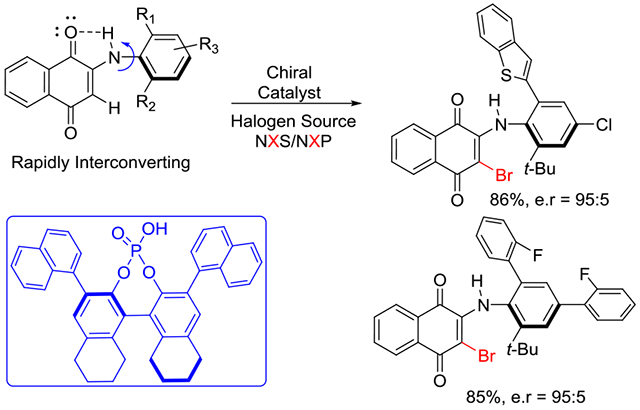
Strong intramolecular hydrogen bonding for high level of stereochemical stability X = Br/CI/I, up to 98:2 e.r. ΔG = ≥ 29 Kcal/mol in protic solvent
Atropisomerism, or axial chirality, is ubiquitous throughout modern drug discovery1–4 and natural products chemistry.5,6 While most chemists recognize atropisomeric axes pertaining to biaryls,7 benzamides,8 and anilides,9 atropisomeric diarylamines are largely overlooked. Despite this, diarylamines are among the most common potentially atropisomeric chemotypes in medicinal chemistry, with FDA-approved binimetinib, bosutinib and afatinib representing examples of diarylamines that exist as rapidly interconverting atropisomers (Scheme 1A).10–12 Indeed, a cursory search in the PDB will reveal thousands of co-crystal structures of diarylamine ligands bound to proteins in an atroposelective manner. With the rapid advancement of synthetic methodology to obtain diarylamines, exemplified by the Buchwald-Hartwig reaction,13,14 the ubiquitous nature of diarylamines in drug discovery will likely continue to grow. Diarylamines are also interesting from a stereochemical perspective as they possess two contiguous atropisomeric axes which leads to a complex conformational profile as well as a potential gearing mechanism that can lead to lower than expected barriers to racemization.15,16
Scheme 1.
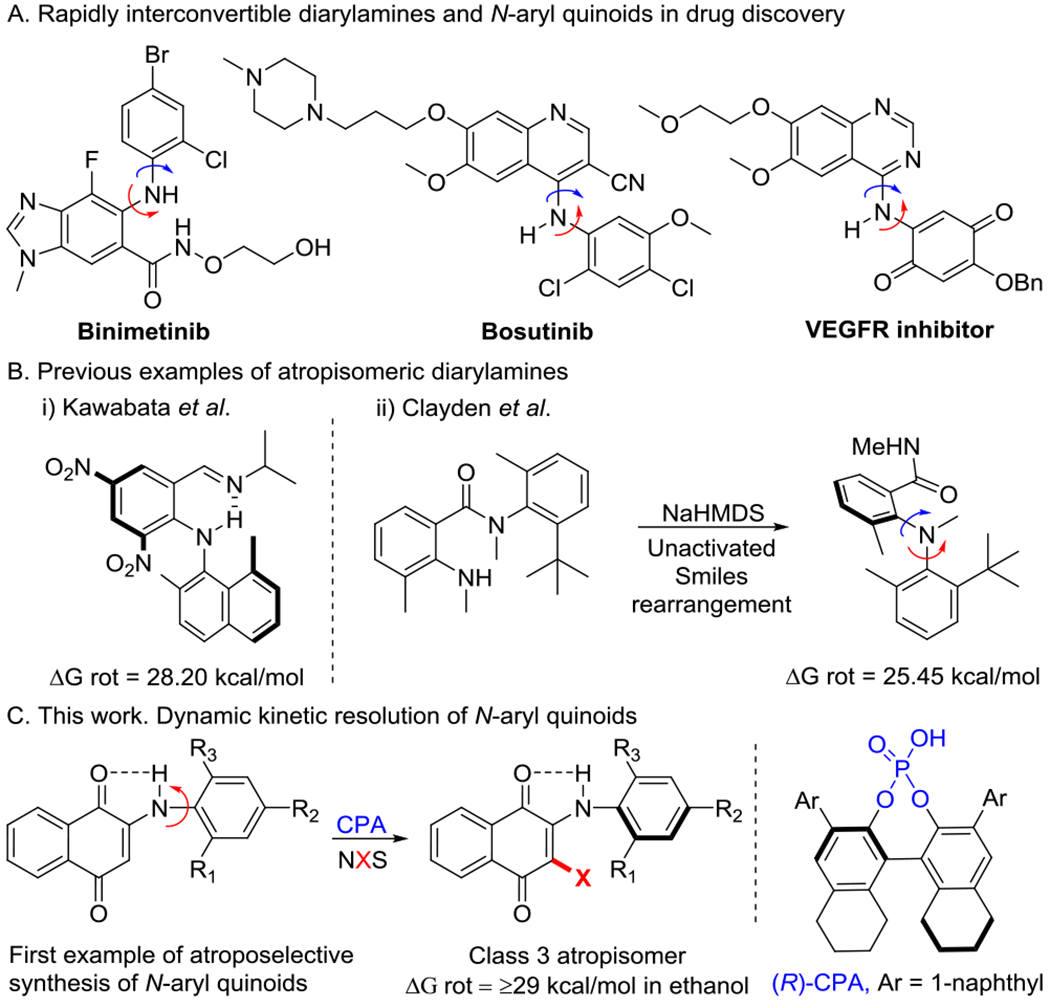
Atropisomerism in Diarylamines and related scaffolds
Relatively little is known about atropisomerically stable diaryl- amines. Kawabata17,18 resolved the atropisomers of a series of diarylamines that possessed an intramolecular N-H-N hydrogen bond that preorganized one of the axes into a planar conformation (Scheme 1B), simplifying diarylamines to a single axis system and resulting in compounds that possessed barriers to racemization of up to ~28 kcal/mol, existing as class 2 atropisomers according to LaPlante’s19 atropisomer stability classification system (t1/2 to racemization on the day to month timescale at 37 °C ). Kawabata’s system proved sensitive to the strength of the intramolecular hydrogen bond, and the stereochemical stabilities quickly decreased upon removal of electron withdrawing groups. More recently, Clayden15 resolved the atropisomers for diarylamines without an internal H-bond, finding them to only have stereochemical stabilities on the hour time scale at room temperature.
As instances of stereochemically stable diarylamines have been scarce, there are no published examples of their enantioselective synthesis. Herein we describe studies that have led to the first catalytic atroposelective synthesis of a diarylamine-like scaffold, finding N-aryl quinoids that possess a 5-membered intramolecular N-H-O hydrogen bond to exist as stereochemically stable class 3 atropisomers with barriers to racemization approaching and exceeding 30 kcal/mol (t1/2 (37° C) > 4.5 years) in both protic and aprotic solvents, a classification that is considered sufficiently stereochemically stable for drug development.
Inspired by Kawabata’s work, as well as a series of N-aryl quinoid-based kinase inhibitors (also in Scheme 1A),20 we synthesized substrate 1a which exists as a stereochemically unstable class 1 atropisomer. Computational studies and X-ray crystallographic analysis (Scheme 2) suggested that the quinoid-nitrogen axis exists in a planar ‘exo’ conformation due to a strong intramolecular N-H-O hydrogen bond, simplifying the 2-axis system into a single axis system in a manner analogous to that of Kawabata. We hypothesized that functionalization of the quinoid C-H could lead to stable atropisomerism. Indeed, Tan and others have pioneered atroposelective synthesis via the addition of aryl nucleophiles into quinones,21–25 and we have studied other nucleophiles in the context of the atroposelective synthesis.16,26 Unfortunately, 1a proved unreactive under these conditions.
Scheme 2.
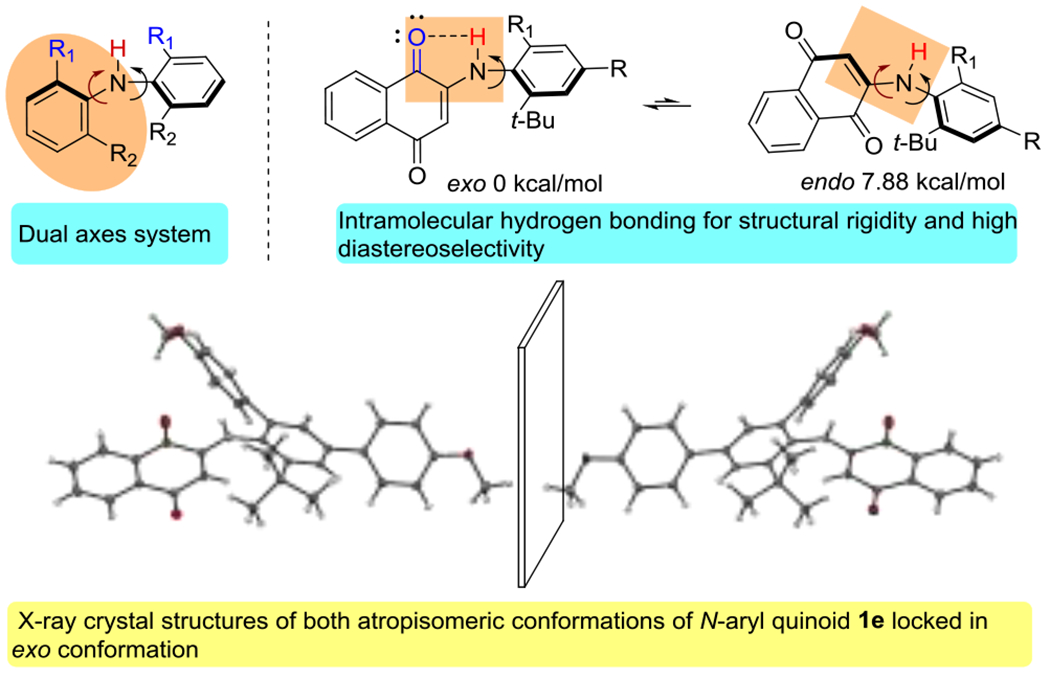
N-Aryl quinoids as a diarylamine surrogate with one chiral axis.
As the amino naphthoquinone moiety could be considered ‘enamine-like’ we next evaluated electrophilic functionalization of 1a, observing facile electrophilic halogenation using achiral Lewis basic catalysts and N-halosuccinimides.27,28 Excitingly, these halogenated products proved quite stereochemically stable (vida infra), confirming that this system may be amenable to atropisomer selective catalysis in which a stereochemical labile axis is rigidified via a ‘net C-H functionalization’, as exemplified in work by Miller and colleagues.7,8,19,29–32
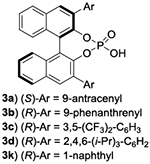
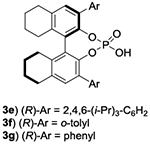
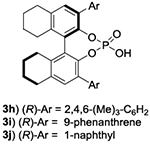
The evaluation of chiral Lewis basic catalysts yielded little to no enantioselectivity (See SI). Inspired by the seminal work of Akiyama33–38 on the atroposelective bromination of biaryl systems, we next evaluated several chiral phosphoric acids (CPA) with N-Bromosuccinimide (NBS) for the bromination of 1a (Table 1, entries 1-4). While many of these catalysts yielded poor selectivity, the venerable CPA (R)-TRIPS 3d effected this reaction to near quantitative conversions with promising enantioselectivity (77:23 e.r.). We next evaluated the less acidic octahydro-BINOL (H8-BINOL) scaffold, finding H8-BINOL (R)-TRIPS (3e, Table 1, entry 5) resulted in a slight increase in enantioselectivity (80:20 e.r.). This result encouraged us to try other H8-BINOL based catalysts (Table 1, entries 6-9). While modifying the 3,3’ positions largely led to minor changes in selectivity, we found that CPA 3j, which possesses 1-naphthyl substitution at the 3,3’ position, to be an effective catalyst yielding brominated product 2a in 94% yield and an e.r. of 91:9 (Table 1, entry 10). We next evaluated other reaction parameters such as temperature, solvent, reaction concentration, catalyst loading and bromination reagent (Table 1, entries 11-13), arriving to conditions that yielded 2a in greater than 96:4 e.r. We finally evaluated 3k, the BINOL based analog of our optimal catalyst, observing a slight loss in enantioselectivity (entry 14).
Table 1.
Condition Optimizationaa
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Br Source | Concentration | Time | Temp. (°C) | Yield (2a%)b | e.r. (2a)c |
| 1 | 3a 5% | NBS | 0.025M | 6 h | 24 | 94 | 45:55 |
| 2 | 3b 5% | NBS | 0.025M | 6 h | 24 | 94 | 51:49 |
| 3 | 3c 5% | NBS | 0.025M | 6 h | 24 | 94 | 56:44 |
| 4 | 3d 5% | NBS | 0.025M | 6 h | 24 | 95 | 77:23 |
| 5 | 3e 5% | NBS | 0.025M | 12 h | 24 | 94 | 80:20 |
| 6 | 3f 5% | NBS | 0.025M | 12 h | 24 | 94 | 82:18 |
| 7 | 3g 5% | NBS | 0.025M | 12 h | 24 | 95 | 74:26 |
| 8 | 3h 5% | NBS | 0.025M | 12 h | 24 | 95 | 75:25 |
| 9 | 3i 5% | NBS | 0.025M | 12 h | 24 | 94 | 50:50 |
| 10 | 3j 5% | NBS | 0.025M | 12 h | 24 | 94 | 91:9 |
| 11 | 3j 10% | NBP | 0.025M | 12 h | 24 | 94 | 92:8 |
| 12 | 3j 10% | NBP | 0.0055M | 12 h | 24 | 95 | 95:5 |
| 13 | 3j 10% | NBP | 0.0055M | 12 h | 24 | 95 | 96:4d |
| 14 | 3k 10% | NBP | 0.0055M | 6 h | 24 | 91 | 90:10d |
 |
 |
 |
|||||
Reaction conditions: Starting material 1a (0.055 mmol), Catalyst (10%), Toluene:Hexane (1:1) 10 mL then bromine source (0.06 mmol) at 24 °C for 12 h.
isolated yield.
Determined by HPLC analysis on a chiral stationary phase.
2 fold 4A MS. NBP = N-Bromophthalimide
With optimal conditions in hand we next sought to define reaction scope. The chemistry proved to be tolerant of diverse aryl substitutions of the 2,4-positions of the aniline. Electron rich aryl substitutions yielding e.r.s above 95:5 (Scheme 3, 2b-2e, 2i, 2j, 2n, 2o) and electron poor aryl substitutions yielding e.r.s around 90:10 (2f-2h, 2k). Notably, aryls groups with ortho fluorine substitutions (2l, 2m) yielded selectivities greater than 95:5 e.r. We were able to obtain a crystal structure of 2l, confirming the stereochemical induction of this reaction to be the (S)-atropisomer in the exo conformation.
Scheme 3. Substrate Scope.
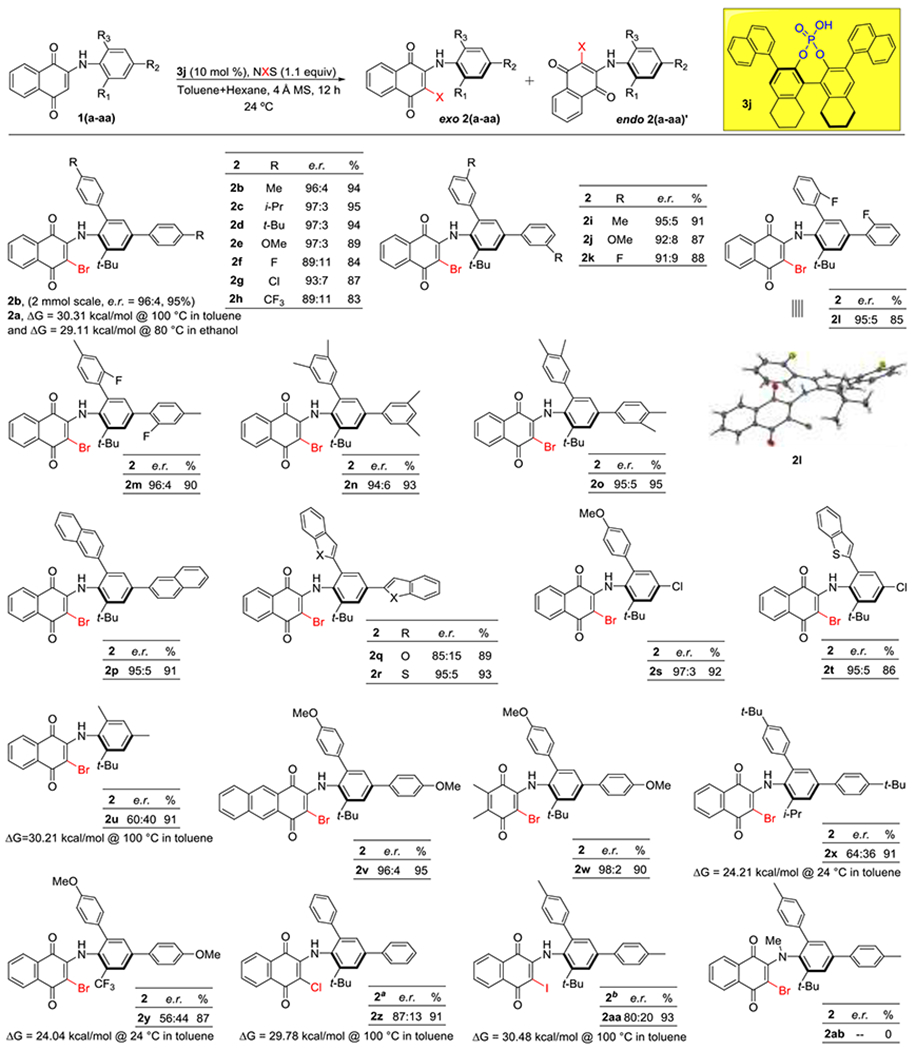
aReaction conditions: Starting material 1a (0.054 mmol), Cat.3e (2.5%), Me3PS (2.5%) Toluene:Hexane (1:1) 10 mL and 2 fold 4 Å MS then NCS (0.06 mmol) 24 °C for 12 h
bStarting material 1a (0.054 mmol). Cat. 3j (10%) Toluene:Hexane (1:1) 10 mL and 2 fold 4 A MS then NIS (0.06 mmol) 24 °C for 24 h .
This chemistry also proved tolerant of fused ring systems including naphthyl (2p, 91% yield with 95:5 e.r.), benzofuran (2q, 89% yield with 85:15 e.r.) and benzothiophene (2r in 93% yield with 95:5 e.r.). We next evaluated substrates that possess 2-aryl-4-chloro substitution, obtaining 2s and 2t in excellent yield and enantioselectivity. We find these results significant, as the ability to transform aryl chlorides into diverse functionalities in a manner orthogonal to bromides23 will allow for significant synthetic utility to obtain diverse analogs. Unfortunately, the synthesis of substrates with halogen substitution at the ortho position of the arene using literature methodologies failed due to an unexpected cyclization (see SI). Replacing the aryl substitution at both positions with methyl groups resulted in a large drop in e.r to 60:40 (2u, 91%), suggesting the ortho- aryl group partakes in an interaction with the catalyst that is crucial for enantiomer discrimination. We next evaluated different quinone fragments, finding an anthraquinone based substrate to give excellent yields and selectivities (2v, 95% yield with 96:4 e.r). A substituted benzoquinone-based substrate also reacted cleanly to give 2w in 90% yield and 98:2 e.r.
We next evaluated anilines that did not possess a t-Bu group at the 6-position, observing that replacing the tert-butyl by isopropyl as in 2x and trifluoromethyl as in 2y resulted in good yield but drastic decreases in e.r. to 63:37 and 56:44 respectively, likely due to a low barrier to racemization (measured to be~24 kcal/mol) for these substrates leading to racemization during the course of the reaction. Finally, we observed that this chemistry was amenable to chlorination in the presence of a Lewis basic catalyst and NCS (2z, 91% yield with 87:13 e.r.) and iodination using NIS (2aa, 93% yield 80:20 e.r.), albeit with somewhat attenuated selectivities.
Racemization studies were also carried out to investigate the stereochemical stability of representative products. Product 2a was found to have a barrier to rotation of 30.31 kcal/mol at 100 °C in toluene, which would be considered a stable class 3 atropisomer. Notably, when the barrier was measured in ethanol at 80 °C the barrier was only slightly lower (29.1 kcal/mol). Furthermore, the stereochemical stability was minimally affected under strongly acidic conditions (28.92 kcal/mol in a 4:1 mixture of EtOH and 0.5M HCl), and was largely unchanged from toluene under buffered aqueous conditions (30.56 kcal/mol at pH 7.5 in 1 M tris buffer). We also measured the barriers for products 2b, 2u, 2z which possess varying substituent sizes about the chiral axis, finding the barrier to rotation tracked well with what is expected with other atropisomeric systems.39 These observed trends are also in line with what Kawabata observed.
Taken together, this data is significant as it suggests that these compounds would be stereochemically stable under biological environments; thus, this work could be a gateway towards the development of atropisomerically stable analogs for the myriad diarylamines in medicinal chemistry. Moreover, these scaffolds would be stable under routine reaction conditions, thus it is possible to embed this scaffold into catalysts to give never before explored chiral environments.
We next studied the ability to modify these products to evaluate their synthetic utility (Scheme 4). We first found that the anilide functionality in 2b could be tosylated to give sulfonamide 4 in 63% yield with no observed racemization. Compound 4, which now lacks the internal H-bond, proved to exist as a class-2 atropiosmer with a barrier to rotation of 27.8 kcal/mol, in line with other literature precedence concerning anilide atropisomers. We also found that the bromide could be modified via Suzuki cross-coupling at room temperature using Organ’s PEPPSI-i-Pr catalyst to afford 5 in 53% yield, also with complete retention of e.r. 95:5. As aryl substitution is significantly smaller than bromide substitution39, 5 also existed as a class-2 atropisomer. While 4 and 5 are less stable, they still possess t (1/2) of racemization at 37 °C on the multi-month timescale. It should be noted that this scaffold proved recalcitrant to reduction to the hydroquinone (see SI).
Scheme 4.
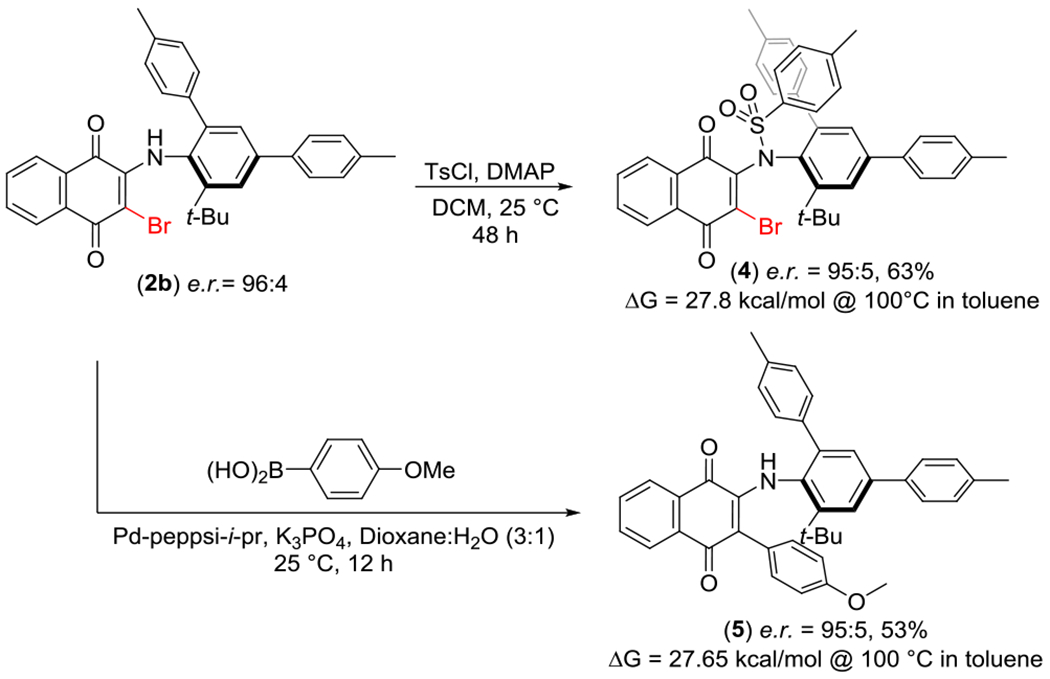
Derivatization of Enantioenriched Products
We next sought out to better understand the unexpected stereochemical stability that was observed. In the X-ray crystal structure of 2l, the naphthoquinone-N axis is locked into a planar exo orientation due to the aforementioned intramolecular hydrogen bonding.
We further explored this by computationally evaluating the relative energy values of other conformations (Scheme 5). Both exo, (Ra) and exo, (Sa) conformations (0 kcal/mol) were significantly lower in energy than their respective endo conformations that possessed no intramolecular hydrogen bonding (+2.87 kcal/mol). Previously reported 2 axes systems are able to undergo concerted “gearing” to racemize, allowing for a lower energy pathway to racemization (barrier to rotations of 25–27 kcal/mol with similar sized substituents). The strong intramolecular hydrogen bonding likely precludes the gearing mechanism for racemization, requiring a more rigid pathway to racemization that is more analogous to biaryl and benzamide systems. Indeed, computational studies predicted barrier to rotations that agreed with our experimentally determined values when freezing the naphthoquinone-N axis; when the system was allowed to rotate both axes, predicted barriers to rotations were significantly lower than the experimental values (See SI).
Scheme 5.
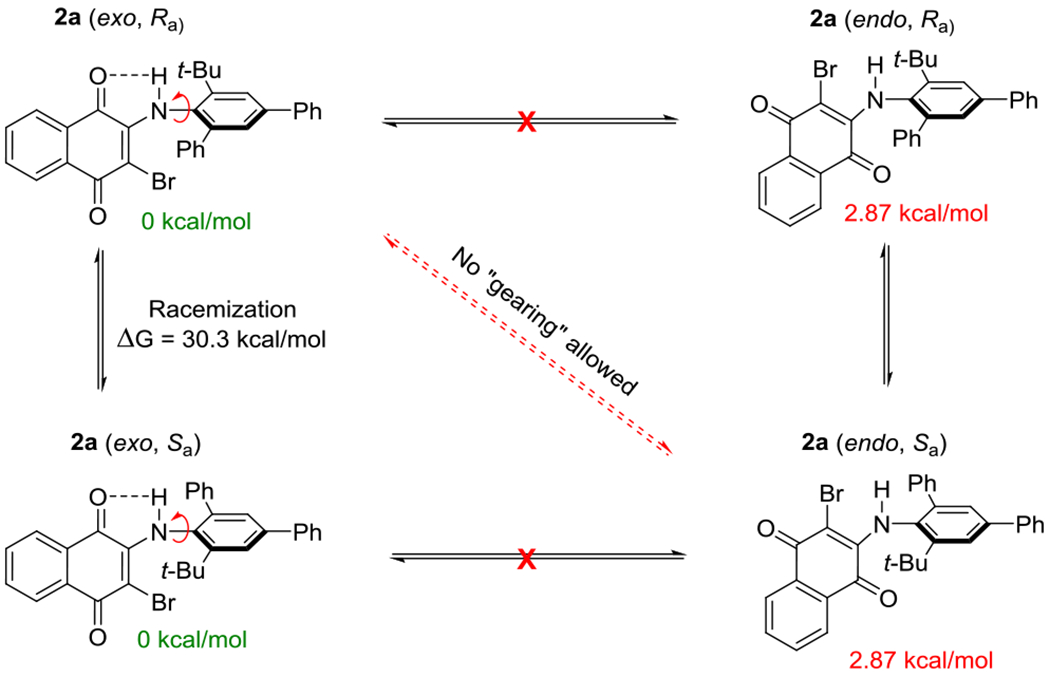
Mechanism of Racemization
Based on our data thus far, we propose the mechanism for stereochemical induction depicted in Scheme 6. We postulate the CPA 3j engages in hydrogen bonding with the substrates O-H-N network with the substrate locked in the exo conformation. This H-bonding interaction would be expected to increase the energy level of the HOMO of the enamine-like amino naphthoquinone. The N-aryl axis would then be expected to rotate as to orientate the t-Bu group away from the catalyst, which would result in this axis being organized into the (Sa) conformation. Notably, the (Sa) conformation would also have the potential for the ortho aryl group to participate in pi-stacking with the catalyst naphthyl group, whereas the enantiomeric conformation would not. Finally, the acidic proton from CPA can activate the electrophilic halogenation reagent via H-bonding which will also allow for direction of bromination towards the naphthoquinone site. Upon electrophilic bromination, the axis will be rigidified selectively into the (Sa) configuration. In support of the importance of the H-bond, a N-methylated substrate analog (1ab) proved unreactive under our optimal conditions (scheme 3).
Scheme 6.
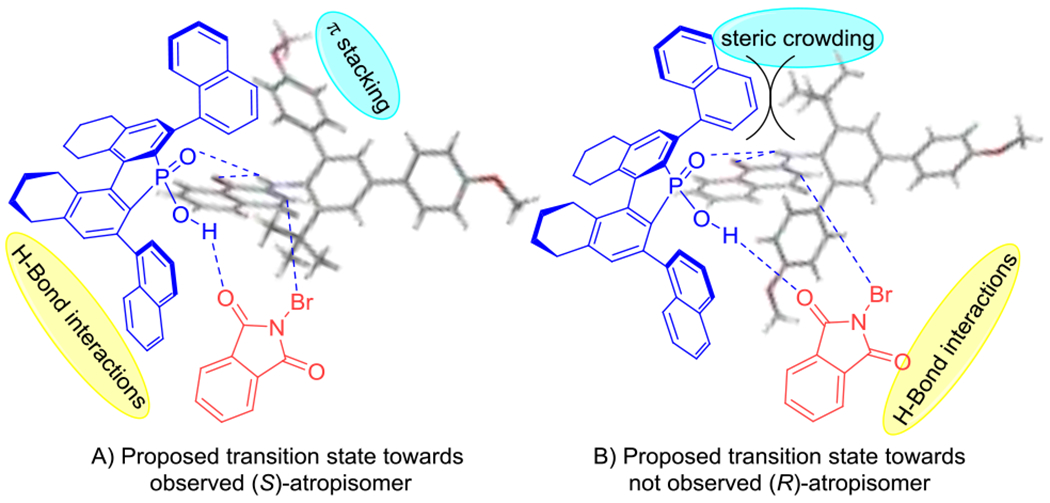
Proposed transition state with substrate, catalyst and N-bromophthalimide
We have described the first catalytic atropisomer selective synthesis of a diarylamine-like scaffold. This chemistry proceeds via phosphoric acid catalyzed halogenation of N-aryl quinoids and yields products in upwards of 95:5 e.r. and 90% yield. Surprisingly, we found these products existed as stereochemically stable class-3 atropisomers in protic and aprotic conditions due to a strong intramolecular H-bond that locks one of the axes into a planar conformation. As diarylamines are among the most ubiquitous scaffolds in drug discovery, the chemistry, concepts and principles put forward can extend far beyond this work and be of use for the design and enantioselective synthesis of diverse chiral diarylamine scaffolds with applications in drug discovery and asymmetric synthesis.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Greg Elliott for assistance with HRMS and Dillan Stengel for assistance with NMR. This work was funded by NIGMS (R35GM124637).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Experimental procedures, and characterization data for all new compounds including 1H- and 13C-NMR spectra, Single-crystal X-ray diffraction, and HPLC traces (PDF).
The authors declare no competing financial interest.
REFERENCES
- (1).Glunz PW Recent Encounters with Atropisomerism in Drug Discovery. Bioorganic Med. Chem. Lett 2018, 28, 53–60. [DOI] [PubMed] [Google Scholar]
- (2).Clayden J; Moran WJ; Edwards PJ; Laplante SR The Challenge of Atropisomerism in Drug Discovery. Angew. Chemie - Int. Ed 2009, 48, 6398–6401. [DOI] [PubMed] [Google Scholar]
- (3).Toenjes ST; Gustafson JL Atropisomerism in Medicinal Chemistry: Challenges and Opportunities. Future Med. Chem 2018, 10, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Toenjes ST; Garcia V; Maddox SM; Dawson GA; Ortiz MA; Piedrafita FJ; Gustafson JL Leveraging Atropisomerism to Obtain a Selective Inhibitor of RET Kinase with Secondary Activities toward EGFR Mutants. ACS Chem. Biol 2019, 14, 1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kozlowski MC; Morgan BJ; Linton EC Total Synthesis of Chiral Biaryl Natural Products by Asymmetric Biaryl Coupling. Chem. Soc. Rev 2009, 38, 3193–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bringmann G; Gulder T; Gulder TAM; Breuning M Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev 2011, 111, 563–639. [DOI] [PubMed] [Google Scholar]
- (7).Gustafson JL; Lim D; Miller SJ Dynamic Kinetic Resolution of Biaryl Asymmetric Bromination. Science (80-. ) 2010, 328, 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Barrett KT; Metrano AJ; Rablen PR; Miller SJ Spontaneous Transfer of Chirality in an Atropisomerically Enriched Two-Axis System. Nature 2014, 508, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Li SL; Yang C; Wu Q; Zheng HL; Li X; Cheng JP Atroposelective Catalytic Asymmetric Allylic Alkylation Reaction for Axially Chiral Anilides with Achiral Morita-Baylis-Hillman Carbonates. J. Am. Chem. Soc 2018, 140, 12836–12843. [DOI] [PubMed] [Google Scholar]
- (10).Levinson NM; Boxer SG Structural and Spectroscopic Analysis of the Kinase Inhibitor Bosutinib and an Isomer of Bosutinib Binding to the Abl Tyrosine Kinase Domain. PLoS One 2012, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Koelblinger P; Dornbierer J; Dummer R A Review of Binimetinib for the Treatment of Mutant Cutaneous Melanoma. Futur. Oncol 2017, 13, 1–12. [DOI] [PubMed] [Google Scholar]
- (12).Liu Q; Sabnis Y; Zhao Z; Zhang T; Buhrlage SJ; Jones LH; Gray NS Developing Irreversible Inhibitors of the Protein Kinase Cysteinome. Chem. Biol 2013, 20, 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Guram AS; Buchwald SL Palladium-Catalyzed Aromatic Animations with in Situ Generated Aminostannanes. J. Am. Chem. Soc 1994, 116, 7901–7902. [Google Scholar]
- (14).Paul F; Patt J; Hartwig JF Palladium-Catalyzed Formation of Carbon-Nitrogen Bonds. Reaction Intermediates and Catalyst Improvements in the Hetero Cross-Coupling of Aryl Halides and Tin Amides. J. Am. Chem. Soc 1994, 116, 5969–5970. [Google Scholar]
- (15).Costil R; Dale HJA; Fey N; Whitcombe G; Matlock JV; Clayden J Heavily Substituted Atropisomeric Diarylamines by Unactivated Smiles Rearrangement of N-Aryl Anthranilamides. Angew. Chemie - Int. Ed 2017, 56, 12533–12537. [DOI] [PubMed] [Google Scholar]
- (16).Dinh AN; Noorbehesht RR; Toenjes ST; Jackson AC; Saputra MA; Maddox SM; Gustafson JL Toward a Catalytic Atroposelective Synthesis of Diaryl Ethers Through C(Sp 2)-H Alkylation with Nitroalkanes. Synlett 2018, 29, 2155–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kawabata T; Jiang C; Hayashi K; Tsubaki K; Yoshimura T; Majumdar S; Sasamori T; Tokitoh N Axially Chiral Binaphthyl Surrogates with an Inner N-H-N Hydrogen Bond. J. Am. Chem. Soc 2009, 131, 54–55. [DOI] [PubMed] [Google Scholar]
- (18).Hayashi K; Matubayasi N; Jiang C; Yoshimura T; Majumdar S; Sasamori T; Tokitoh N; Kawabata T Insights into the Origins of Configurational Stability of Axially Chiral Biaryl Amines with an Intramolecular N-H-N Hydrogen Bond. J. Org. Chem 2010, 75, 5031–5036. [DOI] [PubMed] [Google Scholar]
- (19).Laplante SR; Fader LD; Fandrick KR; Fandrick DR; Hucke O; Kemper R; Miller SPF; Edwards PJ Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem 2011, 54, 7005–7022. [DOI] [PubMed] [Google Scholar]
- (20).Ferguson FM; Gray NS Kinase Inhibitors: The Road Ahead. Nat. Rev. Drug Discov 2018, 17, 353–376. [DOI] [PubMed] [Google Scholar]
- (21).Zhu S; Chen Y-H; Wang Y-B; Yu P; Li S-Y; Xiang S-H; Wang J-Q; Xiao J; Tan B Organocatalytic Atroposelective Construction of Axially Chiral Arylquinones. Nat. Commun 2019, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chen YH; Cheng DJ; Zhang J; Wang Y; Liu XY; Tan B Atroposelective Synthesis of Axially Chiral Biaryldiols via Organocatalytic Arylation of 2-Naphthols. J. Am. Chem. Soc 2015, 137, 15062–15065. [DOI] [PubMed] [Google Scholar]
- (23).Coombs G; Sak MH; Miller SJ Peptide-Catalyzed Fragment Couplings That Form Axially Chiral Non- C 2 -Symmetric Biaryls. Angew. Chemie - Int. Ed 2019, DOI: 10.1002/anie.201913563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Moliterno M; Cari R; Puglisi A; Antenucci A; Sperandio C; Moretti E; Di Sabato A; Salvio R; Bella M Quinine-Catalyzed Asymmetric Synthesis of 2,2′-Binaphthol-Type Biaryls under Mild Reaction Conditions. Angew. Chemie - Int. Ed 2016, 55, 6525–6529. [DOI] [PubMed] [Google Scholar]
- (25).Gao H; Xu QL; Keene C; Yousufuddin M; Ess DH; Kürti L Practical Organocatalytic Synthesis of Functionalized Non-C2-Symmetrical Atropisomeric Biaryls. Angew. Chemie - Int. Ed 2016, 55, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Maddox SM; Dawson GA; Rochester NC; Ayonon AB; Moore CE; Rheingold AL; Gustafson JL Enantioselective Synthesis of Biaryl Atropisomers via the Addition of Thiophenols into Aryl-Naphthoquinones. ACS Catal. 2018, 8, 5443–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Maddox SM; Dinh AN; Armenta F; Um J; Gustafson JL The Catalyst-Controlled Regiodivergent Chlorination of Phenols. Org. Lett 2016, 18, 5476–5479. [DOI] [PubMed] [Google Scholar]
- (28).Maddox SM; Nalbandian CJ; Smith DE; Gustafson JL A Practical Lewis Base Catalyzed Electrophilic Chlorination of Arenes and Heterocycles. Org. Lett 2015, 17, 1042–1045. [DOI] [PubMed] [Google Scholar]
- (29).Kwon Y; Chinn AJ; Kim B; Miller SJ Divergent Control of Point and Axial Stereogenicity: Catalytic Enantioselective C−N Bond-Forming Cross-Coupling and Catalyst-Controlled Atroposelective Cyclodehydration. Angew. Chemie - Int. Ed 2018, 57, 6251–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Diener ME; Metrano AJ; Kusano S; Miller SJ Enantioselective Synthesis of 3-Arylquinazolin-4(3H)-Ones via Peptide-Catalyzed Atroposelective Bromination. J. Am. Chem. Soc 2015, 137, 12369–12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Barrett KT; Miller SJ Enantioselective Synthesis of Atropisomeric Benzamides through Peptide-Catalyzed Bromination. J. Am. Chem. Soc 2013, 135, 2963–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pathak TP; Miller SJ Site-Selective Bromination of Vancomycin. J. Am. Chem. Soc 2012, 134, 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Akiyama T Stronger Brønsted Acids. Chem. Rev 2007, 107, 5744–5758. [DOI] [PubMed] [Google Scholar]
- (34).Kashikura W; Itoh J; Mori K; Akiyama T Enantioselective Friedel-Crafts Alkylation of Indoles, Pyrroles, and Furans with Trifluoropyruvate Catalyzed by Chiral Phosphoric Acid. Chem. - An Asian J 2010, 5, 470–472. [DOI] [PubMed] [Google Scholar]
- (35).Akiyama T; Itoh J; Yokota K; Fuchibe K Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chemie 2004, 116, 1592–1594. [DOI] [PubMed] [Google Scholar]
- (36).Mori K; Ichikawa Y; Kobayashi M; Shibata Y; Yamanaka M; Akiyama T Enantioselective Synthesis of Multisubstituted Biaryl Skeleton by Chiral Phosphoric Acid Catalyzed Desymmetrization/Kinetic Resolution Sequence. J. Am. Chem. Soc 2013, 135, 3964–3970. [DOI] [PubMed] [Google Scholar]
- (37).Merad J; Lalli C; Bernadat G; Maury J; Masson G Enantioselective Brønsted Acid Catalysis as a Tool for the Synthesis of Natural Products and Pharmaceuticals. Chem. - A Eur. J 2018, 24, 3925–3943. [DOI] [PubMed] [Google Scholar]
- (38).Parmar D; Sugiono E; Raja S; Rueping M Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev 2014, 114, 9047–9153. [DOI] [PubMed] [Google Scholar]
- (39).Bott G; Field LD; Sternhell S Steric Effects. A Study of a Rationally Designed System. J. Am. Chem. Soc 1980, 102, 5618–5626. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


