Abstract
The incidence of abdominal and pelvic cancer in pregnancy is low, but it is rising as the population of pregnant women gets older. Depending on disease stage, gestational age and patient’s preference, active surveillance as well as surgery and chemotherapy are feasible options during pregnancy. Correct diagnosis and staging of the tumor is crucial for choosing the best therapeutic approach. Moreover, a reproducible modality to assess the treatment response is requested. Magnetic resonance imaging (MRI) is commonly used with good results for the local staging and treatment response evaluation of most abdominal and pelvic cancers in nonpregnant patients, and it is considered relatively safe during pregnancy. The purpose of this article is to analyze the most relevant topics regarding the use of MRI in pregnant women with abdominal and pelvic cancer. We discuss MRI safety during pregnancy, including the use of gadolinium-based contrast agents (GBCAs), how to prepare the patient for the exam and MRI technique. This will be followed by a brief review on the most common malignancies diagnosed during pregnancy and their MRI appearance.
The diagnosis of cancer during pregnancy is an uncommon event and the estimated incidence is one in 1000 pregnancies (1). In Europe, 2500 to 5000 new diagnoses of cancer in pregnant women are estimated annually (2). Since the age of pregnant women is increasing in developed countries, the diagnosis of cancer will become more frequent during pregnancy. The most frequent cancer diagnosed in pregnant women is breast cancer; among abdominal and pelvic tumors, cervical, ovarian and gastrointestinal cancers are the most frequently reported (3, 4). Symptoms caused by cancer can mimic the physiologic gestational symptoms and this can lead to a delayed diagnosis with a more advanced stage, as reported for gastrointestinal cancers (5). On the other hand, more frequent gynecological examinations in women during pregnancy provides an opportunity for the early diagnosis of cervical cancer (6). For abdominal and pelvic cancers diagnosed during pregnancy chemotherapy and surgical treatment are feasible options; other alternatives are to terminate the pregnancy or to postpone the treatment until after delivery (7). The treatment of cancer in a pregnant woman is complex and a multidisciplinary approach is needed. Correct staging of the disease is necessary in order to plan the treatment and a valid modality to assess the treatment response is requested. The imaging modalities of choice in pregnant women are ultrasonography (US) and magnetic resonance imaging (MRI), while computed tomography (CT) is considered a second-line examination and must be reserved for selected cases, and positron emission tomography (PET) with radioactive isotopes is contraindicated in pregnancy (8). US is safe during pregnancy and is usually the first imaging examination performed in these patients. However, it is operator dependent and may be limited by the body habitus, the presence of intestinal gas and the enlarged uterus in late pregnancy, so it can present low values of sensitivity in the evaluation of deep structures. MRI is commonly used for local staging and treatment response evaluation of cervical, ovarian, colorectal, renal, pancreatic, and liver cancer in nonpregnant patients, with good results. It provides multiplanar images of the abdomen and the pelvis, with an excellent spatial and contrast resolution also without the administration of intravenous contrast agent. Moreover, it does not use ionizing radiation and is considered relatively safe during pregnancy. In this setting, MRI could help the clinicians to achieve the diagnosis and choose the best personalized treatment for patients, avoiding misdiagnosis and waste of time.
We reviewed the literature to analyze the use of MRI in pregnant patients with abdominal and pelvic cancers. In the present article, we discuss MRI safety during pregnancy, including the use of gadolinium-based contrast agents (GBCAs), how to prepare the patient for the exam, and the MRI technique. This will be followed by a brief review on the most common malignancies diagnosed during pregnancy and their MRI appearance.
MRI safety during pregnancy
To date, there is no definitive evidence of detrimental effects of MRI in pregnant women, but its long-term security has not yet been conclusively proven (9).
In the MRI system, there are three types of magnetic fields: static magnetic field, time-varying magnetic field gradients, and radiofrequency pulses. Each of them could put the fetus health at risk (10, 11).
Static magnetic field may affect cell migration, proliferation and differentiation, in particular during the first trimester in which organogenesis occurs, thus having a teratogenic effect. However, this was reported only by few studies performed in animals, while many studies carried out on human subjects have not shown any teratogenic effect after in utero exposure to MRI (10, 12, 13).
Time-varying magnetic field gradients are responsible for the production of acoustic noise and a temporary hearing loss has been reported in patients undergoing MRI without the use of headphones (10). Fetus ear completes its development by 24 weeks of gestation and after this period fetus hearing could be damaged by noise. American Academy of Pediatrics considers 90 dB as the limit above which the ear of the fetus can suffer permanent damage; it has been recorded that the maximum noise produced by MRI reaches 120 dB and also that the mother’s body attenuates about 30 dB (14, 15). Therefore, the limit of 90 dB could be reached and passed during an MRI examination, causing fetal hearing damage. The intensity and frequency of the noise depend on the parameters of the MRI sequences and increase with the reduction of the slice thickness, the field of view, the repetition time and the echo time (10). Currently no cases of fetal hearing damage have been reported in the literature due to an MRI examination during pregnancy (15, 16).
Radiofrequency pulses deposit energy in the body’s tissues in form of heat. The unit that is used to quantify this energy deposition is the specific absorption rate (SAR), expressed in Watts per kilogram (W/kg). The fetal thermoregulation system is strictly dependent on the maternal system and an increase in maternal temperature could produce heat-induced fetal abnormalities (11). The upper limit of SAR permitted during an MRI examination is 4 W/kg for a whole-body scanner for any patient; at this limit the maximum body temperature increase is 0.6°C for 20–30 minutes of exam duration (10). There are no established limits of whole-body SAR for pregnant patients; however, some authors advise against passing the limit of 2 W/kg for imaging pregnant patients (15). The SAR increases with the intensity of the static magnetic field, the flip angle and the number and spacing of radiofrequency pulses (10). To avoid excessively high SAR values, it is recommended to use magnets with a strength not higher than 3 T for imaging pregnant women (11). However, some authors suggest to avoid more than 1.5 T MRI (13, 17). Some pulse sequences, like the single-shot fast spin-echo (SSFSE) sequences that are frequently used to image pregnant patients, are associated with a relatively high SAR when compared with others such as the gradient-echo (GRE) sequences (11).
In 2013, the American College of Radiology (ACR) asserted that pregnant patients can undergo MRI in case of a favorable risk-benefit ratio, and in particular if: 1) the information requested cannot be obtained through other nonionizing imaging techniques, e.g., US; 2) the information obtained can influence the patient’s care during pregnancy; and 3) it is not prudent to postpone the exam until after delivery (18). These conditions are often met if an abdominal or pelvic tumor is to be staged or re-staged in a pregnant woman. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) suggests to be cautious in the use of MRI in the first trimester of pregnancy, during which the sensitivity of the fetus to teratogenic agents is greater, recommending an accurate risk/benefit analysis (19, 20). On the other hand, ACR states that no special consideration is recommended for the first, versus any other, trimester in pregnancy (18). In fact, although there are only few studies in the literature concerning the safety of MRI during the first trimester of pregnancy, there is no evidence of its possible harmful effect on the fetus (13).
Repeated administration of high doses of GBCAs resulted to be teratogenic in some animal studies (21). Gadolinium chelates are able to cross the placenta and enter the fetal circulation; once filtered by the fetal kidney, they are excreted with the urine in the amniotic fluid. The amniotic fluid can be swallowed by the fetus and in this way gadolinium can be absorbed into the gastrointestinal tract and reach the fetal bloodstream. If gadolinium remains in the fetal system for a long time, the probability of a dechelation with release of free toxic gadolinium ions increases.
However, to date there is no scientific evidence of teratogenicity following the administration of recommended doses of GBCAs during pregnancy in human subjects (15, 21). The main international scientific societies agree in recommending caution in the use of GBCAs in pregnant patients: the GBCAs should not be routinely administered to pregnant women and should be administered only if there is a clear benefit from its use, which clearly exceeds possible but unknown risks for the fetus (8, 21, 22). However, a case-by-case assessment by the clinical and radiological working group is necessary. If the decision to administer GBCAs is made, an agent with a low-risk of nephrogenic systemic fibrosis should be used at the lowest possible dosage, such as Gadobenate dimeglumine (MultiHance®, Bracco Diagnostics, 0.2 mL/kg), Gadobutrol (Gadovist® or Gadavist®, Bayer HealthCare, 0.1 mL/kg), Gadoterate meglumine (Dotarem®, Guerbet, 0.2 mL/kg), Gadoteridol (ProHance®, Bracco Diagnostics, 0.2 mL/kg) or Gadoxetate disodium (Primovist® or Eovist®, Bayer Healthcare, 0.1 mL/kg) (22). In any case, for the study of pregnant patients with cancer the excellent contrast resolution of unenhanced MRI in the abdomino-pelvic region often allows to obtain exhaustive information even without the use of contrast agents (23).
Patient preparation
The management of a patient diagnosed with cancer during pregnancy is complex because of ethical, psychological, and religious issues. A multidisciplinary group that includes clinicians, surgeons, oncologists, radiotherapists, radiologists, obstetricians, and even psychologists is needed.
Before the MRI examination, the radiologist must speak with the patient to obtain the informed consent. He must make sure that the patient understands the importance of the examination for the management of the disease and must explain the potential risks for the mother and the fetus. The radiologist must also clarify that to date there is no documented risk from exposure to MRI during pregnancy. A template of an informed consent form for MRI examination in pregnant patients, referring to the most recent guidelines of the ACR, can be found attached to the present article.
In a pregnant patient, the study with MRI of the abdominal and pelvic region may be hindered by intestinal peristalsis and fetal movements, which may degrade the images. For pregnant patients who have to undergo MRI of abdomen and pelvis, fasting 4 hours prior to examination is suggested in order to reduce maternal peristalsis and fetal motion (24).
In nonpregnant patients, to reduce intestinal peristalsis before an MRI examination of the abdomen and pelvis, intravenous (IV) or intramuscular (IM) spasmolytic drugs are frequently administered; among them hyoscine butylbromide (Buscopan®, Boehringer Ingelheim GmbH) is one of the most commonly used. Its use is nowadays being studied to reduce the duration of delivery, favoring the dilatation of the cervical canal and it could be used also as preparation for an MRI examination in a pregnant patient, but the decision should be made on a case-by-case basis (25). Moreover, especially in the third trimester of pregnancy, the enlarged uterus moves the intestinal loops from the pelvic region and the motion artifacts in the pelvic MRI are mainly due to the movements of the mother or the fetus rather than intestinal peristalsis.
MRI technique
The position of the patient during the examination is an important factor to consider in the case of a pregnant woman. The classic supine position can cause discomfort in the patient and the gravid uterus can compress the inferior vena cava causing an impaired venous return that can lead to syncope; in this position the patient may also have difficulty breathing and holding the breath. So the left lateral decubitus is the suggested position for patients in the late stages of pregnancy (24).
The duration of the examination should be as short as possible, both to reduce maternal fatigue and to minimize the potential risks for the fetus, but the MRI should last long enough to allow the necessary information to be obtained. Furthermore, the use of short-duration sequences reduces motion artifacts.
T2-weighted fast spin echo (FSE) and SSFSE are the most used sequences for imaging pregnant patients: they show anatomical structures with high detail and are rapid, thus minimizing artifacts related to fetal movements. Unfortunately, these sequences are associated with relatively high SAR values (26). Low SAR sequences, like T1-weighted spoiled GRE and steady state free precession (SSFP) sequences (FIESTA, TrueFISP or Balanced-FFE), are also employed. In order to minimize the risk to the fetus, it is recommended to alternate between high and low SAR sequences during the examination (15). In particular, FIESTA sequences show a higher signal-to-noise ratio compared with SSFSE sequences, are motion-insensitive and can help in the detection of pathologic lymph nodes and peritoneal carcinomatosis (27). Moreover they can provide good images of vascular structures without GBCAs administration, which could be particularly useful for the presurgical planning in a pregnant patient.
Fat suppression can be useful for studying adnexal masses, while in-phase and opposed-phase GRE imaging is necessary for the characterization of adrenal lesions (24).
Diffusion-weighted imaging (DWI) can be very useful to evaluate tumor extension and to identify lymph nodal and peritoneal metastases and could obviate the need for contrast media administration (1, 28).
Multiple acquisition planes are generally used; in particular, oblique planes with respect to the major axis of the organ can be used to better define the infiltration of adjacent structures, as reported for staging cervical cancer in nonpregnant patients (29).
MR-cholangiopancreatography, MR-angiography or MR-urography could be used, if needed, to obtain high-quality images respectively of the biliary tree, vascular structures or urinary tract, without contrast media administration.
If sequences that cause loud noise (such as fast GRE) are used, limiting the duration of the sequence is necessary in order to avoid harm to fetal hearing (11, 15).
In the Table we suggest the most useful MRI sequences according to the type of cancer in pregnant patients.
Table.
Key MRI sequences for cancer type in pregnant patients
| Type of cancer | Key MRI sequences |
|---|---|
| Cervical | - Axial T2WI in pelvis |
| - Axial T1WI in pelvis | |
| - Sagittal T2WI in pelvis | |
| - Axial oblique T2WI in pelvis (perpendicular to the major axis of the cervix) | |
| - Axial oblique DWI in pelvis (in the same plane of axial oblique T2WI) | |
| - Coronal oblique T2WI in pelvis (parallel to the major axis of the cervix) | |
| - Axial T2WI or SSFP in upper abdomen | |
|
| |
| Ovarian | - Axial T2WI in pelvis |
| - Axial T1WI in pelvis | |
| - Axial T1WI with fat saturation in pelvis | |
| - Axial T2WI or SSFP in upper abdomen | |
| - Axial DWI in pelvis and upper abdomen | |
| - Sagittal T2WI in pelvis | |
|
| |
| Colon | - Axial T2WI or SSFP in pelvis and upper abdomen |
| - Axial T1WI in pelvis and upper abdomen | |
| - Axial T2WI with fat saturation in pelvis and upper abdomen | |
| - Axial DWI in pelvis and upper abdomen | |
| - Coronal T2WI or SSFP in pelvis and upper abdomen | |
|
| |
| Rectal | - Axial T2WI in pelvis |
| - Axial T1WI in pelvis | |
| - Sagittal T2WI in pelvis | |
| - Axial oblique T2WI in pelvis (perpendicular to the major axis of rectal lumen) | |
| - Axial oblique DWI in pelvis (in the same plane of axial oblique T2WI) | |
| - Coronal oblique T2WI in pelvis (parallel to the major axis of rectal lumen) | |
| - Axial T2WI or SSFP in upper abdomen | |
|
| |
| Kidney and adrenal | - Axial T2WI |
| - Axial in-phase and opposed-phase T1WI | |
| - Axial T2WI with fat saturation | |
| - Axial T1WI with fat saturation | |
| - Axial DWI | |
| - Axial SSFP | |
| - Coronal T2WI | |
| - Sagittal T2WI | |
|
| |
| Pancreatic | - Axial T2WI |
| - Axial T2WI with fat saturation | |
| - Axial T1WI with fat saturation | |
| - Axial DWI | |
| - Axial SSFP | |
| - Coronal T2WI | |
| - MR-cholangiopancreatography | |
|
| |
| Liver | - Axial T2WI |
| - Axial in-phase and opposed-phase T1WI | |
| - Axial T2WI with fat saturation | |
| - Axial T1WI with fat saturation | |
| - Axial DWI | |
| - Axial SSFP | |
| - Coronal T2WI | |
No post-contrast sequences are shown as contrast medium should not be routinely administered to pregnant women, but its use should be evaluated on a case-by-case basis.
T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; SSFP, steady state free precession.
Cervical cancer
Cervical cancer is considered the second most common cancer diagnosed during pregnancy (4). The estimated incidence is 1–10 for 10 000 pregnancies (30). After a diagnosis is made, treatment options depend on several factors, such as gestational age, disease stage and the patient’s preference. During the first trimester of pregnancy a conservative approach is often proposed, while in the third trimester, if fetal maturity allows it, a Caesarean section followed by cancer treatment is performed (31). During the second trimester, the possibilities are to wait for fetal maturity or to carry out treatment such as conisation or trachelectomy in early-stage cervical cancer or neoadjuvant chemotherapy (nCT) in locally advanced stages, to preserve pregnancy until fetal maturity (34–35 weeks of gestation), reducing the risks related to prematurity. A rising number of case reports and experiences reported in the literature showed promising results of nCT both in term of maternal and fetal outcome (32). There are no shared guidelines, the treatment is experimental and must be carried out in specialized hospitals. The diagnosis and staging of locally invasive cervical cancer in pregnancy is challenging. The use of staging pelvic lymphadenectomy is still debated and accepted just between 22–25 weeks of gestation (2). Only few studies concerning the use of MRI in this kind of patients are present in the literature, with a low number of patients (30, 33). These studies have reported a good imaging-pathologic correlation and MRI influenced the clinical decision in all cases.
Sagittal, axial, and coronal oblique T2-weighted images are useful in determining tumor extension, dimension and stromal and parametria infiltration; moreover, axial pelvic T1- and T2-weighted and axial abdominal T1- or T2-weighted or FIESTA images are necessary to evaluate the presence of metastatic lymph nodes (29). DWI sequences are very helpful for tumor and lymph nodes detection.
The MRI appearance of cervical cancer during pregnancy is similar to that in nonpregnant patients: the tumor usually appears hyperintense compared with the myometrium (Fig. 1). Anyway, during pregnancy the cervix could become hyperintense and so the tumor appears iso- or weakly hypointense. A possible pitfall could be represented by the dilatation of pelvic veins during pregnancy that could be interpreted as lymph nodes in the axial plane. The error can be avoided by evaluating these structures in other planes. Artifacts related to fetal movement can alter images of the cervix, especially in the third trimester of pregnancy; however, rarely this inconvenience compromises the exam interpretation. MRI determines local extension of the tumor and is useful for staging cervical cancer diagnosed in pregnant women and also is a reproducible tool for determining the response to nCT administered during pregnancy (Fig. 2).
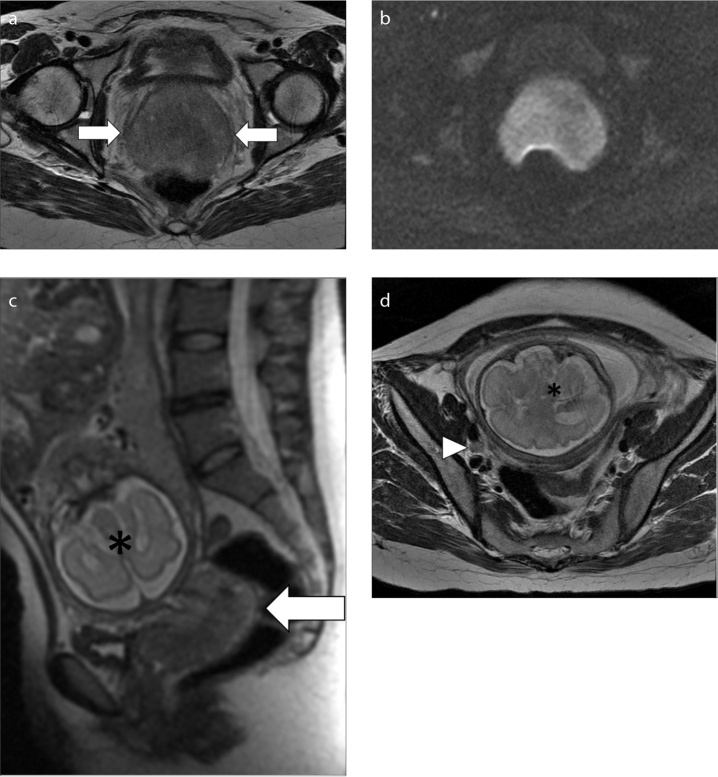
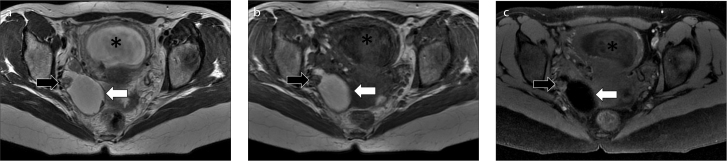
Figure 1. a–d.
MRI in a 35-year-old woman at 28 weeks of pregnancy, diagnosed with cervical cancer: (a, d), axial T2-weighed images; (b), axial DWI image; (c), sagittal T2-weighed image. T2-weighed images show the cancer as a high signal intensity mass in the cervix (a, c, arrows), also hyperintense on DWI image (b). A right obturator metastatic lymph node was also found (d, arrowhead). The gravid uterus is marked with an asterisk.
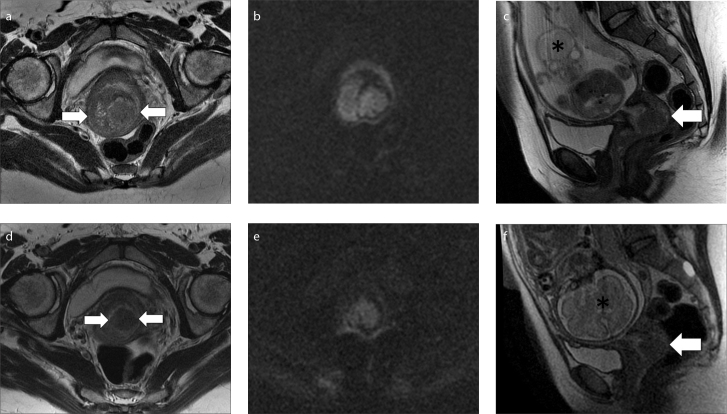
Figure 2. a–f.
MRI performed before (a–c) and after chemotherapy (d–f) in a 37-year-old woman with cervical cancer diagnosed at 21 weeks of pregnancy. Axial oblique T2-weighed images (a, d), axial oblique DWI images (b, e), sagittal T2-weighed images (c, f). At baseline MRI (a–c), T2-weighed sequences show the hyperintense cervical cancer (arrows), also hyperintense on DWI images. No enlarged lymph nodes were detected in abdomen and pelvis. MRI performed after chemotherapy (d–f) shows a reduction in tumor volume, with a partial response. The gravid uterus is marked with an asterisk. At 34 weeks of pregnancy a caesarean section was performed with the extraction of a viable fetus and the patient underwent hysterectomy, bilateral salpingooophorectomy and pelvic lymphadenectomy.
Ovarian cancer
It is estimated that about 2.8–11 ovarian tumors are diagnosed for 100 000 pregnancies (34). Ovarian cancer can be treated by surgery, especially at midgestation, while chemotherapy with platinum compounds and taxanes can be used during the last two trimesters of pregnancy (7, 34). Chemotherapy should be avoided before 14 weeks and after 35 weeks and delivery should be planned at least 3 weeks after the last cycle to avoid hemorrhage, sepsis, and short-term complications. As demonstrated by several studies, when US is not able to characterize an adnexal mass, MRI is the best second-line technique, with an accuracy reported higher than 80% (35).
The MRI study of adnexal masses requires both T2- and T1-weighted sequences and at least two orthogonal imaging planes (usually axial and sagittal) (36). The sagittal view is useful to evaluate local extension of the disease. The T1-weighted sequence must be repeated twice, both with and without fat saturation to detect the hyperintense signal due to hemorrhagic material. We suggest the spectral saturation because the saturation obtained with inversion recovery sequences may be nonhomogeneous and the bleeding tissue may have the same T1 relaxation time of the fat tissue, leading to error in differential diagnosis. Instead, an assessment of the upper abdomen is mandatory, given the possibility that peritoneal carcinomatosis is frequently found in “distant locations” (peri-hepatic or peri-splenic). DWI sequences have shown promising results in the detection of peritoneal implants in patients with gynecological malignancies, and could be particularly useful in this regard if a decision to not administer GBCAs is made (37).
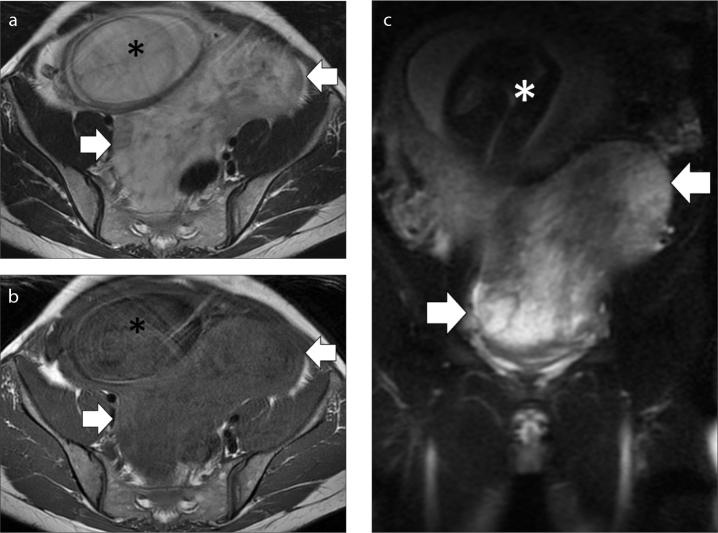
A cystic lesion with papillary projections, wall thickness (>3 mm) and a necrotic solid component is indicative of a malignant lesion, especially if associated with ascites (38). Less frequently, metastases may affect the ovary as in the case of Krukenberg tumors, which are solid metastatic ovarian masses arising most commonly form gastric carcinomas. These tumors may show hyperintensity on T2-weighted images, due to the presence of edema (Fig. 3) (39). However, only about 5% of the ovarian masses diagnosed during pregnancy are malignant and some differential diagnoses must be considered (34). Serous cystadenomas may appear as unilocular cysts with thin walls and a simple fluid content, with high T2 and low T1 signal intensity. Mucinous cystadenomas are usually multilocular cysts with a viscous content that can show various signal intensities on T1- and T2-weighted images (Fig. 4) (39). Teratomas are the most frequent adnexal cystic mass after the sixteenth week of pregnancy (39). Usually they are hyperintense on both T1- and T2-weighted sequences, and fat-suppressed sequences demonstrate the presence of fatty tissue inside the mass (Fig. 5). Other benign ovarian masses that may present during pregnancy are corpus luteum cysts, theca lutein cysts or endometriomas. Uterine subserosal leiomyomas can also mimic ovarian masses, especially at US, while MRI usually manages to characterize the lesion (26).
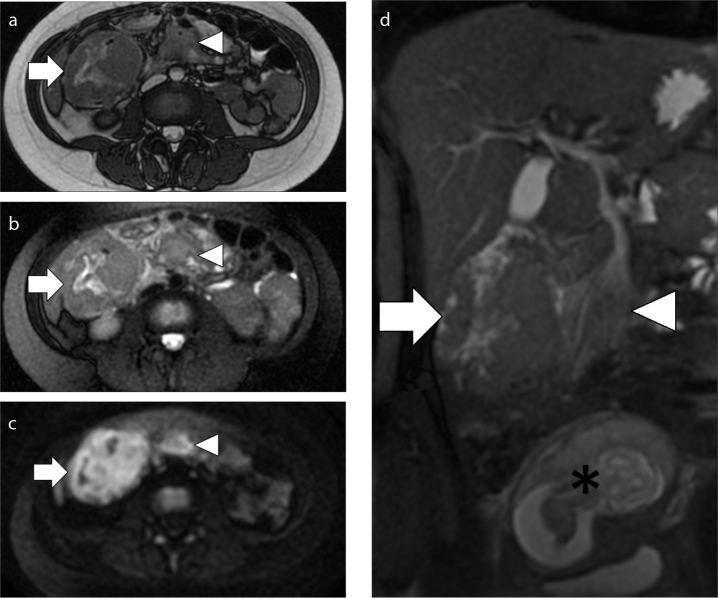
Figure 3. a–c.
MRI performed in a 38-year-old woman at 28 weeks of pregnancy with a Krukenberg tumor. Axial T2-weighed image (a), axial T1-weighed image (b), coronal T2-weighed fat suppressed image (c). The exam shows a big mass in the pelvis (arrows) that displaces the gravid uterus (asterisk) anteriorly and on the right. Notice the high signal intensity of the mass on T2-weighed fat suppressed image (c), related to the presence of edema.
Figure 4. a–c.
MRI in a 39-year-old woman at 12 weeks of pregnancy with an adnexal mucinous cystadenoma. Axial T2-weighed image (a), axial T1-weighed image (b), sagittal T2-weighed image (c). The lesion appears as a multilocular cyst, with a viscous content that is predominantly hyperintense on T2-weighed image and hypointense on T1-weighed image, with some areas of inhomogeneity (arrows). The gravid uterus is marked with an asterisk.
Figure 5. a–c.
MRI performed in a 29-year-old woman at 16 weeks of pregnancy with a right adnexal teratoma. Axial T2-weighed image (a), axial T1-weighed image (b), axial T1-weighed fat suppressed image (c). The major component of the mass (white arrow) shows a high signal intensity both on T2- and T1-weighed images (a and b) and the low signal intensity on fat suppressed image (c) is consistent with the presence of fat tissue. Notice also the presence of a solid lateral component (black arrow). The gravid uterus is marked with an asterisk.
The possible treatment of these tumors, along with chemotherapy (not recommended in the first quarter, when the teratogenicity risk is about 10%), is the surgical resection without abortion (40). In these cases, the MRI is even more important to properly evaluate the controlateral tubes and ovary, which in young patients can (and must) be preserved.
Gastrointestinal cancer
Most of these tumors are represented by colorectal cancers, while gastric or small intestinal cancers are really rare. The estimated incidence of colorectal cancer is 0.8 per 100 000 pregnancies (41). The diagnosis is often delayed and the stage at the diagnosis is advanced, due to nonspecific signs and symptoms that can easily be attributed to pregnancy. Surgery and chemotherapy during pregnancy have been reported for colorectal cancer (41). CT is considered the standard imaging technique for staging nonpregnant patients with colorectal cancer, but MRI has been reported to have a similar accuracy to CT in the diagnosis of colorectal cancer and it is safer during pregnancy (41). There are no specific studies regarding the role of MRI in pregnant patients with colorectal cancer. MRI could be useful for the detection of a colorectal tumor in pregnant women with obstructive symptoms and could be used for re-staging the cancer after the treatment (Fig. 6). The evaluation of the bowel is based on the principles of ultrafast imaging, performing breath-hold SSFSE or GRE sequences (42). To reduce the acquisition time, sequences should be acquired in the coronal plane. Fast imaging with SSFP sequences provide a good anatomical overview with a mixture of both T1 and T2 contrast. It is better not to use only fat saturated sequences as mesenteric and retroperitoneal changes can be visualized without fat saturation. T2-weighted sequences (SSFSE or HASTE) are helpful for the evaluation of colic lesions and to evaluate local extensions; fat suppression is helpful to evaluate extravisceral extension. Moreover, DWI (acquired on axial planes) is very useful for identifying disease areas that may go undetected in morphological sequences, both for the primary cancer and the pathological lymph nodes (Fig. 7).
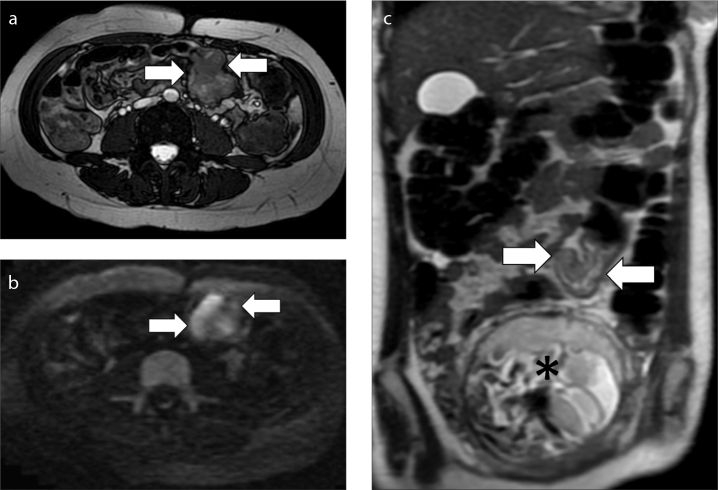
Figure 6. a–c.
MRI in a 31-year-old woman at 19 weeks of pregnancy with an adenocarcinoma of the sigmoid colon. Axial FIESTA image (a), axial DWI image (b) and coronal T2-weighed image (c). The tumor appears as a wall thickening of sigmoid colon that causes stenosis of the lumen and is hyperintense on FIESTA, DWI and T2-weighted images (arrows). Notice how DWI image improves tumor visualization. The gravid uterus is marked with an asterisk. No enlarged lymph nodes were detected in abdomen and pelvis. A week later a sigmoidectomy was performed due to a bowel obstruction; chemotherapy was also started and a cesarean section was performed at 36 weeks of pregnancy.
Figure 7. a–f.
MRI performed before (a–c) and after chemotherapy (d–f) in a 37-year-old woman with cervical cancer diagnosed at 21 weeks of pregnancy. Axial oblique T2-weighed images (a, d), axial oblique DWI images (b, e), sagittal T2-weighed images (c, f). At baseline MRI (a–c), T2-weighed sequences show the hyperintense cervical cancer (arrows), also hyperintense on DWI images. No enlarged lymph nodes were detected in abdomen and pelvis. MRI performed after chemotherapy (d–f) shows a reduction in tumor volume, with a partial response. The gravid uterus is marked with an asterisk. At 34 weeks of pregnancy a caesarean section was performed with the extraction of a viable fetus and the patient underwent hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy.
Less frequent malignancies
Renal cell carcinoma is the most common renal mass diagnosed in pregnancy (43). If a renal mass is identified at US, MRI allows a better characterization and is more accurate in the evaluation of local extension and distant metastases. In this context, the administration of GBCAs has been reported in the literature; however, according to the most recent guidelines, GBCAs should be administered in pregnant patients only if there is a clear benefit from its use, which clearly exceeds possible but unknown risks for the fetus (22, 43). Therefore a case-by-case assessment is always necessary. In addition, MRI could be useful for the surveillance of renal tumors diagnosed during pregnancy, if the decision to postpone the treatment is made.
Adrenal tumors diagnosed during pregnancy are very rare; the most frequently reported are the pheochromocytomas with an estimated incidence of 0.007% (44). MRI is the imaging modality of choice to localize and diagnose pheochromocytomas and usually it is accurate enough even without GBCAs administration (44). The high T2 signal intensity is characteristic for this type of tumor but it can be variable due to necrosis, hemorrhage and calcifications. The use of in-phase and opposed-phase T1-weighted sequences allows the diagnosis of lipid-rich adenomas, thus excluding pheochromocytomas that do not show signal loss in opposed-phase sequences (45). One study reported that DWI can be useful to differentiate malignant pheochromocytomas from benign ones (46). A condition to keep in mind is the adrenal hemorrhage, which may happen during pregnancy and may mimic an adrenal mass (47). Most often it is unilateral and clinically silent. MRI characteristics depend on the age of the bleeding: it is hypointense on both T1- and T2-weighted images in the first week and later can become hyperintense on T1-weighted images due to the paramagnetic effect of free methemoglobin. The presence of hyperintense areas in T2-weighted sequences is caused by the serum. Rarely, bleeding may occur on an existing mass (48).
Only few cases of pancreatic neoplasms diagnosed during pregnancy are reported in the literature (49). MRI has been used almost always to better define the lesion. For nonpregnant patients MRI reported a sensitivity for the detection of pancreatic cancer and an accuracy in determining tumor resectability similar to CT (50).
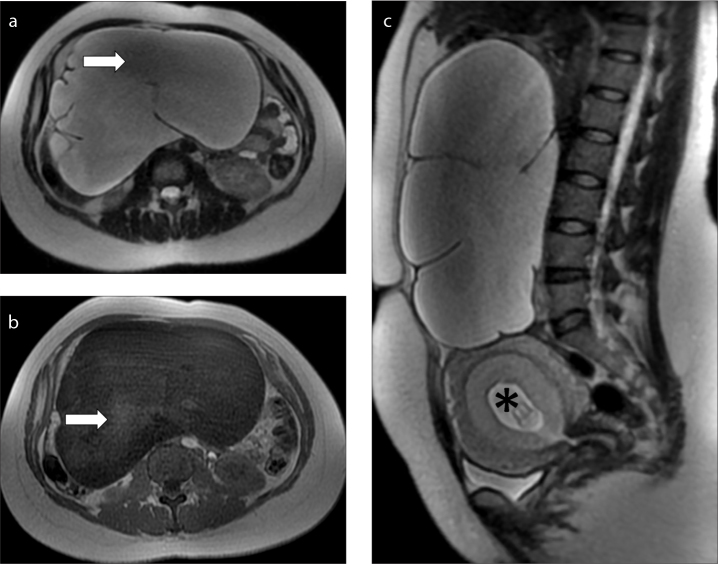
Liver masses in pregnancy are rare, usually asymptomatic and incidentally detected during a routine US exam. The most common masses are the benign ones, like hemangiomas, focal nodular hyperplasia (FNH) or hepatocellular adenoma (51). Usually, they do not show variations during pregnancy. However, growth of adenomas can occur in up to one third of pregnancies, and larger adenomas (>4 cm) are at risk of developing intralesional hemorrhage. Their rupture risk increases in the late months of pregnancy and in the postpartum period, being directly related to size and rate of growth: when adenoma is more than 5 cm the risk is significant (51). MRI findings of these benign lesions do not vary much between pregnant and nonpregnant patients (52). Hepatic metastases are rare lesions during pregnancies. Their MRI appearance is variable, but they usually tend to be hypointense on T1-weighted images and moderately hyperintense on T2-weighted images. They usually show restricted diffusion in the DWI sequence (Fig. 8). Sometimes they can mimic other hepatic lesions, and without contrast media it is difficult to make an exact diagnosis. In these cases, a biopsy can be helpful, despite the absence of literature data regarding the safety of this procedure.
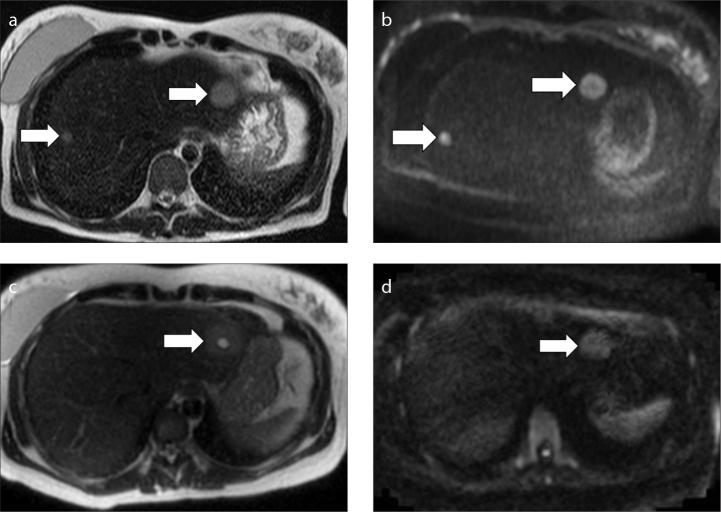
Figure 8. a–d.
MRI performed before (a, b) and after chemotherapy (c, d) in a 38-year-old woman with hepatic metastases by an adenocarcinoma of the colon diagnosed at 13 weeks of pregnancy. Axial T2-weighed images (a, c), axial DWI images (b, d). At baseline MRI (a, b), two round lesions were detected in the liver (arrows). The lesions show an high signal intensity on the T2-weighed image (a) and also on the DWI image (b). A biopsy was performed which confirmed the suspicion of metastases. At MRI performed after chemotherapy (c, d), only one metastasis remains, with a central area of hyperintensity due to treatment-induced necrosis (arrow).
Conclusion
MRI is an excellent reproducible imaging technique in pregnant patients with suspicious abdominal or pelvic cancer. It does not use ionizing radiations and it is therefore generally considered safe for the fetus. However, it presents several potential risks for the health of the fetus, including the ones related to the use of GBCAs, that radiologists must take into account to plan the examination in the best and safest way. Fasting, informed consent, the use of spasmolytic agents and the correct position of the patient must be considered before starting the examination. The MRI technique must be modified according to the type of tumor, in an attempt to ensure the highest diagnostic accuracy while minimizing the risks to the fetus. From the perspective of a modern personalized medicine, MRI could be a fundamental tool in order to plan the most appropriate treatment for the pregnant patient with cancer and to monitor treatment response.
Main points.
Since the age of pregnant women is increasing in developed countries, the diagnosis of cancer will become more frequent during pregnancy. For abdominal and pelvic cancers diagnosed during pregnancy, cervical, ovarian and gastrointestinal cancers are the most frequently reported. The diagnosis and treatment of cancer in a pregnant woman is complex and a multidisciplinary approach is needed.
MRI is an excellent reproducible imaging technique in pregnant patients with suspicious abdominal or pelvic cancer. It is generally considered safe in pregnancy, but it presents several potential risks for fetus health that the radiologists must be aware of.
Issues concerning the preparation of the patient (fasting, informed consent, use of spasmolytic drugs) and the correct position of the patient during the exam must be considered by the radiologist before starting the MRI examination.
The MRI technique must be modified according to the type of the tumor, in an attempt to ensure the highest diagnostic accuracy while minimizing the risks to the fetus.
From the perspective of a modern personalized medicine, MRI could be a fundamental tool in order to plan the most appropriate treatment for the pregnant patient with cancer and to monitor treatment response.
Information sheet and informed consent for magnetic resonance imaging (MRI) during pregnancy
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.De Haan J, Vandecaveye V, Han SN, Van de Vijver KK, Amant F. Difficulties with diagnosis of malignancies in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;33:19–32. doi: 10.1016/j.bpobgyn.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Halaska MJ, Fumagalli M, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer. 2014;24:394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 3.De Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 4.Amant F, Brepoels L, Halaska MJ, Gziri MM, Van Calsteren K. Gynaecologic cancer complicating pregnancy: An overview. Best Pract Res Clin Obstet Gynaecol. 2010;24:61–79. doi: 10.1016/j.bpobgyn.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.McCormick A, Peterson E. Cancer in Pregnancy. Obstet Gynecol Clin North Am. 2018;45:187–200. doi: 10.1016/j.ogc.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Pereg D, Koren G, Lishner M. Cancer in pregnancy: Gaps, challenges and solutions. Cancer Treat Rev. 2008;34:302–312. doi: 10.1016/j.ctrv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Amant F, Han SN, Gziri MM, Vandenbroucke T, Verheecke M, Van Calsteren K. Management of cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29:741–753. doi: 10.1016/j.bpobgyn.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Obstetric Practice. Committee Opinion No. 723: Guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2017;130:e210–216. doi: 10.1097/AOG.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 9.Tsai LL, Grant AK, Mortele KJ, Kung JW, Smith MP. A practical guide to MR imaging safety: what radiologists need to know. Radiographics. 2015;35:1722–1737. doi: 10.1148/rg.2015150108. [DOI] [PubMed] [Google Scholar]
- 10.Ciet P, Litmanovich DE. MR safety issues particular to women. Magn Reson Imaging Clin N Am. 2015;23:59–67. doi: 10.1016/j.mric.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Tirada N, Dreizin D, Khati NJ, Akin EA, Zeman RK. Imaging pregnant and lactating patients. Radiographics. 2015;35:1751–1765. doi: 10.1148/rg.2015150031. [DOI] [PubMed] [Google Scholar]
- 12.De Wilde JP, Rivers AW, Price DL. A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the fetus. Prog Biophys Mol Biol. 2005;87:335–353. doi: 10.1016/j.pbiomolbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316:952–961. doi: 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Committee on Environmental Health. Noise: a hazard for the fetus and newborn. Pediatrics. 1997;100:724–727. doi: 10.1542/peds.100.4.724. [DOI] [PubMed] [Google Scholar]
- 15.Woitek R, Prayer D, Hojreh A, Helbich T. Radiological staging in pregnant patients with cancer. ESMO Open. 2016;1:e000017. doi: 10.1136/esmoopen-2015-000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strizek B, Jani JC, Mucyo E, et al. Safety of MR imaging at 1.5 T in fetuses: a retrospective case-control study of birth weights and the effects of acoustic noise. Radiology. 2015;275:530–537. doi: 10.1148/radiol.14141382. [DOI] [PubMed] [Google Scholar]
- 17.Gomes M, Matias A, Macedo F. Risks to the fetus from diagnostic imaging during pregnancy: review and proposal of a clinical protocol. Pediatr Radiol. 2015;45:1916–1929. doi: 10.1007/s00247-015-3403-z. [DOI] [PubMed] [Google Scholar]
- 18.Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530. doi: 10.1002/jmri.24011. [DOI] [PubMed] [Google Scholar]
- 19.Wang PI, Chong ST, Kielar AZ, et al. Imaging of pregnant and lactating patients: part 1, evidence-based review and recommendations. AJR Am J Roentgenol. 2012;198:778–784. doi: 10.2214/AJR.11.8223. [DOI] [PubMed] [Google Scholar]
- 20.International Commission on Non-Ionizing Radiation Protection. Medical magnetic resonance (MR) procedures: protection of patients. Health Phys. 2004;87:197–216. doi: 10.1097/00004032-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Puac P, Rodríguez A, Vallejo C, Zamora CA, Castillo M. Safety of contrast material use during pregnancy and lactation. Magn Reson Imaging Clin N Am. 2017;25:787–797. doi: 10.1016/j.mric.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 22.ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media Version 10.3. American College of Radiology; 2018. [Google Scholar]
- 23.Tannus JFK, Dagoglu G, Oto A. Magnetic resonance imaging of maternal diseases of the abdomen and pelvis in the pregnant patient. Am J Perinatol. 2008;25:605–610. doi: 10.1055/s-0028-1090596. [DOI] [PubMed] [Google Scholar]
- 24.Leyendecker JR, Gorengaut V, Brown JJ. MR imaging of maternal diseases of the abdomen and pelvis during pregnancy and the immediate postpartum period. Radiographics. 2004;24:1301–1316. doi: 10.1148/rg.245045036. [DOI] [PubMed] [Google Scholar]
- 25.Rohwer AC, Khondowe O, Young T. Antispasmodics for labour. Cochrane Database Syst Rev. 2013;(6):CD009243. doi: 10.1002/14651858.CD009243.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oto A, Ernst R, Jesse MK, Chaljub G, Saade G. Magnetic resonance imaging of the chest, abdomen, and pelvis in the evaluation of pregnant patients with neoplasms. Am J Perinatol. 2007;24:243–250. doi: 10.1055/s-2007-973444. [DOI] [PubMed] [Google Scholar]
- 27.Bhosale P, Ma J, Choi H. Utility of the FIESTA pulse sequence in body oncologic imaging: review. AJR Am J Roentgenol. 2009;192:S83–93. doi: 10.2214/AJR.07.7062. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz JM, Hotalen IM, Miller ES, Barber EL, Shahabi S, Miller FH. How can pelvic MRI with diffusion-weighted imaging help my pregnant patient? Am J Perinatol. 2019 doi: 10.1055/s-0039-1685492. Published online 12 April 2019. [DOI] [PubMed] [Google Scholar]
- 29.Balleyguier C, Sala E, Da Cunha T, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21:1102–1110. doi: 10.1007/s00330-010-1998-x. [DOI] [PubMed] [Google Scholar]
- 30.Balleyguier C, Fournet C, Ben Hassen W, et al. Management of cervical cancer detected during pregnancy: role of magnetic resonance imaging. Clin Imaging. 2013;37:70–76. doi: 10.1016/j.clinimag.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Han SN, Mhallem Gziri M, Van Calsteren K, Amant F. Cervical cancer in pregnant women: treat, wait or interrupt? Assessment of current clinical guidelines, innovations and controversies. Ther Adv Med Oncol. 2013;5:211–219. doi: 10.1177/1758834013494988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricci C, Scambia G, De Vincenzo R. Locally advanced cervical cancer in pregnancy: overcoming the challenge. a case series and review of the literature. Int J Gynecol Cancer. 2016;26:1490–1496. doi: 10.1097/IGC.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 33.Zanetta, Pellegrino, Vanzulli, Di Lelio, Milani, Mangioni Magnetic resonance imaging of cervical cancer in pregnancy. Int J Gynecol Cancer. 1998;8:265–269. doi: 10.1046/j.1525-1438.1998.09802.x. [DOI] [Google Scholar]
- 34.Boussios S, Moschetta M, Tatsi K, Tsiouris AK, Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: Diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1–9. doi: 10.1016/j.jare.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomassin-Naggara I, Fedida B, Sadowski E, et al. Complex US adnexal masses during pregnancy: Is pelvic MR imaging accurate for characterization? Eur J Radiol. 2017;93:200–208. doi: 10.1016/j.ejrad.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Forstner R, Thomassin-Naggara I, Cunha TM, et al. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. Eur Radiol. 2017;27:2248–2257. doi: 10.1007/s00330-016-4600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy A, Medjhoul A, Caramella C, et al. Interest of diffusion-weighted echo-planar MR imaging and apparent diffusion coefficient mapping in gynecological malignancies: a review. J Magn Reson Imaging. 2011;33:1020–1027. doi: 10.1002/jmri.22546. [DOI] [PubMed] [Google Scholar]
- 38.Oto A, Ernst R, Jesse MK, Saade G. Magnetic resonance imaging of cystic adnexal lesions during pregnancy. Curr Probl Diagn Radiol. 2008;37:139–144. doi: 10.1067/j.cpradiol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Yacobozzi M, Nguyen D, Rakita D. Adnexal masses in pregnancy. Semin Ultrasound CT MR. 2012;33:55–64. doi: 10.1053/j.sult.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 2001;7:394–403. doi: 10.1093/humupd/7.4.394. [DOI] [PubMed] [Google Scholar]
- 41.Kocián P, de Haan J, Cardonick EH, et al. Management and outcome of colorectal cancer during pregnancy: report of 41 cases. Acta Chir Belg. 2019;119:166–175. doi: 10.1080/00015458.2018.1493821. [DOI] [PubMed] [Google Scholar]
- 42.Kinner S, Hahnemann ML, Forsting M, Lauenstein TC. Magnetic resonance imaging of the bowel: today and tomorrow. Rofo. 2015;187:160–167. doi: 10.1055/s-0034-1385453. [DOI] [PubMed] [Google Scholar]
- 43.Putra LGJ, Minor TX, Bolton DM, Appu S, Dowling CR, Neerhut GJ. Improved assessment of renal lesions in pregnancy with magnetic resonance imaging. Urology. 2009;74:535–539. doi: 10.1016/j.urology.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 44.van der Weerd K, van Noord C, Loeve M, et al. Endocrinology in pregnancy: Pheochromocytoma in pregnancy: case series and review of literature. Eur J Endocrinol. 2017;177:R49–58. doi: 10.1530/EJE-16-0920. [DOI] [PubMed] [Google Scholar]
- 45.Elsayes KM, Emad-Eldin S, Morani AC, Jensen CT. Practical approach to adrenal imaging. Radiol Clin North Am. 2017;55:279–301. doi: 10.1016/j.rcl.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Dong Y, Liu Q. Differentiation of malignant from benign pheochromocytomas with diffusion-weighted and dynamic contrast-enhanced magnetic resonance at 3.0 T. J Comput Assist Tomogr. 2012;36:361–366. doi: 10.1097/RCT.0b013e31825975f8. [DOI] [PubMed] [Google Scholar]
- 47.Jordan E, Poder L, Courtier J, Sai V, Jung A, Coakley FV. Imaging of nontraumatic adrenal hemorrhage. AJR Am J Roentgenol. 2012;199:W91–98. doi: 10.2214/AJR.11.7973. [DOI] [PubMed] [Google Scholar]
- 48.Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36:75–82. doi: 10.1007/s00268-011-1338-6. [DOI] [PubMed] [Google Scholar]
- 49.Boyd CA, Benarroch-Gampel J, Kilic G, Kruse EJ, Weber SM, Riall TS. Pancreatic neoplasms in pregnancy: diagnosis, complications, and management. J Gastrointest Surg. 2012;16:1064–1071. doi: 10.1007/s11605-011-1797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J Sudbury Mass. 2017;23:333–342. doi: 10.1097/PPO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 51.Noels JE, van Aalten SM, van der Windt DJ, et al. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54:553–558. doi: 10.1016/j.jhep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Siegelman ES, Chauhan A. MR characterization of focal liver lesions: pearls and pitfalls. Magn Reson Imaging Clin N Am. 2014;22:295–313. doi: 10.1016/j.mric.2014.04.005. [DOI] [PubMed] [Google Scholar]