Abstract
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the most common primary liver malignancies. HCC and ICC have characteristic imaging findings, but a number of benign entities can appear similar and can cause diagnostic dilemma. Ideally, accurate and timely diagnosis of these conditions can help the patient to avoid a needle biopsy or even unnecessary treatment. In this article, we present various benign liver lesions that display imaging characteristics that are similar to HCC and ICC on magnetic resonance imaging (MRI) and discuss salient features that may assist in accurate diagnosis.
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the most common primary malignancies arising in the liver. Hepatocellular carcinoma is the fifth most common adult cancer and the second leading cause of cancer-related death globally, while cholangiocarcinoma is the second most prevalent primary liver cancer.
There are a number of benign and malignant liver lesions that can mimic HCC and ICC on diagnostic imaging of the liver (Table). Careful review of the clinical history, physical examination, hepatitis serology and imaging studies are helpful to narrow down differential diagnoses. Accurate characterization of benign lesions is important to avoid misdiagnosing them as cancers, which may lead to unwarranted surgery or intervention.
Table.
Benign lesions mimicking HCC and ICC on imaging studies covered in this review
| Mimicking HCC: |
| Arterio-portal shunt |
| Hemangioma |
| FNH and FNH-like nodule (hypervascular hyperplastic nodule) |
| Hepatic angiomyolipoma |
| Intrahepatic splenosis |
| Benign intrahepatic lymphoid nodule |
| Hepatocellular adenoma |
|
|
| Mimicking ICC: |
| Sclerosing hemangioma |
| Inflammatory pseudotumor |
| Bile duct adenoma |
HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; FNH, focal nodular hyperplasia.
Accurate imaging diagnosis is particularly important in the context of patients with high risk for developing HCC, namely cirrhosis and chronic viral Hepatitis B or C, because treatment can be instituted without a histopathological diagnosis if the characteristic features of arterial hyper-enhancement and washout appearance are detected (1).
The liver imaging reporting and data systems (LI-RADS) document outlines several ancillary features that would favor HCC or malignancy. These ancillary features should also be carefully sought out in the evaluation of a nodule. Failure to adhere to well-established guidelines such as LI-RADS may result in the erroneous diagnosis and treatment of non-HCC lesions. To address this, radiologists should first exclude benign entities such as cysts, hemangiomas and arterioportal shunts. A liver mass with a targetoid appearance is denoted as LR-M in LI-RADS as it is likely to represent an adenocarcinoma rather than HCC. Interestingly, benign entities such as sclerosing hemangiomata or abscesses can be misclassified as LR-M. As such, observations classified as LR-M require a core biopsy to obtain the histological diagnosis. High specificity is crucial for clinical practice in North America where liver transplantation is the treatment of choice for suitable patients with HCC.
Focal liver lesions seen in patients without known malignancy or risk factors for HCC have a higher likelihood for benignity, with most of them being detected incidentally. A small proportion of these lesions can be malignant although the incidence of HCC in patients with no risk factors is very low (2).
Ultrasound is commonly used for surveillance of HCC in cirrhotic patients; however, the characterization of focal liver lesions by ultrasound is limited. Multiphasic computed tomography (CT) or MRI is routinely performed for definitive imaging diagnosis of focal liver lesions. MRI is superior to CT in the evaluation of focal liver lesions due to lack of ionizing radiation, high contrast resolution and availability of hepatobiliary contrast agents (3).
This article reviews MRI features, which may assist in the differentiation of various benign focal liver lesions from HCC and ICC.
MRI with hepatobiliary contrast agents
Hepatocyte-specific, gadolinium-based (hepatobiliary) contrast agents are increasingly being used in MRI studies of the liver. These agents are taken up and excreted by the hepatocytes of the liver via the organic anion-transporting polypeptide (OATP) group of molecules (in particular, OATP8) and multidrug resistance-associated proteins (MRP)-2, respectively.
The hepatobiliary phase is obtained 10–20 minutes after contrast injection with gadoxetic acid (Gd-EOB-DTPA, Primovist in Europe and Asia; Eovist in the USA, Bayer) or after 60–120 minutes in the case of Gadobenate dimeglumine (MultiHance, Bracco). In the hepatobiliary phase, malignant lesions that do not contain normal, functioning hepatocytes such as metastases, cholangiocarcinomas and HCC, are not expected to take up the contrast agent and will therefore show up as a hypointense defect against a background of hyperintense liver parenchyma. This increases sensitivity in the detection of metastasis and early HCC (4).
However, benign lesions such as hemangiomas, cysts and hepatic adenomas will also appear as a defect during the hepatobiliary phase because they do not contain functioning hepatocytes. Conversely, other benign lesions such as focal nodular hyperplasia (FNH) or nodular regenerative hyperplasia (NRH) contain normal functioning hepatocytes and will be hyperintense during the hepatobiliary phase (4).
Mimickers of HCC
Arterioportal shunts
Arterioportal shunts (APS) are usually due to localized disordered perfusion and are common hypervascular pseudolesions that mimic HCC in the imaging of the cirrhotic liver (5, 6).
No specific or discrete parenchymal abnormality is found on histopathology. The characteristic imaging features of APS are subcapsular or peripheral location, wedge-shaped appearance and homogeneous enhancement in the hepatic arterial phase (Fig. 1). They are typically sub-centimeter in size. Differences in scan timing may result in the false impression that the lesion is new or is enlarging over time. MRI tends to be more sensitive for detection of APS, mainly due to the higher soft tissue contrast and consequently higher sensitivity for subcentimeter enhancing nodules. Key associated imaging features on MRI include absence of portovenous phase and/or equilibrium phase washout. These lesions are expected to show normal uptake of hepatobiliary contrast on MRI using gadoxetic acid since they are areas of differential perfusion but not impaired hepatocyte function (7). On unenhanced imaging, they usually show signal that is isointense to surrounding liver parenchyma, and no diffusion restriction is seen. Since early HCC may display arterial phase hyperenhancement, attention to other features on MRI such as T2 hyperintensity and restricted diffusion or for threshold growth over serial imaging becomes important (8). APS are benign and classified as either LR-1 or LR-2. Hence, careful evaluation is required to avoid misclassifying APS as an LR-4 observation.
Figure 1. a–c.
Arterioportal shunt (APS) in a 48-year-old man with chronic hepatitis B. Axial contrast-enhanced magnetic resonance image (a) of the upper abdomen reveals a wedge shaped subcapsular, enhancing area (arrow) in the right lobe of the liver in the arterial phase. The corresponding area was isointense to the rest of the liver in T2-weighted image (b) and in venous phase (c). The characteristic location, morphology and enhancement pattern of the lesion is in keeping with APS.
Hemangiomas
Hemangiomas are the most common focal benign liver lesion. They show characteristic MRI features such as bright hyperintense signal on T2-weighted sequences, peripheral, discontinuous, nodular enhancement in the arterial phase with centripetal filling-in during the portal venous and delayed phases. Hemangiomas can sometimes be confused with HCC when gadoxetic acid is used as a contrast agent in the evaluation of patients with cirrhosis. Hemangiomas in cirrhotic livers tend to be smaller in size and show flash enhancement. Both HCC and small hemangiomas are hyperintense in the arterial phase. Progressed HCC is expected to have a wash-out appearance in the portal venous and transitional phases. However, owing to the rapid uptake of gadoxetic acid by hepatocytes, flash enhancing hemangiomas may appear hypointense in the portal venous and transitional phases and thus may be misinterpreted as having a wash-out appearance (9) (Fig. 2). This would result in the erroneous diagnosis of HCC. Careful examination of the other MRI sequences is required in order to differentiate a flash enhancing hemangioma from HCC. Hemangiomas are very hyperintense on T2-weighted sequences while HCC often show intermediate T2 signal. Furthermore, HCC shows restricted diffusion while hemangiomas will demonstrate T2 shine through. Alternatively, an extracellular contrast agent can be used (9, 10).
Figure 2. a, b.
Flash enhancing hemangioma in a 60-year-old woman with hepatitis B. Axial contrast-enhanced arterial phase image (a) of the upper abdomen reveals a homogeneously enhancing lesion in the subcapsular region of segment 2 in the left lobe of the liver (arrow). Axial contrast-enhanced image (b) of upper abdomen in hepatobiliary phase shows the corresponding lesion slightly hypointense to liver, thus mimicking HCC. However, bright hyperintensity of the lesion in T2-weighted imaging (not shown) helped to accurately characterize it as flash enhancing hemangioma.
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is a common benign focal lesion of the liver. FNHs occur as a result of hyperplastic growth of normal hepatocytes but are characterized by a malformed biliary drainage. On MRI, FNHs are isointense to the liver on T1-weighted sequences and are mildly hyperintense on T2-weighted sequences. A central scar is seen in FNH, which is classically hyperintense on T2-weighted images, although this finding is not always present. Following intravenous contrast administration, FNHs show brisk homogeneous arterial hyperenhancement. The lesion subsequently becomes isointense in the portal venous and transitional phases. On gadoxetic acid enhanced MRI, FNH characteristically shows intense and homogeneous uptake of contrast, appearing hyperintense on the hepatobiliary phase (Fig. 3). The central scar, however, is depicted as hypointense on the hepatobiliary phase, given that it does not contain functioning hepatocytes. Just considering lesional arterial hyperenhancement and the presence of a central scar, FNH can theoretically be mistaken for fibrolamellar HCC. However, fibrolamellar HCC shows T2 hypointense central scar and heterogeneous enhancement (11). Approximately 20% of HCC show uptake of hepatobiliary contrast on delayed imaging and can therefore be mistaken for FNH (12–14). However, on dynamic imaging, FNHs do not display washout appearance, as opposed to HCC. FNH may show atypical hypointensity in hepatobiliary phase when there is substantial fat component or large radiating scar thus mimicking HCC (15). However, the arterial enhancement in FNH is typically homogeneous, while the arterial enhancement seen in HCC is usually heterogeneous with a mosaic or nodule-in-nodule appearance (16).
Figure 3. a–c.
Focal nodular hyperplasia (FNH) in a 46-year-old woman without chronic liver disease. Axial contrast-enhanced arterial phase image (a) of the upper abdomen shows a homogeneously enhancing lesion in right lobe of the liver (arrow). Axial contrast-enhanced images (b, c) of the upper abdomen show that the corresponding lesion becomes isointense to liver in the in venous phase (b, arrow) and retains contrast in the hepatobiliary phase (c, arrow). Note that the lesion retains contrast in the hepatobiliary phase, a key imaging finding in FNH that will help in differentiating it from non-hepatocyte-containing lesions. That said, uptake of hepatobiliary phase contrast can be seen in a minority of HCCs and FNH may show atypical hypointensity in hepatobiliary phase when it contains substantial fat and large central scar. Assessing washout on the portal phase would be key in the diagnosis of HCC.
Hepatic angiomyolipoma
Hepatic angiomyolipoma (HAML) is a benign tumor that contains varying amounts of fat, smooth muscle fibers and blood vessels (17). HAMLs are associated with tuberous sclerosis in less than 10% of cases. Patients with HAML are usually asymptomatic, although some patients may present with vague abdominal discomfort or pain due to intratumoral hemorrhage. Due to the presence of fat within the lesion, HAMLs are hyperintense on T1-weighted sequences and loose signal following frequency selective fat-suppression sequences. HAMLs also show relative signal loss on opposed phase sequences as compared to in-phase sequences due to the presence of intravoxel fat. Enhancement of the soft tissue elements is also seen following gadolinium base contrast media. However, 50% of HAML do not have a substantial fat component. In such lipid-poor HAMLs, the enhancement of the soft tissue elements may be mistaken for arterial hyperenhancement (18) (Fig. 4). More recently, it has been shown that HAMLs can be indistinguishable from HCC on the basis of enhancement profiles on gadoxetic acid enhanced MRI of the noncirrhotic liver (19). High grade dysplastic nodules and early HCC may contain intratumoral foci of fat, which can also show loss of signal on opposed phase images. These can often be followed up with serial close imaging. In some cases, needle biopsy or surgical resection may be required to establish the diagnosis. Patients with nonalcoholic fatty liver disease (NAFLD) may develop steatohepatitic HCCs, which also contain macroscopic areas of fat and show arterial hyperenhancement, mimicking HAMLs. Hence, although HAMLs are considered benign entities, in areas where HCC and chronic liver disease is endemic, it may be prudent to biopsy fat containing liver lesions to exclude HCC, especially if they show threshold growth (20).
Figure 4. a–d.
Lipid-poor hepatic angiomyolipoma (HAML) in a 48-year-old woman without chronic liver disease. In-phase axial image (a) of the upper abdomen shows a well-defined, subcapsular lesion in segment VI in the right lobe of the liver (arrow). The lesion does not show loss of signal in out-of-phase image (b, arrow). Axial contrast-enhanced images (c, d) of upper abdomen show the corresponding lesion enhancing in the arterial phase (c, arrow) and seen as a defect in the hepatobiliary phase (d, arrow). The enhancement characteristics mimic hepatoma; however, there are no risk factors for HCC in this patient. The lesion was finally proven to be lipid-poor HAML by histopathological examination (not shown).
Intrahepatic splenosis
Intrahepatic splenosis is a rare condition due to auto-transplantation of splenic tissue within the liver. It is thought to be due to implantation of splenic tissue into the liver secondary to trauma or surgery and, rarely, due to hematogenous spread (21). Intrahepatic splenosis is often confused with a primary hepatic nodule on imaging. It shows hyperenhancement in the arterial phase and subsequent de-enhancement, which may mimic HCC, especially if the patient has a history of chronic liver disease or hepatitis (22). However, intrahepatic splenules mirror the signal intensity of the spleen on all sequences. Furthermore, HCCs will have a washout appearance. Hence, intrahepatic splenosis is a differential diagnosis that should be kept in mind when an arterially enhancing intrahepatic lesion is encountered in a patient with previous splenic trauma or splenectomy (Fig. 5). Nuclear scintigraphy using technetium-99m (99mTc) labeled heat denatured erythrocytes is a useful imaging test that can be used to confirm the diagnosis of intrahepatic splenosis (23).
Figure 5. a–c.
Intrahepatic splenosis in a 56-year-old man with previous history of splenic injury. T1-weighted axial image (a) of the upper abdomen shows a nodular T1 hypointense focus in the left lobe of the liver (arrow). Axial contrast-enhanced images (b, c) of the upper abdomen show the corresponding focus enhancing similar to spleen in portal venous phase (b, arrow) and seen as a defect in the hepatobiliary phase (c, arrow). There is a history of splenic injury and the lesion is similar to the remnant spleen (arrowhead) in all pulse sequences; these are key to the diagnosis.
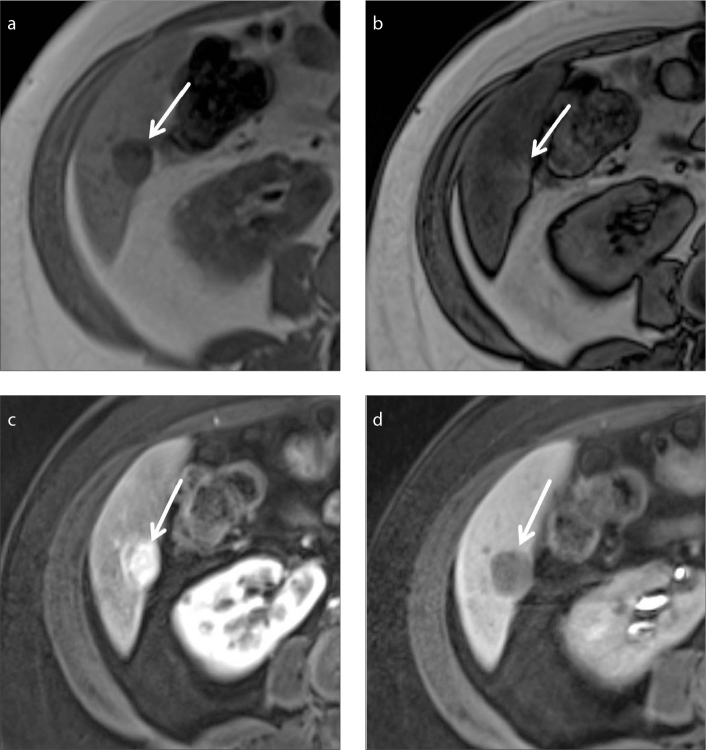
Benign intrahepatic lymphoid nodule
Benign intrahepatic lymphoid nodules are also known as pseudolymphomas. These are rare, benign lesions, characterized by proliferation of polyclonal lymphocytes forming follicles with an active germinal center. Patients with such lesions are usually asymptomatic and are incidentally detected on cross-sectional imaging. Only 51 cases have been reported in English literature (24). According to a review of 9 histologically proven pseudolymphomas by Yoshida et al. (25), pseudolymphomas show increased T2 signal, are hypointense on T1-weighted sequences and demonstrate restricted diffusion. These lesions also show arterial hyperenhancement with wash-out appearance. Furthermore, they present as a defect on the hepatobiliary phase on gadoxetic acid enhanced MRI. These imaging features can be very similar to HCC and hypervascular metastases (Fig. 6a–6c). Understandably, it is easy to mistake pseudolymphomas for a malignant liver tumor on imaging. However, even in the presence of characteristic features of arterial phase hyperenhancement and washout appearance, in patients with no history of chronic liver disease, the imaging diagnosis of HCC cannot be made and a biopsy is mandated. Histologically, pseudolymphomas consist of marked lymphoid follicles and germinal centers with no significant atypia (Fig. 6d) (24–26).
Figure 6. a–d.
Benign intrahepatic lymphoid nodule in a 51-year-old woman without chronic liver disease. Axial contrast-enhanced images (a–c) of the upper abdomen show the lesion (arrow) enhancing in the arterial phase (a), washing out in the portal venous phase (b) displaying a capsule appearance, and as a filling defect in the hepatobiliary phase (c). Based on the enhancement characteristics, possibility of malignant mass such as HCC was considered, but absence of risk factors caused a diagnostic dilemma; the patient opted for excision biopsy, which proved the lesion to be benign intrahepatic lymphoid nodule. Photomicrograph (d) (original magnification, ×40; hematoxylin-eosin [H-E] stain) higher power view shows reactive secondary lymphoid follicles containing reactive germinal centres (arrows).
Hepatocellular adenoma
Hepatocellular adenoma (HCA) is a benign liver tumor that predominantly occurs in young and middle-aged women with history of oral contraceptive intake. Inflammatory, hepatocyte nuclear factor-1α-mutated, β-catenin-mutated, and unclassified are the four subtypes of HCA. The imaging findings of HCA may vary according to the subtype and presence of complications such as hemorrhage. HCA characteristically shows mild T2 hyperintensity, arterial phase enhancement, washout in the venous phase, and appear as a defect in the hepatobiliary phase thus mimicking HCC (Fig. 7). Useful imaging findings of HCA that may help in differentiation from HCC include presence of large amounts of fat, a band of peripheral T2 hyperintensity (atoll sign) (Fig. 7a), and occurrence in a noncirrhotic liver (27, 28). With extracellular contrast agents HCA can be confused with FNH, as both are hyperenhancing benign lesions and occur in a similar population group; however, hepatobiliary contrast agents will help in differentiation, as HCA will appear hypointense to liver in the hepatobiliary phase, while FNH will appear hyper- or isointense to background liver (29) (Fig. 7d).
Figure 7. a–d.
Hepatocellular adenoma (HCA) in a 44-year-old woman with no risk factors for HCC. Axial T2-weighted image (a) of the upper abdomen shows a mild T2 hyperintense lesion in the right lobe of the liver with peripheral hyperintense rim (atoll sign) (a, arrow). Axial contrast-enhanced arterial phase image (b) of the upper abdomen shows a homogeneously enhancing lesion in the right lobe of the liver (arrow). Axial contrast-enhanced images (c, d) of the upper abdomen show the corresponding lesion washed out in venous phase (c, arrow) and hypointense to liver in the hepatobiliary phase (d, arrow), thus mimicking HCC. Note that the lesion does not retain contrast in the hepatobiliary phase, a key imaging finding that will differentiate HCA from FNH.
Mimickers of ICC
Sclerosing hemangioma
Sclerosed or sclerosing hemangioma is a rare variant and it develops as a result of extensive hyalinization of an existing hemangioma. The pathogenesis of sclerosing hemangiomas is not well studied but thought to be due to hemorrhage or thrombosis within the hemangioma, resulting in fibrosis and eventually leading to sclerosing hemangioma. Histologically, sclerosing hemangiomas are characterized by extensive fibrosis and hyalinization with narrowing and obliteration of the vascular spaces. The extent of sclerosis and hyalinization may vary, depending on the degree of fibrosis that has occurred (30). Sclerosing hemangiomas can show irregular rim enhancement and patchy central enhancement in the early phases of contrast-enhanced CT or MRI. The atypical enhancement pattern seen in sclerosing or sclerosed hemangiomata could be attributed to the heterogeneity of fibrosis and the degree of hyaline sclerosis occurring within the lesion. Patchy enhancement of the sclerosing hemangioma in the hepatic arterial phase can be mistaken for the arterial hyperenhancement seen in HCC. Imaging findings of sclerosing hemangioma that can be useful to differentiate it from HCC are capsular retraction, decrease in size over time, presence of transient hepatic attenuation difference, loss of previously seen regions of enhancement at follow-up and absence of washout in the delayed phase (31, 32). While capsular retraction and absence of washout in the delayed phase help in differentiating it from HCC, the downside is that it can be potentially confused with ICC (33) (Fig. 8). On T2-weighted MRI, sclerosing hemangiomas tend to show heterogeneously lower signal intensity than other hemangiomas. However, the signal intensity is still higher than expected in most malignancies (31).
Figure 8. a–c.
Sclerosing hemangioma in a 58-year-old woman without chronic liver disease. Axial T2-weighted fast spin-echo image of the upper abdomen (a) shows a mild T2 hyperintense lesion in the right lobe of the liver (arrow). Axial images of upper abdomen after administration of gadoterate meglumine show the corresponding region minimally enhancing in the arterial phase (b, arrow) and progressively enhancing in the delayed phase (c, arrow). The findings were inconsistent with a classic hemangioma, and the diagnosis of a malignant primary lesion such as ICC needed to be excluded.
Inflammatory pseudotumor
Inflammatory pseudotumor (IPT) of the liver is a rare benign lesion due to chronic inflammation and fibroblast proliferation. Patients usually present with symptoms of fever and abdominal pain (34). Infection and autoimmune diseases are associated with IPT of the liver (34). Few studies have reported that hepatopancreatobiliary autoimmune diseases, such as IgG4 sclerosing cholangitis can cause IPT of the liver (35). An increased incidence of IPT is encountered in patients with recurrent pyogenic cholangitis (36). IPT can be confused with malignant liver tumors and hepatic abscess on imaging (37). This is due to the varied enhancement pattern of IPT. IPTs have been described to show heterogeneous or peripheral enhancement in the arterial phase, progressive enhancement or washout appearance in the delayed phase (34, 37) (Fig. 9). As such, it is difficult to differentiate IPT of liver from more aggressive lesions like HCC and ICC on imaging alone, particularly in a patient with chronic liver disease. Typically, biopsy is necessary to obtain a definitive diagnosis (38).
Figure 9. a–c.
Inflammatory pseudotumor in a 70-year-old man with constitutional symptom and no risk factors for HCC. Axial contrast-enhanced image of the upper abdomen in arterial phase (a) shows a rim enhancing lesion in the right lobe of liver (arrow). Axial contrast-enhanced images (b, c) of upper abdomen show the corresponding lesion with progressive enhancement in portal venous (b, arrow) and delayed phase (c, arrow) images. Note minimal right pleural effusion and right basal atelectasis. Presence of constitutional symptoms and absence of risk factors were against the diagnosis of malignant liver lesions such as ICC and a close follow-up or biopsy was advised. The lesion regressed on follow-up studies (not shown) and presumed to be due to spontaneous regression of IPT.
Bile duct adenoma
Bile duct adenoma (BDA) is a rare benign focal liver lesion. It arises from the bile duct epithelium secondary to trauma or inflammation (39). The reported incidence of bile duct adenoma is 1.3% among benign primary liver tumors (40). BDA is detected incidentally during imaging, surgery or during autopsy. It is usually a solitary, subcapsular, solid lesion, less than 2 cm in size (39). On MRI it may appear hyper- or hypointense in T2-weighted image. It usually shows enhancement in the arterial and venous phases. Enhancement may be observed in delayed images, depending on the degree of fibrosis (41) (Fig. 10). BDAs also appear as a defect in the hepatobiliary phase on gadoxetic acid enhanced MRI and thus may be confused with HCC. However, the lack of diffusion restriction in BDA can potentially help in differentiating it from HCC (42). Histologically, BDA is characterized by inflammation, fibrosis, and proliferation of bile ductules usually reactive to focal injury and may be associated with chronic liver disease (39).
Figure 10. a–c.
Bile duct adenoma in a 49-year-old man. Axial contrast-enhanced images (a, b) of upper abdomen show rim enhancing subcapsular lesion in the arterial phase (a, arrow) and central enhancement of the lesion in the venous phase (b, arrow) in the right lobe of the liver. Resected liver segment (c) shows solitary, subcapsular lesion in right lobe of the liver (arrow). The lesion was proven to be a bile duct adenoma by histopathological examination (not shown).
Conclusion
Various benign conditions in the liver can show imaging features similar to HCC and ICC. The recognition of the characteristic MRI findings of various benign lesions is pertinent to avoid false-positive diagnosis of HCC or ICC and subsequent unnecessary invasive management. A careful imaging approach along with a review of clinical and histopathological findings is helpful in differentiating these lesions from HCC and ICC.
Main points.
Accurate characterization of benign mimics of HCC is important to avoid unnecessary biopsy or intervention, since HCC can be treated without histological confirmation when the characteristic imaging features are encountered in at risk patients.
Arterial phase hyperenhancement with washout in subsequent phases is characteristic of HCC and progressive enhancement is characteristic of cholangiocarcinoma. However, these enhancement characteristics are commonly encountered in nonmalignant lesions.
T2 signal intensity, diffusion-weighted imaging and hepatobiliary contrast uptake are useful adjunct imaging features that, when interpreted alongside with multiphasic enhanced imaging, aid in differentiating benign from malignant entities.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 2.Cogley JR, Miller FH. MR imaging of benign focal liver lesions. Radiol Clin North Am. 2014;52:657–682. doi: 10.1016/j.rcl.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Algarni AA, Alshuhri AH, Alonazi MM, Mourad MM, Bramhall SR. Focal liver lesions found incidentally. World J Hepatol. 2016;8:446–451. doi: 10.4254/wjh.v8.i9.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 5.O’Malley ME, Takayama Y, Sherman M. Outcome of small (10–20 mm) arterial phase-enhancing nodules seen on triphasic liver CT in patients with cirrhosis or chronic liver disease. Am J Gastroenterol. 2005;100:1523–1528. doi: 10.1111/j.1572-0241.2005.41814.x. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu A, Ito K, Koike S, Fujita T, Shimizu K, Matsunaga N. Cirrhosis or chronic hepatitis: evaluation of small (<or=2-cm) early-enhancing hepatic lesions with serial contrast-enhanced dynamic MR imaging. Radiology. 2003;226:550–555. doi: 10.1148/radiol.2262011967. [DOI] [PubMed] [Google Scholar]
- 7.Motosugi U, Ichikawa T, Sou H, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid–enhanced MR imaging. Radiology. 2010;256:151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Joo I, Lee JM. Atypical appearance of hepatocellular carcinoma and its mimickers: how to solve challenging cases using gadoxetic acid-enhanced liver magnetic resonance imaging. Korean J Radiol. 2019;20:1019–1041. doi: 10.3348/kjr.2018.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dioguardi Burgio M, Ronot M, Paulatto L, Terraz S, Vilgrain V, Brancatelli G. Avoiding pitfalls in the interpretation of gadoxetic acid-enhanced magnetic resonance imaging. Semin Ultrasound CT MR. 2016;37:561–572. doi: 10.1053/j.sult.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Kim TK, Lee E, Jang H-J. Imaging findings of mimickers of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:326–343. doi: 10.3350/cmh.2015.21.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganeshan D, Szklaruk J, Kundra V, Kaseb A, Rashid A, Elsayes KM. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 2014;202:544–552. doi: 10.2214/AJR.13.11117. [DOI] [PubMed] [Google Scholar]
- 12.Kitao A, Zen Y, Matsui O, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid–enhanced MR imaging–correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817–826. doi: 10.1148/radiol.10092214. [DOI] [PubMed] [Google Scholar]
- 13.Lee SA, Lee CH, Jung WY, et al. Paradoxical high signal intensity of hepatocellular carcinoma in the hepatobiliary phase of Gd-EOB-DTPA enhanced MRI: initial experience. Magn Reson Imaging. 2011;29:83–90. doi: 10.1016/j.mri.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin MD, Dobson JE, Sirlin CB, Lim BG, Stella DL. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics. 2011;31:1547–1568. doi: 10.1148/rg.316115528. [DOI] [PubMed] [Google Scholar]
- 15.Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 16.Hussain SM, Zondervan PE, Ijzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023–1036. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 17.Du S, Li Y, Mao Y, et al. Diagnosis and treatment of hepatic angiomyolipoma. Hepatobiliary Surg Nutr. 2012;1:19–24. doi: 10.3978/j.issn.2304-3881.2012.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Kim SY, Kim KW, et al. Hepatic angiomyolipoma with minimal fat, mimicking hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:330–335. doi: 10.3350/cmh.2012.18.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Kim SY, Kim KW, et al. Hepatic angiomyolipoma versus hepatocellular carcinoma in the noncirrhotic liver on gadoxetic acid-enhanced MRI: a diagnostic challenge. AJR Am J Roentgenol. 2016;207:562–570. doi: 10.2214/AJR.15.15602. [DOI] [PubMed] [Google Scholar]
- 20.Chang Z, Zhang JM, Ying JQ, Ge YP. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis. 2011;20:65–69. doi: 10.1007/s11749-010-0230-2. [DOI] [PubMed] [Google Scholar]
- 21.Kwok CM, Chen YT, Lin HT, et al. Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses. 2006;67:1330–1332. doi: 10.1016/j.mehy.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 22.Wang W-C, Li X-F, Yan Z-L, et al. Intrahepatic splenosis mimics hepatocellular carcinoma in a patient with chronic hepatitis B: A case report and literature review. Medicine (Baltimore) 2017;96:e8680. doi: 10.1097/MD.0000000000008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan I, Hopkins R, Lyburn I. Superior demonstration of splenosis by heat-denatured Tc-99m red blood cell scintigraphy compared with Tc-99m sulfur colloid scintigraphy. Clin Nucl Med. 2006;31:463–466. doi: 10.1097/01.rlu.0000226907.36840.b3. [DOI] [PubMed] [Google Scholar]
- 24.Kwon YK, Jha RC, Etesami K, Fishbein TM, Ozdemirli M, Desai CS. Pseudolymphoma (reactive lymphoid hyperplasia) of the liver: A clinical challenge. World J Hepatol. 2015;7:2696–2702. doi: 10.4254/wjh.v7.i26.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida K, Kobayashi S, Matsui O, et al. Hepatic pseudolymphoma: imaging-pathologic correlation with special reference to hemodynamic analysis. Abdom Imaging. 2013;38:1277–1285. doi: 10.1007/s00261-013-0016-6. [DOI] [PubMed] [Google Scholar]
- 26.Yang CT, Liu KL, Lin MC, Yuan RH. Pseudolymphoma of the liver: Report of a case and review of the literature. Asian J Surg. 2017;40:74–80. doi: 10.1016/j.asjsur.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 27.van Aalten SM, Thomeer MG, Terkivatan T, et al. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172–181. doi: 10.1148/radiol.11110023. [DOI] [PubMed] [Google Scholar]
- 28.Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182–1191. doi: 10.1002/hep.24147. [DOI] [PubMed] [Google Scholar]
- 29.Roux M, Pigneur F, Baranes L, et al. Correction to: Differentiating focal nodular hyperplasia from hepatocellular adenoma: Is hepatobiliary phase MRI (HBP-MRI) using linear gadolinium chelates always useful? Abdom Radiol (NY) 2018;43:2212. doi: 10.1007/s00261-017-1423-x. [DOI] [PubMed] [Google Scholar]
- 30.Song JS, Kim YN, Moon WS. A sclerosing hemangioma of the liver. Clin Mol Hepatol. 2013;19:426–430. doi: 10.3350/cmh.2013.19.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle DJ, Khalili K, Guindi M, Atri M. Imaging features of sclerosed hemangioma. AJR Am J Roentgenol. 2007;189:67–72. doi: 10.2214/AJR.06.1076. [DOI] [PubMed] [Google Scholar]
- 32.Ridge CA, Shia J, Gerst SR, Do RK. Sclerosed hemangioma of the liver: concordance of MRI features with histologic characteristics. J Magn Reson Imaging. 2014;39:812–818. doi: 10.1002/jmri.24228. [DOI] [PubMed] [Google Scholar]
- 33.Andeen NK, Bhargava P, Park JO, Moshiri M, Westerhoff M. Cavernous hemangioma with extensive sclerosis masquerading as intrahepatic cholangiocarcinoma—A pathologist’s perspective. Radiol Case Rep. 2014;9:937. doi: 10.2484/rcr.v9i2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001;48:1118–1123. [PubMed] [Google Scholar]
- 35.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon KH, Ha HK, Lee JS, et al. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT-histopathologic correlation. Radiology. 1999;211:373–379. doi: 10.1148/radiology.211.2.r99ma36373. [DOI] [PubMed] [Google Scholar]
- 37.Park KS, Jang BK, Chung WJ, et al. Inflammatory pseudotumor of liver--a clinical review of 15 cases. Korean J Hepatol. 2006;12:429–438. [PubMed] [Google Scholar]
- 38.Jeong JY, Sohn JH, Kim TY, et al. Hepatic inflammatory pseudotumor misinterpreted as hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:239–244. doi: 10.3350/cmh.2012.18.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1998;12:708–715. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Edmondson HA. Tumors of the liver and intrahepatic bile ducts. Washington DC: Armed Forces Institute of Pathology; 1958. Atlas of tumor pathology, fascicle 25; pp. 19–29. [Google Scholar]
- 41.Song KD, Jeong WK. Benign nodules mimicking hepatocellular carcinoma on gadoxetic acid-enhanced liver MRI. Clin Mol Hepatol. 2015;21:187–191. doi: 10.3350/cmh.2015.21.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–775. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]