Abstract
PURPOSE
We aimed to assess the diagnostic performance of transluminal attenuation difference (TAD) in predicting the severity of internal carotid artery (ICA) stenosis.
METHODS
The study cohort consisted of 48 patients with <50% stenosis, 50%–69% stenosis, 70%–99% stenosis, and 51 controls without plaque development in ICA. A total of 143 measurements were performed through right and left internal and common carotid arteries. The TAD ratio was calculated as the difference between the mean attenuation values of the common carotid artery (CCA) and ICA, divided by the MAV of the CCA, multiplied by 100.
RESULTS
TAD ratio was significantly higher in severe (>70%) stenosis compared with control arteries and low-moderate stenosis. A TAD ratio cutoff of 4.5 predicted 70%–99% stenosis with a sensitivity of 100% and specificity of 93%. The inter- and intraobserver agreements in TAD measurements were almost perfect (ICC, 0.89–0.86).
CONCLUSION
Assessment of TAD ratio predicts the degree of stenosis in concordance with NASCET system.
Stroke is the fifth leading cause of death and the leading cause of long-term disability (1, 2). Each year, approximately 800 000 people experience an ischemic attack (2). Atherosclerosis in the extracranial carotid system is a significant cause of stroke (3). It is important to determine the accurate degree of stenosis in selecting the most appropriate therapy (4, 5). Several methods can be used to measure the degree of stenosis in cross-sectional imaging. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) method determines the stenosis degree by comparing the residual stenotic internal carotid artery (ICA) luminal diameter to the distal post-stenotic ICA diameter, which has a normal luminal diameter. The European Carotid Surgery Trial (ECST) compares the stenotic luminal diameter to the estimated normal diameter of the carotid bulb. The common carotid method measures the residual luminal diameter of the most stenotic part of the ICA and it estimates the degree of stenosis by comparing with the luminal diameter of the proximal common carotid (CCA) artery (6). A strong correlation between the NASCET and ECST methods has been reported with perfect inter- and intraobserver agreements (3).
Conventional digital subtraction angiography (DSA) is considered the gold standard method for determining the degree of stenosis (6). However, at present, less invasive imaging techniques, such as Doppler ultrasonography, computed tomography angiography (CTA), and magnetic resonance angiography (MRA) are used in the evaluation of stenosis degree (5). In addition to its invasiveness, another disadvantage of DSA is a risk of embolization with subsequent stroke during the procedure (6). In recent years, CTA has emerged as a primary modality in the evaluation of the degree of stenosis. Besides the possibility of imaging intracranial arteries at the same time, CTA is faster compared with other techniques and it is operator independent (3, 6). However, it has been assumed that CTA may over or underestimate the degree of stenosis, especially in heavily calcified plaques (6, 7). In an in vitro model study, Smith et al. (7) showed that CTA underestimates the stenosis degree in severe stenosis. These false CTA results have led us to investigate another method to estimate the degree of carotid stenosis. Based on the principle of transluminal attenuation gradient in coronary arteries on coronary CTA (8), we hypothesized that the transluminal attenuation difference (TAD) on CTA might be used in the evaluation of degree of stenosis in the ICA as a complementary method of confirming stenosis on CTA. The aim of this study is to assess the diagnostic performance of TAD in predicting the severity of ICA stenosis by performing the NASCET quantification method as a reference test. To the best of our knowledge, the application of TAD method to quantify stenosed ICA on CTA is presented for the first time.
Methods
Patient population
This retrospective study was approved by the Clinical Research Ethical Committee of our institution (no:18-1188-18). Informed consent has been obtained from all patients. We reviewed medical records of patients with a history of stroke to determine the study population. CTA images of 176 patients were evaluated for the presence of carotid artery stenosis between November 2015 and April 2018. The patients who had i) a significant stenosis in the major intracranial arteries, ii) a history of dissection and stent replacement, iii) image artifacts due to dental apparatus and motion and iv) totally occluded ICA were excluded from the study. Patients with advanced tortuous ICA were also excluded from the study based on the unfavorable effects on cerebral blood flow. Dolichoarteriopathies including tortuous ICA can be associated with reduced blood supply to the brain (9, 10). The control group consisted of age-matched patients without any calcific or fibrofatty plaque on CTA. Finally, 99 subjects (48 patients, 51 controls) were enrolled in the study, and 143 measurements in total were performed through right and left internal and common carotid arteries. The patients were classified into three groups according to the presence of low (<50%), moderate (50%–69%), and high grade (70%–99%) stenosis (defined later in the method of measurement subsection).
Examinations were obtained by a 64-slice CT scanner (Toshiba Aquilion 64). During the examinations, the patients were told to hold their breath and not swallow. A 70 mL non-ionic contrast agent (350/100 Omnipaque, GE healthcare) and 40 mL of saline were injected at a rate of 4 mL/s through an antecubital vein using an 18–20 G cannula. The area between the aortic arch and vertex was scanned in the supine position. CTA images were obtained with an automatic scan start triggered by contrast enhancement at 90 HU threshold in the arcus aorta. CT images were acquired with the following parameters: 64×0.5 detector collimation, 120 kVp tube voltage, 0.5 s gantry rotation time, 0.5 mm section thickness, and 0.3 reconstruction interval. The patients were scanned with a pitch factor of 0.828 that represents a helical pitch of 53 with 64×0.5 mm collimation. Axial source data images and multiplanar reformatted images were analyzed on a workstation (Vitrea 4.1.14 Vital Images).
Grading of stenosis
Two observers (A.G.C. and E.P.) with more than 10 years of experience in head and neck imaging evaluated the images to determine the degree of stenosis based on NASCET in consensus. The observers measured the narrowest lumen of the ICA by manually placing the calipers from the inner surface to the opposite inner wall and proportioned it to the distal normal lumen diameter (inner to inner lumen) without stenosis. All calculations were performed on the plane perpendicular to the lumen centerline, and magnification tools were used in the measurements (×3.5). The patient group was classified into three categories: <50% stenosis, 50%–69% stenosis, and 70%–99% stenosis.
Method of measurement
Luminal mean attenuation value measurements (MAVs) were performed with an interval of at least 6 weeks following the classification of the degree of stenosis to avoid bias. The luminal MAV through a 3 cm long carotid artery segment was calculated by measuring the density (HU) 1 cm below and 2 cm above the right and left carotid bifurcation, respectively, by two observers (A.G.C. and E.P.) in all patients at different times, independently. Observer 1 (principal observer, A.G.C.) repeated the analyses for the second time 60 days after her initial measurement to avoid bias. The reference points of measurements, through the 3 cm long carotid artery segment, were defined based on either coronal or sagittal reformatted images by the consultant radiologist. In addition, the luminal attenuation values of the CCA and ICA were measured by two observers (different from the consultant one) on the axial images at the points determined on reformatted images (Fig. 1). A circular region of interest (ROI) was placed within the center of the lumen and care was taken to avoid the area next to calcific plaque. Both radiologists used the same size of ROI for the CCA and ICA separately to prevent variability. In patients with plaque, the plaque length and morphology were also recorded. The patient population was also grouped into two (short and long segment stenosis within the low, moderate and high stenotic groups) in accordance with the length of stenosis at the ICA. A plaque length of >5 mm was defined as a long-segment stenosis. The TAD ratio was calculated as follows:
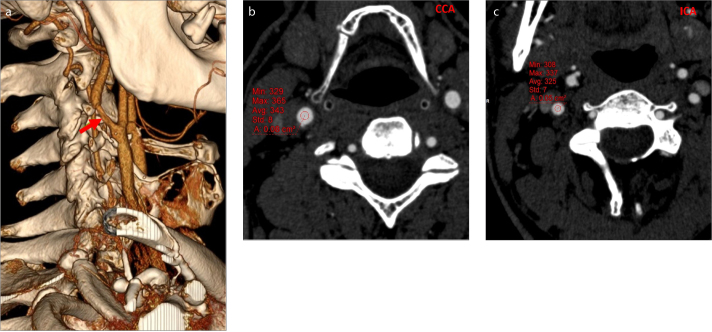
Figure 1. a–c.
CT angiogram of right common and internal carotid arteries of a 45-year-old female patient without any plaque (control group). Measurement of mean attenuation values of right common artery (a) and internal carotid artery (b) were performed on axial images and reference points were determined from the sagittal images (c). TAD ratio, 341-339/341×100=0.6.
Statistical analysis
The intraclass correlation coefficient (ICC) was used for evaluating the agreement between both observers, and the two-way analysis of variance (ANOVA) was used to evaluate the influence of two different categorical independent variables on one continuous dependent variable. The main effect of each independent variable, as well as any interaction between them was tested by two-way ANOVA. Receiver operating characteristic (ROC) curves were used to describe the performance of the diagnostic value of a method. An area of 0.50 implies that the variable adds no information. The areas under the ROC curves (AUC) and 95% confidence intervals (CI) for all variables were calculated in the manner described by Hanley and McNeil (11). The difference between two groups for continuous variables was evaluated by Student’s t test or Mann Whitney U test, where applicable. Cutoff values were determined according to the Youden index. P values of less than 0.05 were considered statistically significant.
Results
Out of 143 measurements, 76 were obtained from the right carotid system. The patient group and control group consisted of 62 and 81 measurements, respectively. The median TAD ratio of <50% stenosis, 50%–69% stenosis, and 70%–99% stenosis were 2.5 (min–max; 0.5–5.5), 3.9 (min–max; 1.7–7), and 9.9 (4.6–22.3), respectively. The median TAD ratio was 1.4 (−6.3 to 9.8) for the control group, and there was a statistically significant difference between the control and patient groups (P < 0.001, P = 0.001, and P < 0.001, respectively). The TAD ratio in patients with 70%–99% stenosis (Fig. 2) was significantly higher compared with patients with 50%–69% stenosis (Fig. 3), <50% stenosis, and the control group (P < 0.001). The patients with 50%–69% stenosis had higher TAD ratios compared with patients with <50% stenosis, but the values did not reach a statistical difference (P = 0.532). Table 1 shows the TAD ratios for both readers.
Figure 2. a–d.
A 70-year-old male patient with hemiplegia. Severe stenosis (70%–99%) of the left internal carotid artery (arrow) shown by volume rendering (a) and sagittal image (b). The measurements of mean attenuation values reveal a 34 HU difference between common carotid artery (c) and internal carotid artery (d). The TAD ratio was calculated as 10.7 (315-281/315×100 = 10.7).
Figure 3. a–c.
Volume rendering (a) and axial images of a 65-year-old male patient with a history of hemiparesis. A stenosis of 50%–69% was detected on right ICA (arrow). Measurements of mean attenuation values of right common (b) and internal carotid artery (c) were performed on axial images. TAD ratio was calculated as 5.2 (343-325/343×100 = 5.2).
Table 1.
The median TAD ratios of study population
| Vessels (n) | TAD ratio Median (min–max) (Obs 1+Obs 2) | Obs 1 TAD ratio Median (min–max) (1st measurement) | Obs 1 TAD ratio Median (min–max) (2nd measurement) | Obs 2 TAD ratio Median (min–max) | |
|---|---|---|---|---|---|
| Control | 81 | 1.4a (−6.3–9.8) | 1.2 (−5.2–9.5) | 1.6 (−9.2–10.5) | 1.5 (−7.9–9.7) |
| <50% stenosis | 33 | 2.5 (0.5–5.5) | 2.8 (0.5–7.3) | 2.8 (0–7.6) | 2.3 (−2.4–5.4) |
| 50%–69% stenosis | 9 | 3.9 (1.7–7) | 4.2 (2–7.8) | 3.8 (1.5–6.7) | 3.8 (1.7–6.5) |
| 70%–99% stenosis | 20 | 9.9b (4.6–22.3) | 9.9 (4.2–22.1) | 9.8 (4.9–32.7) | 9.5 (4.6–25.7) |
TAD, transluminal attenuation difference; Obs, observer.
Significantly different from <50% stenosis, 50%–69%, and 70%–99% stenosis (P < 0.001, P = 0.001, P < 0.001, respectively)
Significantly different from 50%–69% and <50% stenosis (P < 0.001 for both).
There was an interaction between the plaque length and stenosis degree (P < 0.001). Moreover, there was a statistically significant difference in TAD ratios between short- and long-segment stenosis in patients with 70%–99% stenosis compared with those with 50%–69% stenosis, <50% stenosis, and the control group (P < 0.001). The TAD ratios for the patients with 70%–99% stenosis were 6.7 (min–max, 4.6–9.6) and 16.1 (min–max, 9.1–22.3) for short- and long-segment stenosis, respectively. Table 2 presents median TAD ratios of study population, obtained from the average of the two readers, in accordance with the length of the plaque.
Table 2.
The TAD ratios in accordance with the length of the stenosis
| Median* | Minimum | Maximum | n | ||
|---|---|---|---|---|---|
| <5 mm | <50% stenosis | 2.4 | 0.5 | 5.5 | 16 |
| >5 mm | <50% stenosis | 3.0 | 0.6 | 5.5 | 17 |
| <5 mm | 50%–69% stenosis | 3.9 | 1.7 | 6.1 | 7 |
| >5 mm | 50%–69% stenosis | 5.3 | 3.6 | 6.9 | 2 |
| <5 mm | 70%–99% stenosis | 6.7 | 4.6 | 9.6 | 9 |
| >5 mm | 70%–99% stenosis | 16.1 | 9.1 | 22.3 | 11 |
Obtained from the average of two readers.
A TAD ratio of 3.6 was identified as a cutoff value for patients with ≥50% stenosis via ROC analysis (P < 0.001; AUC±SE, 0.95±0.02; 95% CI, 91%–99%). This cutoff value had a sensitivity of 97% (83%–99%), specificity of 88% (80%–92%), positive predictive value (PPV) of 67% (58%–74%), and negative predictive value (NPV) of 99% (95%–100%) in discriminating ≥50% stenosis. The cutoff value of the TAD ratio to predict 70%–99% stenosis was 4.5. With the 4.5 cutoff value (P < 0.001, AUC±SE: 0.98±0.01, 95% CI: 97%–100%), the sensitivity, specificity, PPV, and NPV were 100% (95% CI, 84%–100%), 93% (95% CI, 87%–96%), 69% (95% CI, 61%–76%), and 100% (95% CI, 97%–100%), respectively.
The intra- and interobserver agreement in TAD measurements were almost perfect (ICC, 0.89 [95% CI, 0.84–0.92]; ICC, 0.86 [95% CI, 0.81–0.90]).
Discussion
TAD is based on looking at contrast kinetics on CTA (12–14). It has been shown that, even in normal coronary arteries, there is a small drop off in the enhancement from the proximal part of the coronary artery to the distal part throughout its length which was referred as transluminal attenuation gradient (TAG) (14). With severe stenosis, this drop off becomes larger. It has been assumed that the difference in the contrast kinetics may convey information about the flow through the coronary artery (12, 13). A significantly lower TAG value was observed in vessels with significant stenosis in the coronary system (15, 16). Likewise, Choi et al. (17) showed that the decrease in luminal attenuation value was significant, ranging from mild to severe occlusion. They also indicated that the diagnostic accuracy was improved by adding TAG to the evaluation of coronary CTA. The coronary artery stenosis classification was changed by adding TAG to the evaluation of the coronary CTA in a significant number of vessels with calcified plaque (net reclassification improvement, 0.095) (17).
Based on these studies, we suggest that the TAD ratio can give information about the stenosis degree, and contrast kinetics would act similarly in the carotid system as in coronary arteries; to our knowledge, this had not been evaluated thus far. In the present study, a significant decrease in luminal attenuation was observed in the 70%–99% stenotic group compared with the 50%–69%, <50%, and control groups. Likewise, TAD ratio increased progressively according to the severity of stenosis in ICA and there was a significant difference in terms of the TAD ratio between the control and patient groups (70%–99% stenosis, 50%–69% stenosis, <50% stenosis). In comparison with the 50%–69% and <50% stenosis groups, the TAD ratio was lower in the <50% stenotic group, but this difference did not reach statistical significance. This might be due to the insignificant difference in flow dynamics between those two stenotic groups. Additionally, although a statistically significant difference was observed between the control group and <50% stenosis, the TAD ratios of these two groups were close to each other. In this circumstance, it may be hard to discriminate the control group and the mild stenotic group by using the TAD ratio.
The embolic and hemodynamic mechanisms are the main factors responsible for stroke in the setting of ICA atherosclerosis. Reduced cerebral blood flow promotes the risk of stroke, and it has been shown that plaque length is another indicator of cerebral flow in addition to stenosis (18, 19). This may be delineated by Poiseuille law. Poiseuille law expresses that the resistance in a tube to a fluid is determined by the formula 8 η L/π(d/2)4. In this formula, L refers to the length of a tube and d is the diameter. According to this formula, longer carotid plaques would induce an increase in resistance and a decrease in cerebral blood flow (19). In MRA study, Douglas et al. (19) showed that patients with longer plaque lengths had a significant decrease in brain blood flow compared with shorter plaque lengths in patients with >70% stenosis. Concordant with these findings, the present study obtained a significant increase in the TAD ratio in >70% stenotic patients with longer plaque lengths compared with >70% stenotic patients with shorter plaque lengths. Also, among 20 patients with severe stenosis, only two patients did not show significant neurologic symptoms and those patients had smaller TAD ratios compared with symptomatic ones. Considering these points, we assume that the TAD ratio may be used as an indirect indicator of cerebral blood flow. With a larger prospective study cohort, these results could be strengthened by using the perfusion studies. The most important factor that determines the intraluminal attenuation is the cardiac output. In this study, the attenuation value difference between ICA and CCA was divided into CCA and this ratio, which we referred to as TAD ratio, was used to eliminate the variability due to cardiac output based on different patients.
The present study has some limitations. This study is a retrospective single-center study with a relatively small sample size. Further studies in larger groups are needed to validate the role of TAD ratio in carotid stenosis. Another limitation of this study is that this method may not be applicable to every patient. The patients with occluded ICA and major intracranial vessels, patients who had a history of stent replacement and patients with advanced tortuous ICA were excluded from the study. These situations have variable effects on cerebral blood flow; they also affect hemodynamics and may cause turbulence inside the lumen. The blood flow is the main factor that affects luminal attenuation value in vessels on CT. This is why these factors were considered as exclusion criteria. Unfortunately, due to retrospective design of the study we could not perform perfusion studies to strengthen our hypothesis and this would be valuable especially in patients who were excluded from the study because of the hemodynamic alterations. An additional limitation is close TAD ratios of the control and mild stenotic group. Discrimination of these two groups by using TAD ratio may not be accurate. We think that, the discrimination of stenotic and non-stenotic groups would be more valuable in practice. We did not correlate our results with Doppler and DSA findings. DSA is still the gold standard method for identifying stenosis; however, in an in vitro model study, Smith et al. (7) claimed that DSA tends to overestimate the stenosis area (7). The combination of TAD findings on CTA, DSA, and Doppler findings might allow for the accurate grading of carotid artery stenosis.
In conclusion, assessment of the TAD ratio with 64-detector CT provides an impression of stenosis degree in concordance with the NASCET system. The TAD ratio may provide a correct estimation of stenosis, especially in calcified vessels, without the need for invasive carotid angiography. Patients with severe stenosis and longer plaque lengths show a greater increase in the TAD ratio. Further studies using a large cohort of patients are warranted to determine the feasibility of using the TAD ratio in carotid artery stenosis grading.
Main points.
The transluminal attenuation difference (TAD) between common and internal carotid arteries might help to predict the degree of internal carotid artery stenosis (low, moderate and high).
TAD ratio significantly increases in severe stenosis.
TAD ratio significantly differs between short and long plaques in severe stenosis of the internal carotid artery.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ovbiagele B, Nguyen-Huynh NM. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–329. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saba L, Mallarini G. A comparison between NASCET and ECST methods in the study of carotids: evaluation using multi-detector-row CT angiography. Eur J Radiol. 2010;76:42–47. doi: 10.1161/CIR.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 4.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1016/j.ejrad.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 5.Waaijer A, Weber M, van Leeuwen MS, et al. Grading of carotid artery stenosis with multidetector-row CT angiography: visual estimation or caliper measurements? Eur Radiol. 2009;19:2809–2818. doi: 10.1056/NEJM199108153250701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adla T, Adlova R. Multimodality imaging of carotid stenosis. Int J Angiol. 2015;24:179–184. doi: 10.1007/s00330-009-1508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JC, Watkins GE, Smith DC, et al. Accuracy of digital subtraction angiography, computed tomography angiography, and magnetic resonance angiography in grading of carotid artery stenosis in comparison with actual measurement in an in vitro model. Ann Vasc Surg. 2012;26:338–343. doi: 10.1055/s-0035-1556056. [DOI] [PubMed] [Google Scholar]
- 8.Wong DT, Ko BS, Cameron JD, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol. 2013;61:1271–1279. doi: 10.1016/j.avsg.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Derrick JR, Smith T. Carotid kinking as a cause of cerebral insufficiency. Circulation. 1962;25:849–853. doi: 10.1016/j.jacc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Inahara T. Management of the tortuous internal carotid artery. Am J Surg. 1985;149:651–655. doi: 10.1161/01.CIR.25.5.849. [DOI] [PubMed] [Google Scholar]
- 11.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1983;143:29–36. doi: 10.1016/S0002-9610(85)80149-5. [DOI] [PubMed] [Google Scholar]
- 12.Steigner ML, Mitsouras D, Whitmore AG, et al. Iodinated contrast opacification gradients in normal coronary arteries imaged with prospectively ECG-gated single heart beat 320-detector row computed tomography. Circ Cardiovasc Imaging. 2010;3:179–186. doi: 10.1148/radiology.143.1.7063747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lackner K, Bovenschulte H, Stützer H, et al. In vitro measurements of flow using multislice computed tomography (MSCT) Int J Cardiovasc Imaging. 2011;27:795–804. doi: 10.1161/CIRCIMAGING.109.854307. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YE, Choi JH, Kim JH, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging. 2012;5:1088–1096. doi: 10.1007/s10554-010-9728-7. [DOI] [PubMed] [Google Scholar]
- 15.Ko BS, Wong DT, Nørgaard BL, et al. Diagnostic performance of transluminal attenuation gradient and noninvasive fractional flow reserve derived from 320-detector row CT angiography to diagnose hemodynamically significant coronary stenosis: an NXT substudy. Radiology. 2016;279:75–83. doi: 10.1016/j.jcmg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Nagata K, Tanaka R, Takagi H, et al. Improved diagnostic performance of transluminal attenuation gradient in combination with morphological evaluation of coronary artery stenosis using 320-row computed tomography. Jpn J Radiol. 2018;36:51–58. doi: 10.1148/radiol.2015150383. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Min JK, Labounty TM, et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc Imaging. 2011;4:1149–1157. doi: 10.1007/s11604-017-0699-7. [DOI] [PubMed] [Google Scholar]
- 18.de Labriolle A, Mohty D, Pacouret G, et al. Comparison of degree of stenosis and plaque volume for the assessment of carotid atherosclerosis using 2-D ultrasound. Ultrasound Med Biol. 2009;35:1436–1442. doi: 10.1016/j.jcmg.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Douglas AF, Christopher S, Amankulor N, et al. Extracranial carotid plaque length and parent vessel diameter significantly affect baseline ipsilateral intracranial blood flow. Neurosurgery. 2011;69:767–773. doi: 10.1016/j.ultrasmedbio.2009.03.013. [DOI] [PubMed] [Google Scholar]