Abstract
PURPOSE
We aimed to investigate the clinical and magnetic resonance imaging (MRI) characteristics of uterine adenomyosis, which presents as an extensive area of high signal intensity in the myometrium on T2-weighted MRI.
METHODS
This retrospective radiographic study reviewed a case series of six patients (mean age, 36 years) with adenomyosis. These patients were selected because, unlike classical adenomyosis, T2-weighted images showed a larger area of high signal intensity than that of low signal intensity in the myometrium. The morphology of the myometrial lesions, patterns of contrast enhancement (n=4), intramyometrial hemorrhaging, diffusion restriction (n=5), endometrial lesions, and imaging findings after treatment (n=3) were evaluated on MRI.
RESULTS
The patients’ clinical symptoms included vaginal bleeding and severe anemia. Four were administered hormonal therapy, one underwent hysterectomy, and one underwent enucleation. On T2-weighted images, all showed endometrial thickening and a high signal intensity area in the myometrium that was divided up by a mesh of low signal intensity bands, with an appearance reminiscent of a fish caught in a net. Other findings included gradual centripetal enhancement with contrast defects in multicystic areas (4/4), an intramyometrial hemorrhage (1/6), and increased diffusion (5/5). Following hormonal therapy, the uteruses decreased in size and were similar to those of classical adenomyosis on MRI (3/3). The lesions were diagnosed as adenomyosis with a proliferation of adenomyotic glandular tissue and a proliferative endometrial polyp.
CONCLUSION
This case series suggests that there is a subgroup of uterine adenomyosis that shows a characteristic “fish-in-a-net” appearance on T2-weighted images.
Uterine adenomyosis is a pathological condition characterized by the presence of ectopic endometrial glands and stroma within the myometrium, with adjacent myometrial hyperplasia and hypertrophy. It is thought that adenomyosis results from the invagination of the basal endometrium into the inner layer of the myometrium; however, its exact pathogenesis is unknown (1, 2). Adenomyosis has been considered to be a hormone-dependent disease and is associated with other gynecological disorders such as uterine leiomyomas, endometriosis, and endometrial polyps (1, 3, 4).
Adenomyosis can be diagnosed on magnetic resonance imaging (MRI) with high accuracy (5, 6). On T2-weighted images, adenomyosis typically presents as an ill-defined area of low signal intensity with uterine enlargement due to myometrial hypertrophy, with small foci of high signal intensity because of the ectopic endometrium (7, 8). Usually, on T2-weighted images, the area of low signal intensity covers most of the lesion; however, areas of high signal intensity can increase in number or expand in size because of an increase in the glandular tissue, decidualization, hemorrhage, edema, or a malignant transformation (7–11). Diffuse adenomyosis with dilated endometrial glands within the myometrium can appear as a “Swiss cheese” appearance on T2-weighted images (12, 13).
Funaki et al. (14) reported a case of multicystic adenomyosis with simple endometrial hyperplasia for which T2-weighted images showed an extensive area of multicystic high signal intensity in the myometrial lesion, with low signal intensity bands intervening in it. Preoperative MRI diagnosis was endometrial stromal sarcoma based on the suggestion of myometrial invasion from the endometrial lesion. Postoperative histological examination showed dense endometrial glands with cystic dilatation and edematous stroma in a myometrial and endometrial lesion (14). To the best of our knowledge, no other cases of adenomyosis in which proliferated glandular tissue covered most of the lesion have been reported. Here, we present the first case series of adenomyosis showing similar imaging findings with detailed clinical information.
The purpose of this study was to investigate the clinical and MRI characteristics of uterine adenomyosis with a myometrial lesion presenting as an extensive area of high signal intensity on T2-weighted images, which has been sporadically mentioned as “Swiss-cheese appearance”.
Methods
Patients
The institutional review boards of the University of Tokyo Hospital and Teikyo University Hospital approved this study and, because of the retrospective nature of the study design, they waived the requirement for informed patient consent. Both of the hospitals have radiologic database systems in which rare diseases and those with unusual imaging findings are classified by site at the time of imaging interpretation and/or after diagnosis. We searched these databases for cases of uterine adenomyosis where T2-weighted images showed an extensive area of high signal intensity in the myometrial lesion either in the University of Tokyo Hospital between January 2002 and April 2018 or in Teikyo University Hospital between April 2013 and March 2017. From these, we selected the cases where the T2-weighted high signal intensity area in the myometrial lesion was greater than the low signal intensity area, contrary to the case in classical adenomyosis. We excluded patients where the high signal intensity area was due to massive bleeding, including cystic adenomyosis. The presence or absence of bleeding was evaluated on T1-weighted images. Finally, five cases from the University of Tokyo Hospital and one from Teikyo University Hospital were selected (mean age, 36 years; range, 24–50 years).
Adenomyosis was diagnosed by histological findings and/or the reduction of lesions by hormonal therapy. One patient underwent hysterectomy, one underwent enucleation of the myometrial lesion after endometrial polypectomy and uterine artery embolization, and four received hormonal therapy after polypectomy and total endometrial curettage. The reduction of the lesion after hormonal therapy was confirmed by MRI and/or transvaginal sonography. MRI was performed 7–10 months after hormonal therapy and transvaginal sonography was performed at intervals of one to several months. Clinical findings and serological data (serum hemoglobin, carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 125 levels) were collected at the patient’s first visit. In addition, minimum serum hemoglobin levels during the course were recorded.
Imaging techniques
The MRI studies for the uterine lesions used 0.4 T, 1.5 T, or 3.0 T scanners. The following scans were acquired for all the patients: axial T2-weighted (TR range, 3000–6762 ms; TE range, 82–105 ms), sagittal T2-weighted (TR range, 3380–5469 ms; TE range, 88–120 ms), and axial two-dimensional T1-weighted images (TR range, 250–622 ms; TE range, 2.3–22 ms) and/or axial three-dimensional T1-weighted images (TR, 4.4 or 7.3 ms; TE, 2.3 or 4.8 ms). In addition, axial diffusion-weighted images were acquired for five patients (with b values of 800, 1000, or 750 and 1500 s/mm2); and axial or sagittal dynamic contrast-enhanced MRI was performed for four patients. Because of the differing examination protocols used at other institutions, the MRI parameters varied.
Imaging and histological analyses
The images were interpreted by two radiologists with 6 and 15 years of experience in gynecologic imaging, respectively. The MRI findings were evaluated for the following features: morphology of the high signal intensity area in myometrial lesion on T2-weighted images; the patterns of dynamic contrast enhancement; hemorrhaging on T1-weighted images; diffusion restriction; and the endometrial lesion, assessing its continuity with the myometrial lesion and the extent of development (from corpus uteri to vagina). In addition, the imaging findings of adenomyosis following hormonal therapy were investigated. Board-certified pathologists reevaluated all the histological specimens.
Results
The clinical characteristics and the MRI findings of the six cases reviewed in this study are summarized in Tables 1 and 2, respectively. None of the patients received hormonal therapy at first visit. On the T2-weighted images, all patients showed endometrial thickening with high signal intensity and an extensive high signal intensity area spreading continuously from the endometrial lesion to the myometrium (Figs. 1–4). In the myometrium, the area of high signal intensity was divided by a mesh of low signal intensity bands similar to those in muscles. This characteristic mesh pattern with endometrial thickening at the center was reminiscent of a fish caught in a fishing net (Fig. 2) and so is referred from here on as a “fish-in-a-net” appearance. In the five patients for whom diffusion-weighted imaging was performed, the apparent diffusion coefficient value was increased in the myometrial lesions (Fig. 1). In the four patients for whom dynamic contrast enhancement scans were acquired, gradual centripetal enhancement and contrast defects of the multicystic areas on the delayed phase were observed (Fig. 1). In the four cases with hormonal therapy, the uterus decreased in size on MRI or transvaginal sonography after treatment. In the three cases with follow-up MRI, the abnormal signal in the myometrium improved and the imaging findings after treatment were similar to those of classical adenomyosis (Fig. 3).
Table 1.
Clinical characteristics of the patients
| Case | Age, years | Gravidity | Parity | Clinical symptoms | Medication history | Treatment | Serum Hb, g/dL | Minimum serum Hb during the course, g/dL | Serum CEA, ng/mL | Serum CA19-9, U/mL | Serum CA125, U/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 0 | 0 | Irregular vaginal bleeding and menorrhagia | - | Polypectomy, total endometrial curettage and hormonal therapy | 9.3 | 3.4 | 0.3 | 49 | 119 |
| 2 | 40 | 0 | 0 | Irregular vaginal bleeding and menorrhagia | Olmesartan medoxomil and azelnidipine | Polypectomy, total endometrial curettage and hormonal therapy | 10.6 | 6.0 | 1.1 | 30 | 112 |
| 3 | 50 | NA | NA | Irregular vaginal bleeding and rapidly enlarging abdominal mass | - | Hysterectomy | 9.1 | 9.0 | 1.0 | 110 | 195 |
| 4 | 36 | 0 | 0 | Irregular vaginal bleeding | - | Polypectomy, UAE and enucleation | 10.8 | 6.4 | 1.0 | 65 | 272 |
| 5 | 33 | 0 | 0 | Irregular vaginal bleeding | - | Polypectomy, total endometrial curettage and hormonal therapy | 13 | 7.6 | 1.8 | 65 | 154.6 |
| 6 | 35 | 0 | 0 | Irregular vaginal bleeding | - | Polypectomy, total endometrial curettage and hormonal therapy | 13.1 | 5.7 | 0.8 | 17 | 51 |
Hb, hemoglobin; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; NA, not available; UAE, uterine artery embolization.
Table 2.
MRI findings for uterine adenomyosis
| Case | Morphology on T2-weighted images | Hemorrhage in the myometrial lesion | Contrast enhancement pattern | Diffusion | Progress range of the endometrial polyp | Imaging findings after hormonal therapy |
|---|---|---|---|---|---|---|
| 1 | Fish-in-a-net appearancea | - | N/A | Increasedb | Vagina | Decreased in size and looks like usual adenomyosis |
| 2 | Fish-in-a-net appearancea | - | Gradual centripetal enhancement and delayed contrast defects in multicystic areas | Increasedb | Vagina | Decreased in size and looks like usual adenomyosis |
| 3 | Fish-in-a-net appearancea | A small amount | Gradual centripetal enhancement and delayed contrast defects in multicystic areas | Increasedb | Corpus uteri | N/A |
| 4 | Fish-in-a-net appearancea | - | N/A | N/A | Cervix | N/A |
| 5 | Fish-in-a-net appearancea | - | Gradual centripetal enhancement and delayed contrast defects in multicystic areas | Increasedb | Cervixc | Decreased in size and looks like usual adenomyosis |
| 6 | Fish-in-a-net appearancea | - | Gradual centripetal enhancement and delayed contrast defects in multicystic areasd | Increasedb | Vagina | N/A |
MRI, magnetic resonance imaging; N/A, not applicable.
Fish-in-a-net appearance on T2-weighted images refers to endometrial thickening and a high signal intensity area in the myometrium divided into partitions by mesh-like low signal intensity bands similar to those in muscle.
Increased diffusion means high signal intensity on diffusion-weighted images (b = 800, 1000, or 1500) and high apparent diffusion coefficient values.
MRI was acquired after two resections of the endometrial polyp drooping in the vagina.
Dynamic contrast-enhanced imaging was acquired one year after the initial MRI, with no treatment other than polypectomy and endometrial curettage.
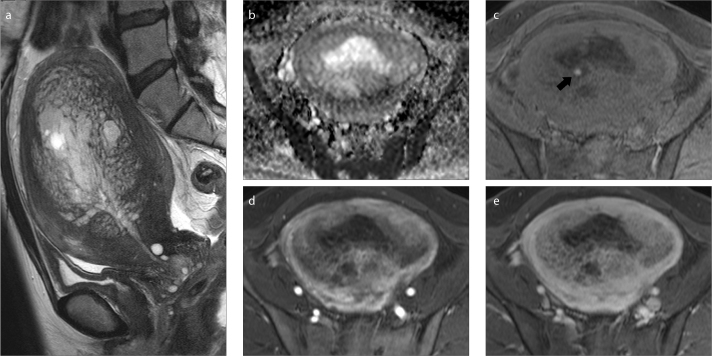
Figure 1. a–e.
A 50-year-old woman with uterine adenomyosis and an endometrial polyp (Case 3). Sagittal T2-weighted image (a) shows endometrial thickening and mesh-like hyperintensity in the myometrium giving the fish-in-a-net appearance. Apparent diffusion coefficient map (b) shows no diffusion restriction in the lesion. Much bleeding occurred in the endometrial lesions (not shown); but, in the myometrial lesion, only a slight amount was observed in the vicinity of the endometrium on fat suppressed T1-weighted images (c, arrow). With dynamic contrast enhancement, early (d) and delayed phase (e) images show gradual centripetal enhancement and delayed contrast defects in multicystic areas.
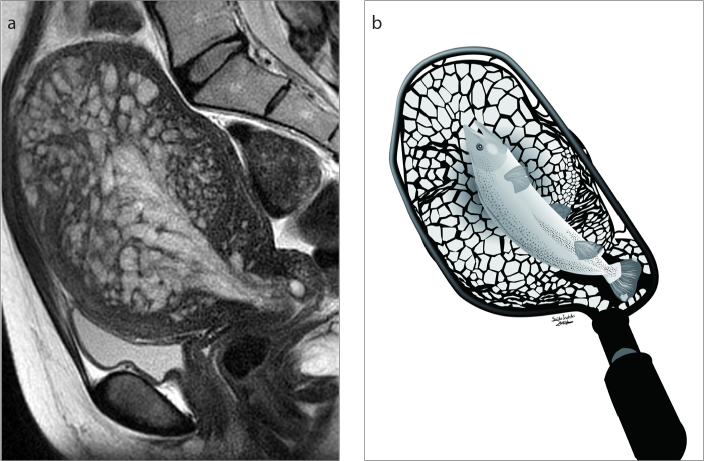
Figure 2. a, b.
A 33-year-old woman with uterine adenomyosis and an endometrial polyp (Case 5). Sagittal T2-weighted image (a) shows endometrial thickening and mesh-like hyperintensity in the myometrium giving the fish-in-a-net appearance. Illustration (b) shows fish-in-a-net appearance, where the fish refers to the thickened endometrium and the net refers to the mesh-like high signal intensity area in the myometrial lesion. The illustration is our own work, drawn by the author (S.I.).
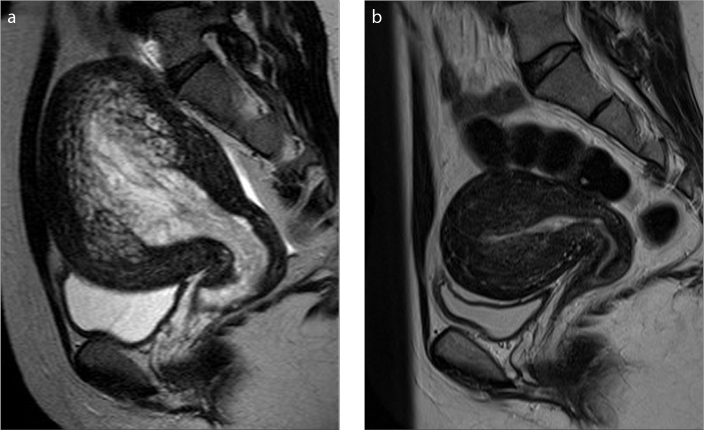
Figure 3. a, b.
A 24-year-old woman with uterine adenomyosis and an endometrial polyp (Case 1). Sagittal T2-weighted image (a) shows endometrial thickening and mesh-like hyperintensity in the myometrium giving the fish-in-a-net appearance. Sagittal T2-weighted image (b) acquired 7 months after polypectomy and total endometrial curettage and 5 months after hormonal therapy, shows that the uterus has decreased in size and the abnormal signal in the myometrium has improved, with an appearance similar to that of classical adenomyosis.
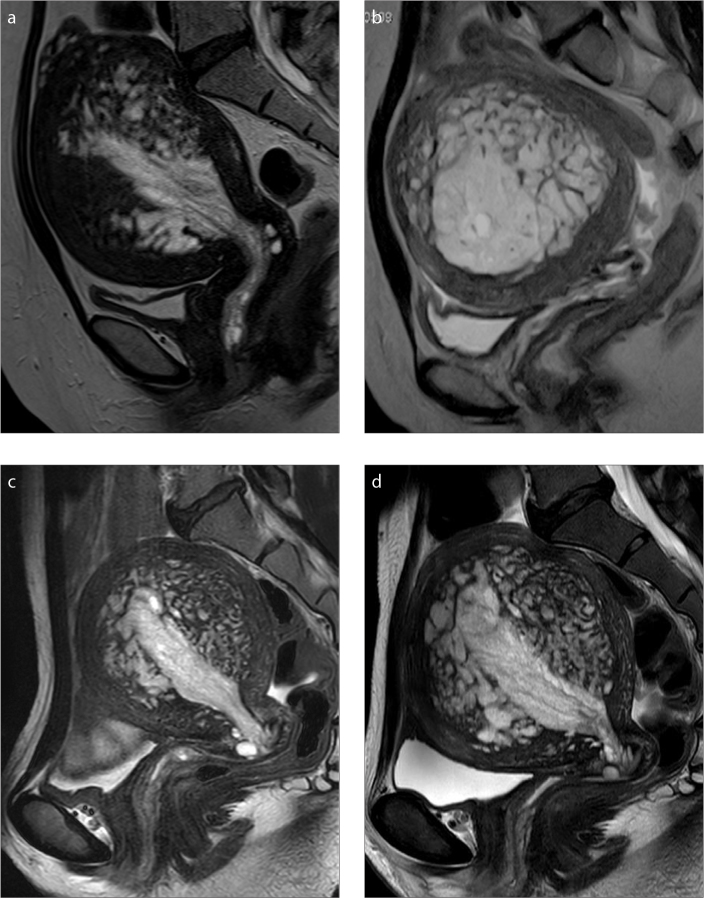
Figure 4. a–d.
Sagittal T2-weighted images (a–c) for Cases 2, 4, and 6, respectively, show endometrial thickening and mesh-like hyperintensity in the myometrium giving the fish-in-a-net appearance. In Case 6, T2-weighted image (d) after 1-year follow-up without treatment other than polypectomy and endometrial curettage showed expansion of the high signal intensity area in the myometrial lesion and recurrence of the endometrial polyp.
In case 3, an endometrial biopsy revealed no malignancy but endometrial stromal sarcoma was suspected radiologically and a hysterectomy was performed. In Case 4, a uterine tumor was found five years ago (MRI not shown) and it was reduced by hormonal therapy at the original institution. The patient was referred to our hospital because the uterine tumor had increased after cessation of hormonal therapy. Uterine artery embolization was performed before enucleation of the myometrial lesion because the patient’s anemia progressed rapidly after hospitalization. In 12 months of follow-up after surgery, no recurrence was observed in the absence of hormonal therapy.
In Cases 1, 2, 5, and 6, the endometrial polyps recurred after polypectomy. Subsequently, these patients received hormonal therapy (leuprorelin acetate, ethinyl estradiol/norethindrone, leuprorelin acetate, and goserelin acetate, respectively). In case 6, MRI examination at 1-year follow-up revealed expansion of myometrial lesion and recurrence of the endometrial polyp in the absence of hormonal therapy (Fig. 4). In Case 5, after stopping hormonal therapy, the endometrial polyp recurred, and the recurrent endometrial polyp was resected many times. Her condition was stable for 10 months after resuming hormonal therapy. The disease remained stable in Cases 1, 2, and 6 through continuation of the hormonal therapy for 42, 26, and 6 months, respectively.
Four patients underwent endometrial polypectomy and total endometrial curettage, one underwent hysterectomy, and one underwent endometrial polypectomy and enucleation of the myometrial lesion, resulting in a diagnosis of endometrial polyp with proliferative endometrial glands showing ductal dilatation and branching without atypia, with the exception of Case 1, for which the diagnosis was simple endometrial hyperplasia coexistent with the endometrial polyp. Cases 2–6 showed proliferation of glandular tissue in the endometrial polyps similar to simple endometrial hyperplasia. In all the cases, no features of malignancy were found either in the surface of the polyp or in glandular proliferation inside the polyp.
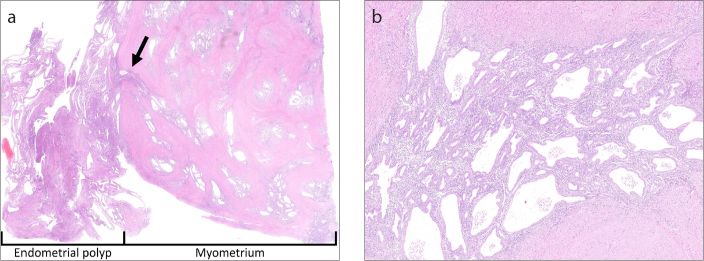
In Cases 3 and 4, in which the patients underwent hysterectomy or enucleation of the myometrial lesion, glandular tissue was observed to have branched into the myometrium from the endometrial polyp (Fig. 5). In these two cases, glandular tissue grew in a mesh pattern in the myometrium without atypia. In addition, branching and dilatation of the glandular ducts in the myometrium, indicating proliferation similar to that of their endometrial polyps, was observed. Hemorrhaging in the myometrium was rarely seen. The lesions were diagnosed as uterine adenomyosis coexisting with an endometrial polyp.
Figure 5. a, b.
Histological findings of uterine adenomyosis and endometrial polyp evaluated by hematoxylin-eosin staining in a 50-year-old woman (Case 3). Image (a) (original magnification, ×1) shows endometrial glandular tissue branching from the endometrial polyp into the myometrium (arrow). In addition, there are many islands of ectopic endometrial tissue and cystic dilation of glands in the myometrium. Image (b) (original magnification, ×40) shows conspicuous branching and dilation of the glandular ducts compared with classical adenomyosis; the ectopic endometrial tissue in the myometrium showed proliferation. No cellular atypia was observed.
Discussion
This report describes six cases of uterine adenomyosis with a characteristic fish-in-a-net appearance on T2-weighted images. A comparison of the radiology and pathology showed that the high signal intensity area on T2-weighted images and the contrast defects in multicystic areas in the myometrium were proliferative glandular tissue with cystic dilatation, the low signal intensity bands were smooth muscle, and the endometrial thickening was an endometrial polyp. In fish-in-a-net appearance, fish corresponds to an endometrial polyp and net corresponds to normal smooth muscle intervening in multicystic glandular tissue. Continuity of the glandular tissue in the myometrium and endometrial polyp was also demonstrated radiologically and pathologically. A few review articles reported that diffuse adenomyosis with dilated endometrial glands within the myometrium can appear as a “Swiss cheese” appearance on T2-weighted images without cited reference about MRI and pathological findings (12, 13). The present cases may be an extreme form of adenomyosis with “Swiss cheese” appearance. However, all the present cases had large endometrial polyps in addition to adenomyosis. To the best of our knowledge, only one case with similar imaging and pathological findings has been previously reported as an exceedingly rare disease (14). Our report showed the features of clinical, pathological, and MRI findings in this extremely rare form of adenomyosis, which may be called “proliferative uterine adenomyosis,” as a case series.
On T2-weighted images, classical adenomyosis can show high signal intensity linear striations radiating from the endometrium to the myometrium due to benign invasion of endometrial tissue into the myometrium (8). However, extensive myometrial invasion from the endometrial lesion usually indicates malignancy. Therefore, the radiological differential diagnosis of proliferative uterine adenomyosis includes low-grade endometrial stromal sarcoma, adenosarcoma, leiomyosarcoma, and endometrial cancer invading adenomyosis. On T2-weighted images, low-grade endometrial stromal sarcoma shows as intramyometrial worm-like nodular extension with high signal intensity, with the normal muscle layer between lesions showing as a low signal band, thus, presenting a similar appearance to the findings in the present cases (15–17). In low-grade endometrial stromal sarcoma, the high signal lesions on T2-weighted images are sarcomatous components and exhibit obvious contrast enhancement, diffusion restriction, and occasionally extension of the tumor along the vessels or ligaments, although cystic degeneration or necrosis can sometimes be observed (16–20). In contrast, the high signal lesion on T2-weighted images in proliferative uterine adenomyosis in the present study was glandular tissue with cystic dilatation, which showed gradual centripetal enhancement with contrast defects in multicystic areas, increased diffusion, and no extension along the vessels or ligaments. We speculate that proliferative uterine adenomyosis has a finer and more orderly patterned mesh-like lesion than that seen in previously reported cases of low-grade endometrial stromal sarcoma (15–20). On T2-weighted images, adenosarcoma exhibits a multiseptated cystic mass with low signal intensity solid areas (15, 21). However, it is endometrial-based, and we speculate that the solid part of adenosarcoma shows greater heterogeneity of signal intensity, and to the best of our knowledge, no findings of a mesh-like invasion of the myometrium have been reported for adenosarcoma. Leiomyosarcoma shows a heterogeneous mass that contains areas of necrosis and hemorrhage (15). Usually, endometrial cancer shows lower apparent diffusion coefficient values than adenomyosis does, and it rarely has a multicystic or mesh-like appearance (22). Therefore, characteristic imaging findings including a fish-in-a-net appearance in proliferative uterine adenomyosis may help preventing the radiological overdiagnosis in the clinical setting.
Kishi et al. (23) reported that adenomyosis consist of three distinct subtypes with different causes: direct endometrial invasion, endometriotic invasion from outside, and de novo metaplasia. The present cases of adenomyosis may have been caused by direct endometrial invasion because of pathological continuity of endometrial and myometrial lesions. However, it was unclear whether the proliferative glandular tissue in the endometrial polyp had invaded normal myometrium or already existing adenomyosis, or the glandular tissue within existing adenomyosis and an endometrial polyp had proliferated.
Adenomyosis and endometrial polyp have been considered to be hormone-dependent diseases (1, 4, 24). In addition, the selective estrogen receptor modulator tamoxifen causes adenomyosis and endometrial polyps; these polyps tend to be larger and, microscopically, adenomyosis and polyps show proliferative activity with cystic glandular dilatation (25, 26). The findings of the present study were consistent with these reports. Furthermore, the lesions decreased in all four patients who underwent hormonal therapy, suggesting that sex steroid hormone aberrations may be linked to glandular proliferation. However, the expression of the hormone receptor in the lesion was not examined, and the cause of the change in hormonal state is unclear.
For patients with symptoms, the endometrial polyps should be removed. If there is heavy bleeding, additional treatment should be considered, such as a levonorgestrel intrauterine device or a concomitant endometrial resection to reduce blood loss (27). The patients in the present study had severe anemia during the course and recurrence of the endometrial polyps after polypectomy, prior to the hormonal therapy. The condition became stable after hormonal therapy. The risk factors for malignant endometrial polyps include being postmenopausal, abnormal bleeding, and perhaps a larger size of polyps; however, cell atypia was not found in the present cases (28, 29). Furthermore, as described above, sarcomas can be differentially diagnosed from proliferative uterine adenomyosis. Therefore, we speculate that polypectomy should be performed before hormonal therapy to relieve symptoms and exclude malignancy, but that hysterectomy is not necessary if there are no malignant findings. However, because polypectomy alone cannot rule out the possibility of malignant myometrial lesions, it is speculated that reduction of myometrial lesion after hormonal therapy should be confirmed by transvaginal ultrasonography at short intervals. Other conditions, including malignancy, may have to be considered if the lesion does not shrink since our cases responded well to hormonal therapy. Even if the response to hormonal therapy is good, the termination of treatment may be difficult, at least before menopause, because the endometrial polyp recurred during the interruption of hormonal therapy in Case 5. MRI in addition to transvaginal ultrasonography may not be necessary for follow-up, but it is considered useful for evaluating the overview of adenomyosis, residual lesions, and recurrent lesions after hormonal therapy.
The present study had some limitations. First, this was a case series that included a limited number of non-consecutive patients. Second, there may be an intermediate type of lesion between the classical and proliferative adenomyosis; however, only patients with an extensive myometrial lesion were selected in this study. Third, differences between proliferative adenomyosis and other uterine lesions were not examined. Forth, the frequency of proliferative adenomyosis is unknown since the number of classical adenomyosis were not counted during the study period.
In conclusion, there is a rare form of uterine adenomyosis that presents with a characteristic fish-in-a-net appearance on T2-weighted images, with proliferation of adenomyotic glandular tissue and a proliferative endometrial polyp. Although the pathogenesis of this condition is unknown, understanding the imaging findings may help to avoid unnecessary treatment.
Main points.
A subgroup of proliferative uterine adenomyosis shows proliferation of adenomyotic glandular tissue and proliferative endometrial polyp.
The characteristic appearance on T2-weighted images is endometrial thickening and an extensive high signal intensity area in the myometrium divided by a mesh of low signal intensity bands, giving a “fish-in-a-net” appearance.
The characteristic imaging findings help to avoid radiological overdiagnosis.
The condition can be treated with polypectomy and hormonal therapy; however, continued treatment may be necessary.
Acknowledgement
The authors would like to thank Prof. Tomoyuki Fujii and Prof. Yutaka Osuga (Department of Obstetrics and Gynecology, Graduate School of Medicine, The University of Tokyo) for reviewing the present cases.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592–601. doi: 10.1016/j.rbmo.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–322. doi: 10.1093/humupd/4.4.312. [DOI] [PubMed] [Google Scholar]
- 3.McElin TW, Bird CC. Adenomyosis of the uterus. Obstet Gynecol Annu. 1974;3:425–441. [PubMed] [Google Scholar]
- 4.Rizner TL. The important roles of steroid sulfatase and sulfotransferases in gynecological diseases. Front Pharmacol. 2016;7:30. doi: 10.3389/fphar.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novellas S, Chassang M, Delotte J, et al. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR Am J Roentgenol. 2011;196:1206–1213. doi: 10.2214/AJR.10.4877. [DOI] [PubMed] [Google Scholar]
- 6.Togashi K, Ozasa H, Konishi I, et al. Enlarged uterus: differentiation between adenomyosis and leiomyoma with MR imaging. Radiology. 1989;171:531–534. doi: 10.1148/radiology.171.2.2704819. [DOI] [PubMed] [Google Scholar]
- 7.Tamai K, Togashi K, Ito T, Morisawa N, Fujiwara T, Koyama T. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics. 2005;25:21–40. doi: 10.1148/rg.251045060. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi M, Matsuzaki K. Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics. 2011;31:99–115. doi: 10.1148/rg.311105110. [DOI] [PubMed] [Google Scholar]
- 9.Shitano F, Kido A, Fujimoto K, et al. Decidualized adenomyosis during pregnancy and post delivery: three cases of magnetic resonance imaging findings. Abdom Imaging. 2013;38:851–857. doi: 10.1007/s00261-013-9988-5. [DOI] [PubMed] [Google Scholar]
- 10.Motohara K, Tashiro H, Ohtake H, Saito F, Ohba T, Katabuchi H. Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol. 2008;13:266–270. doi: 10.1007/s10147-007-0725-3. [DOI] [PubMed] [Google Scholar]
- 11.Troiano RN, Flynn SD, McCarthy S. Cystic adenomyosis of the uterus: MRI. J Magn Reson Imaging. 1998;8:1198–1202. doi: 10.1002/jmri.1880080603. [DOI] [PubMed] [Google Scholar]
- 12.Wolfman DJ, Ascher SM. Magnetic resonance imaging of benign uterine pathology. Top Magn Reson Imaging. 2006;17:399–407. doi: 10.1097/RMR.0b013e31805003f5. [DOI] [PubMed] [Google Scholar]
- 13.Agostinho L, Cruz R, Osorio F, Alves J, Setubal A, Guerra A. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549–556. doi: 10.1007/s13244-017-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funaki K, Fukunishi H, Maeda T, Ohbayashi C, Yamaguchi S. Adenomyosis with extensive glandular proliferation simulating infiltrating malignancy on magnetic resonance imaging. Jpn J Radiol. 2011;29:272–275. doi: 10.1007/s11604-010-0538-6. [DOI] [PubMed] [Google Scholar]
- 15.Tirumani SH, Ojili V, Shanbhogue AK, Fasih N, Ryan JG, Reinhold C. Current concepts in the imaging of uterine sarcoma. Abdom Imaging. 2013;38:397–411. doi: 10.1007/s00261-012-9919-x. [DOI] [PubMed] [Google Scholar]
- 16.Koyama T, Togashi K, Konishi I, et al. MR imaging of endometrial stromal sarcoma: correlation with pathologic findings. AJR Am J Roentgenol. 1999;173:767–772. doi: 10.2214/ajr.173.3.10470920. [DOI] [PubMed] [Google Scholar]
- 17.Ueda M, Otsuka M, Hatakenaka M, et al. MR imaging findings of uterine endometrial stromal sarcoma: differentiation from endometrial carcinoma. Eur Radiol. 2001;11:28–33. doi: 10.1007/s003300000541. [DOI] [PubMed] [Google Scholar]
- 18.Li HM, Liu J, Qiang JW, Gu WY, Zhang GF, Ma FH. Endometrial stromal sarcoma of the uterus: magnetic resonance imaging findings including apparent diffusion coefficient value and its correlation with ki-67 expression. Int J Gynecol Cancer. 2017;27:1877–1887. doi: 10.1097/IGC.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 19.Fujii S, Kaneda S, Tsukamoto K, et al. Diffusion-weighted imaging of uterine endometrial stromal sarcoma: a report of 2 cases. J Comput Assist Tomogr. 2010;34:377–379. doi: 10.1097/RCT.0b013e3181cfc676. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa R, Akahane M, Yamada H, et al. Endometrial stromal sarcoma located in the myometrium with a low-intensity rim on T2-weighted images: report of three cases and literature review. J Magn Reson Imaging. 2010;31:975–979. doi: 10.1002/jmri.22126. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi M, Matsuzaki K, Yoshida S, et al. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009;33:244–247. doi: 10.1016/j.clinimag.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi M, Matsuzaki K, Nishitani H. Diffusion-weighted magnetic resonance imaging of endometrial cancer: differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol. 2009;50:947–953. doi: 10.1080/02841850903099981. [DOI] [PubMed] [Google Scholar]
- 23.Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, Taniguchi F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207:114e1–7. doi: 10.1016/j.ajog.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Pal L, Niklaus AL, Kim M, Pollack S, Santoro N. Heterogeneity in endometrial expression of aromatase in polyp-bearing uteri. Hum Reprod. 2008;23:80–84. doi: 10.1093/humrep/dem346. [DOI] [PubMed] [Google Scholar]
- 25.Ascher SM, Imaoka I, Lage JM. Tamoxifen-induced uterine abnormalities: the role of imaging. Radiology. 2000;214:29–38. doi: 10.1148/radiology.214.1.r00ja4429. [DOI] [PubMed] [Google Scholar]
- 26.McCluggage WG, Desai V, Manek S. Tamoxifen-associated postmenopausal adenomyosis exhibits stromal fibrosis, glandular dilatation and epithelial metaplasias. Histopathology. 2000;37:340–346. doi: 10.1046/j.1365-2559.2000.01001.x. [DOI] [PubMed] [Google Scholar]
- 27.Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand. 2010;89:992–1002. doi: 10.3109/00016349.2010.493196. [DOI] [PubMed] [Google Scholar]
- 28.Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:1197–1205. doi: 10.1097/AOG.0b013e3181f74864. [DOI] [PubMed] [Google Scholar]
- 29.Baiocchi G, Manci N, Pazzaglia M, et al. Malignancy in endometrial polyps: a 12-year experience. Am J Obstet Gynecol. 2009;201:462.e1–4. doi: 10.1016/j.ajog.2009.05.055. [DOI] [PubMed] [Google Scholar]