Abstract
Flow modification has caused a paradigm shift in the management of intracranial aneurysms. Since the FDA approval of the Pipeline Embolization Device (Medtronic, Dublin, Ireland) in 2011, it has grown to become the modality of choice for a range of carefully selected lesions, previously not amenable to conventional endovascular techniques. While the vast majority of flow-diverting stents operate from within the parent artery (ie, endoluminal stents), providing a scaffold for endothelial cells growth at the aneurysmal neck while inducing intra-aneurysmal thrombosis, a smaller subset of endosaccular flow disruptors act from within the lesions themselves. To date, these devices have been used mostly in Europe, while only utilized on a trial basis in North America. To the best of our knowledge, there has been no dedicated review of these devices. We therefore sought to present a comprehensive review of currently available endosaccular flow disruptors along with high-resolution schematics, presented with up-to-date available literature discussing their technical indications, procedural safety, and reported outcomes.
Keywords: Aneurysm, Endovascular, Flow disruption, Endosaccular device, Embolization
ABBREVIATIONS
- CE
Conformité Européenne
- CLARYS
Clinical Assessment of WEB Device in Ruptured AneurYSms
- ISAT
International Subarachnoid Aneurysm trial
- ISUIA
International Study of Unruptured Intracranial Aneurysm
- MCA
middle cerebral artery
- MED
Medina Embolic Device
- WEB
Woven EndoBridge
- WEB-IT
WEB® Intrasaccular Therapy
Since the emergence of the Guglielmi detachable coil in the late 1980s and early 1990s, treatment of intracranial aneurysms has entered an endovascular era which has served as a crucial adjunct to the gold standard of microsurgical clipping. The International Subarachnoid Aneurysm trial (ISAT) and International Study of Unruptured Intracranial Aneurysms (ISUIA) have established the exponential increase in utility of endovascular procedures for aneurysms treatment. Results of the ISAT showed that 1-yr disability or death occurred in 30.9% of patients treated via clipping vs only in 23.5% of patients in the coiling group.1 Likewise, in patients with no prior history of subarachnoid hemorrhage, the ISUIA reported overall 1-yr morbidity and mortality of 12.6% in clipping vs 9.8 for endovascular coiling.2 Since then, continuous research and development have led to the generation of more innovative coil designs with improved results, such as those seen with hydrophilic coils. Adjunctive to this was the increased use of balloon and stent-assisted coiling, enabling higher packing densities while reducing coil herniation into the parent artery. Nevertheless, these innovations left room for improvement in the management of certain aneurysms subgroups, such as nonsaccular aneurysms, as well as saccular aneurysms with wide necks, and dome-to-neck ratios under 2, all of which tend to recanalize more often, with rates up to 18.2% in some series.3,4 In an attempt to address this challenge, the neurovascular community has developed a number of flow-diverting stents, most of which are endoluminal. While endoluminal flow diverters function from within the parent artery by providing a scaffold for endothelial cell growth at the neck of the aneurysm and induction of intra-aneurysmal thrombosis,5 endosaccular devices mimic the endoluminal devices but within the aneurysmal sac itself.6 Our focus will be on endosaccular flow disruptors, reviewing the devices available
and up to date, along with the available literature discussing their procedural safety and treatment outcomes.
ADVENT OF FLOW DIVERSION AND DISRUPTION
Aneurysmal flow stagnation secondary to stent use was first noted related to stent-assisted coiling, with numerous studies describing accelerated thrombosis specifically felt to be related to the altered flow. This phenomenon of flow stagnation is 1 of 2 central tenets of flow diversion, the other being neointimal proliferation and endothelialization over the aneurysm neck beginning at approximately 6 mo postprocedurally. The latter phenomenon is a unique advantage of flow diversion, which theoretically eliminates the possibility of recurrence that exists with coiling. While endoluminal flow diversion can be combined with coiling safely, a novel competing technology is that of endosaccular disruption, wherein the device occupies the lesion rather than the vessel thus promoting endothelialization with simultaneous mechanical occlusion. Moreover, lack of presence in the parent vessel is felt to imply that dual antiplatelet therapy is not required. This represents a substantial theoretical benefit because of potential issues such as medications interactions, safety concerns related to medical comorbidities, and prescription adherence, thus increasing ischemic stroke risk from in-stent thrombosis as well as pharmacological hemorrhagic stroke risk.
Several endosaccular flow disruptors have been developed, including the Woven EndoBridge (WEB; Microvention, Aliso Viejo, California), which was introduced in Europe in 2011, and Medina (Medtronic, Dublin, Ireland), Artisse (formerly LUNA; Medtronic),7,8 and the Contour and Cerus devices (Cerus Endovascular, Fremont, California). While not yet FDA-approved, these devices have received the Conformité Européenne (CE) mark, which signifies adherence to the health and safety standards of the European Economic Area. These standards are divided into implantable devices, external devices, and in vitro diagnostic devices each with separate directives.
Flow disruption technology has relatively scant evidence reporting long-term outcomes, although available data suggest that complications beyond 6 mo postprocedure are rare. Meanwhile, device technology is evolving rapidly, encompassing advances in control and precision of device placement, as well as growing operator experience and skill, all of which contributes to greater procedural safety. To our knowledge, this represents the most comprehensive review of technical specifications and representative outcome data for endosaccular flow disruptors to inform this fast-moving field.
AVAILABLE FLOW DISRUPTORS
Woven EndoBridge
The WEB (Microvention) device is designed to be placed completely within the aneurysm sac and span the ostium, where it disrupts local flow.7 It is composed predominantly of a braided nitinol wire that holds a globular shape (Figure 1), which is intended to be used as a stand-alone therapy, where its properties and intrasaccular positioning should obviate antiplatelet prophylaxis. The original version consisted of a double layer; however, all second-generation configurations employ a single layer only (Table 1). This device has been comparatively well studied, with 8 prospective series in patients mostly harboring bifurcation aneurysms showing short- to mid-term occlusion rates of approximately 60% and generally low morbidity/mortality (Table 2).9-17
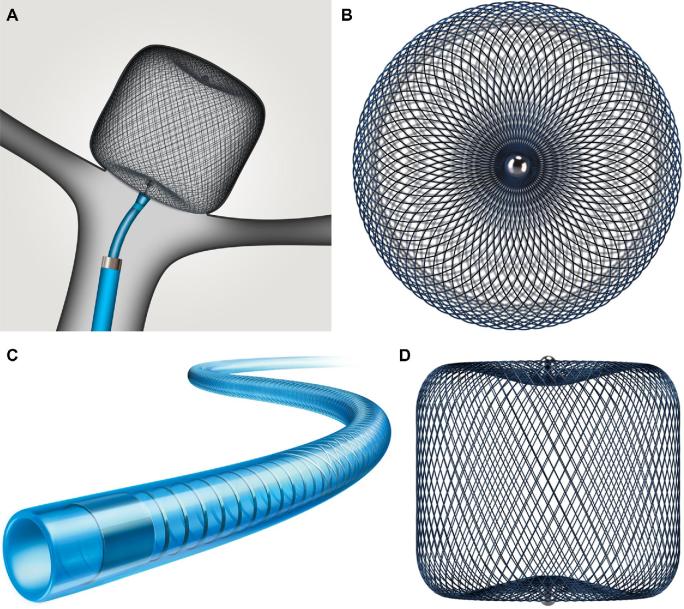
FIGURE 1.
Woven EndoBridge (WEB; Microvention) device with A, deployed view, and B, overhead view showing lowest porosity in highest-flow areas. The device is delivered with the C, proprietary VIA catheter, which possesses internal coils for stability and a Polytetrafluoroethylene liner. The D, high-density braiding has conceived for both neck and dome sealing.
TABLE 1.
Main Features of Intrasaccular Flow-Disrupting Devices
| Device | Dimensions, mm | Structure | Coveragea, % | Porosity, % | Pore size μm | Deployment | Resheathable | Comments |
|---|---|---|---|---|---|---|---|---|
| WEB | Diameter 3–11 Height 2–9 | Available in 2 configurations (SL, SLS), both with EV | 60 | Not reported | Not reported | Electrothermal detachment; delivered via a ≥0.17-inch microcatheter | Yes, completely | Proximal and distal radiopaque markers |
| Medina | Not reported | 3D coil with “petal” filamentsb | Not reported | Not reported | Not reported | Mechanical detachment | Yes, completely | Hybrid flow disruption device and detachable coil with filaments that have shape memory |
| Artisse (LUNA) | Not reported | Not reported | Not reported | Not reported | Not reported | Mechanical detachment | Not reported | Oval shape designed to treat small aneurysms |
| Contour | Not reported | Dual-layer radiopaque shape-memory mesh | Not reported | Not reported | Not reported | Electrolytical detachment | Yes, completely | Flat disc which after deployment, assumes a tulip-like configuration |
| Cerus Intrasaccular Stent | Not reported | Dual-layer radiopaque shape-memory mesh | Not reported | Not reported | Not reported | Electrolytical detachment | Yes, completely | Can be coiled through per operator preference |
EV, enhanced visualization; SL, single layer (standard; barrel shaped); SLS, single layer sphere; WEB, Woven EndoBridge.
At aneurysm outflow.
Available in framing and filling styles (filler softer and designed to fill internal space after framing device has been deployed to provide a suitable basket).
TABLE 2.
Outcomes From Key Trials of Intrasaccular Flow-Disrupting Devices
| Device | Study | Description | Patients, N | Aneurysms, N | Indication(s) | Occlusion rate, % | Morbidity, % | Mortality, % |
|---|---|---|---|---|---|---|---|---|
| WEB | Pierot et al9 | WEBCAST: prospective, multicenter, European trial of WEB DL | 51 | 51 | Predominantly unruptured, wide-neck (≥4 mm); bifurcation aneurysms, mean neck diameter 5.6 mm | 56.1a(6 mo) | 2.0 (1 mo) | 0 (1 mo) |
| Pierot et al11 | French observatory: prospective, multicenter, French GCP study of WEB-DL (30 patients/31 aneurysms) and WEB SL/SLS (32 patients with 32 aneurysms) | 62 | 63 | Predominantly unruptured, wide-neck (≥4 mm) bifurcation aneurysms | 51.7b (1 yr) | 3.2 (1 mo) | 0 (1 mo) | |
| Pierot et al10 | WEBCAST 2: prospective, multicenter, European Registry study of WEB SL/SLS and EV | 55 | 55 | Predominantly unruptured, wide-neck (≥4 mm) bifurcation aneurysms, mean neck diameter 4.6 mm | 54.0 | 1.8 (1 mo) | 0 (1 mo) | |
| Papagiannaki et al12 | Retrospective, multicenter, French cohort study of WEB DL | 83 | 85 | Predominantly unruptured, wide-neck (>4 mm) aneurysms, mean neck diameter 5.6 mm | 56.9 (mean follow-up, 5.3 mo)c | 1.3 | 0 | |
| Clajus et al13 | Retrospective, single-center cohort study of WEB DL (49 devices), WEB SL (44 devices), and WEB SLS (17 devices) | 108 | 114d | Predominantly wide-neck (dome:neck <2 or neck ≥4 mm), anterior aneurysms, 47 patients with SAH | 57.8 (mean follow-up, 13.4 mo)e | 5.3f | 8.5f | |
| Mine et al14 | Retrospective, multicenter cohort study of WEB DL | 48 | 49 | Predominantly unruptured, wide-neck, mostly MCA bifurcation aneurysms, mean neck diameter 4.9 mm | 74.3g (12 mo)72i (mean follow-up, 39 mo) | 6.2h | 0 | |
| Caroff et al15 | Retrospective, multicenter, European cohort study of WEB SL | 90 | 98 | Predominantly wide-neck, bifurcation aneurysms, 66% unruptured, mean neck 5.2 mm, including 11 patients requiring planned or unplanned additional treatment | 65j (average follow-up, 3.3 mo) | 2.2k (average follow-up, 3.8 mo) | 1.1k (average follow-up, 3.8 mo) | |
| Lawson et al16 | Retrospective, multicenter, UK registry safety study | 109 | 109 | Predominantly unruptured, wide-neck, saccular aneurysms, 45% bifurcation, mean neck diameter 6.18 mm | – | 0 | 0 | |
| Popielski et al22 | Retrospective, 2-center, European cohort study of WEB SL and WEB SLS | 101 | 102 | Predominantly wide-neck and fundus width between 3 and 10 mm MCA and anterior communicating aneurysm (AComA) aneurysms, 63.7% unruptured, median neck diameter 4.6 mm | 80.7% (3 mo)l77.6% (12 mo)m | 4 | 1 | |
| van Rooij et al21 |
Retrospective, single-center European cohort | 100 | 100 | Predominantly wide-neck (66% ≥4 mm) AComA (44%) and posterior communicating aneurysm (22%) aneurysms, all ruptured | 96% (at 3 mo)n | 3 | 1 | |
| van Rooij et al17 | Retrospective, single-center European cohort | 40 | 46 | Predominantly ruptured (54%) very small AcomA and MCA aneurysms (height/dome ratio ≤0.5) | 72% (at 3 mo)o | 4 | 1 | |
| Medina | Sourour et al28 | Retrospective, single-center cohort study | 12 | 13 | Predominantly unruptured, wide-neck (dome:neck <2 or neck diameter ≥4 mm) aneurysms | 83p (mean follow-up, 5.2 mo) | 0? | 0? |
| Aguilar Perez et al26 | Retrospective, single-center cohort study | 15 | 16 | Predominantly unruptured aneurysms with a fundus diameter ≥5 mm | 81q (mean follow-up, 1.7 mo) | 0? | 0? | |
| Bhogal et. al29 | Retrospective, single-center cohort study | 13 | 14 | Predominantly unruptured spherical or ovoid aneurysms >5 mm | 71r (mean follow-up, 3.2 mo) | 23 | 0 | |
| Artisse (LUNA) | Piotin et al32 | Prospective, multicenter European cohort | 63 | 64 | Predominantly unruptured bifurcation or terminal aneurysms, mean neck diameter 3.8 mm | 78.0%s (12 mo)79.2%t (36 mo) | 0 (12 mo)1.6 (36 mo) | 1.6 |
EV, enhanced visualization; MCA, middle cerebral artery; SAH, subarachnoid hemorrhage; SL, single layer (standard; barrel shaped); SLS, single layer sphere; WEB, Woven EndoBridge; WEBCAST, WEB Clinical Assessment of Intrasaccular Aneurysm Therapy.
aForty-one patients (29.3% had neck remnants).
bFifty-eight patients (27.6% had neck remnants).
cSixty-five aneurysms (35.4% had neck remnants).
dOne hundred ten devices successfully deployed.
eNinety aneurysms (17.8% neck remnants).
fNinety-four patients.
gThirty-five aneurysms (including 2 retreated).
hForty-eight patients.
iTwenty-five aneurysms (including 2 retreated).
jSixty-nine patients.
kFifty-two patients.
lSeventy-eight patients.
mForty-nine patients.
nSeventy-four patients (23% had neck remnants).
oThirty-nine aneurysms (23% had neck remnants).
pThirteen aneurysms.
qEleven aneurysms (45% had neck remnants).
rFourteen aneurysms (45% had neck remnants).
sFifty-nine patients.
tFifty-three patients.
These prospective studies evaluated either or both the original double-layer and the second-generation single-layer versions, and outcomes do not appear to discriminate between them. There are multiple other smaller studies reporting short-term outcomes, which do not add new revelations. The largest series to date with longest follow-up reported the cumulative anatomical and clinical data of the 3 landmark prospective studies (WEBCAST-1, French Observatory, and WEBCAST-2), comprising 168 patients with 169 aneurysms, with reported treatment feasibility of 96.4% and 1-mo morbidity and mortality rates of 1.2 and 0%, respectively. The 1-yr treatment success (defined as complete aneurysmal occlusion or neck remnant) was achieved in 78% of patients. This was further corroborated by a recent systematic review comprising 940 patients who were harboring 962 aneurysms, reporting that the WEB device has a potentially large learning curve, and while the device seemed to demonstrate acceptable mid-term occlusion rates (81%), mortality and procedural-related complication rates were not negligible, warranting further comparisons with other treatment options.18 The ability to draw definite conclusions was hindered by lack of long-term follow-up. The WEB® Intrasaccular Therapy study (WEB-IT; NCT02191618) is a US investigational device exemption study evaluating the WEB device in wide-neck bifurcation aneurysms; this study is well underway, with a primary outcome completion due in March 2021. Preliminary findings showed a high level of procedural safety and technical success.19
As is the case with most flow diverters, the majority of treated aneurysms are unruptured. Given delayed occlusion following flow diversion, the potential risk of aneurysmal rebleeding following rupture necessitates a rapid securing of the aneurysm. Moreover, with dual antiplatelet therapy the potential need for additional invasive procedures over the course of subarachnoid hemorrhage management (eg, ventriculostomy insertions) could carry a theoretical higher risk of complications. However, with the WEB device being completely intrasaccular, a putative advantage is the lack of mandatory dual antiplatelet prophylaxis, which should mitigate concerns surrounding hemorrhagic complication. While still scant, there is growing evidence supporting the use of WEB in ruptured aneurysms.15,20-22 The biggest cohort of 100 patients was reported by van Rooij et al.21 in which the authors reported adequate occlusion (complete occlusion or neck remnant) in 96% of patients (71 of 74). However, thromboembolic complication rates appeared to be high (9%), which is consistent with previous meta-analysis23 and suggests the need for further optimization of antiplatelet regimens. Moreover, the study was limited by a short follow-up period (3 mo). The CLARYS (Clinical Assessment of WEB Device in Ruptured AneurYSms; NCT02687607) is an ongoing prospective trial currently in France, with a target follow-up of 12 mo, evaluating the safety and efficacy of WEB in patients with ruptured aneurysms. The primary outcome is rebleeding rates at 1 mo.
It is also essential that operators know what can be done in the context of recurrences. In the available literature, a variety of treatment options have been utilized to retreat recurrences after initial treatment with the WEB device. Since the WEB device is placed within the aneurysmal sac, the parent and branch vessels are unimpeded and can be accessed for any further retreatment or subsequent deployment of adjunctive devices during retreatment.19 The WEBCAST, French Observatory, and WEBCAST 2 studies report a cumulative retreatment rate of 6.9%. The breakdown of retreatment approaches is as follows: 4 aneurysms with stents and coils, 4 aneurysms with flow diverters, 1 aneurysm with a stent only, 1 aneurysm with additional WEB and stent, and 1 aneurysm with additional WEB.10,24 Concomitant flow diversion at the time of initial treatment way also be done at the cost of requiring dual antiplatelet therapy.25 With the available data and our experience, we feel that WEB is best suited for the classical wide-necked and bifurcation aneurysms for which it was initially indicated. WEB, because of its compact nature and variable sizes, may be better suited for distal applications, such as the middle cerebral artery (MCA) bifurcation, relative to the other devices discussed. In the largest study to date, the Cumulative WEB good clinical practice (GCP) Study of 169 aneurysms, the MCA predominated at 50.9% of all lesions.10
Medina Embolic Device
The Medina (Medtronic) is a CE Mark-approved hybrid flow disruptor and coil system comprising a 3-dimensional, layered structure made from a radiopaque shape-set core wire, and shape-memory alloy filaments that form a self-expanding mesh (Figures 2A and 2B). The mesh resembles multiple “petals” that provide flow diversion; these lie along the axis of the core wire, and the device assumes a spherical shape upon deployment. This device is inserted by using a 0.021-inch microcatheter and can be resheathed and redeployed in a similar manner to standard coils (Table 1).26 The smallest Medina currently available is 5 mm in diameter, limiting its use to aneurysms of at least this size.27
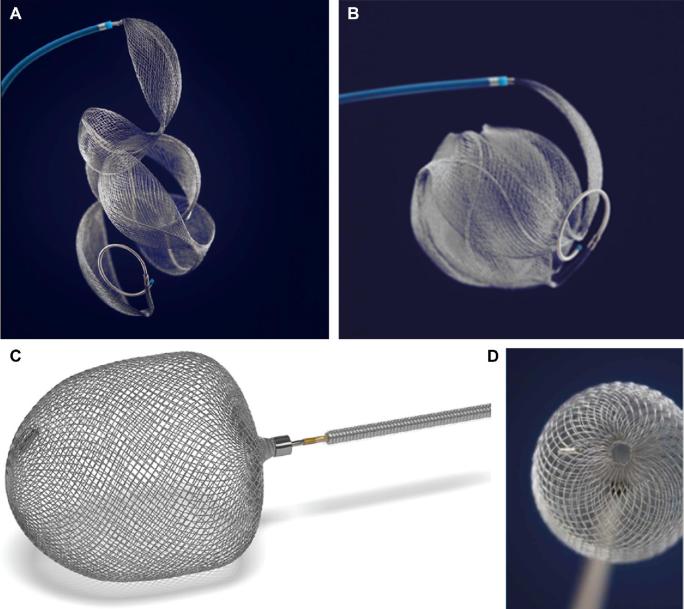
FIGURE 2.
The Medina (Medtronic) device forms A, a mesh scaffold of nitinol-platinum petals at the aneurysm neck by B, self-orienting along the axis of the core wire, whereas Artisse (Medtronic) deploys as a semi-fixed construct which may be C, flared or D, spheroid in nature.
Preliminary clinical results from a small series of 15 patients with wide-neck aneurysms are mixed and difficult to interpret because follow-up was relatively short in most cases, and in several patients, the Medina system was intended from the outset to be the first stage of a complex treatment strategy involving other implants (eg, endoluminal flow diverters or bifurcation stents). A small study (N = 12) with slightly longer follow-up found 83% complete occlusion rate at 6 mo (Table 2), but 85% of patients had adjunctive standard coiling, and aneurysms were heterogeneous, making the contribution of the Medina uncertain.28
Likewise, another small series study reported similar findings, with no short-term complications.29 Whether the Medina may have a role alongside endoluminal devices in irregularly shaped aneurysms in achieving a synergistic occlusive effect and minimizing the overall number of devices used will be interesting to establish.26 The same authors recently published their 25 patients series experience of patients treated only with a combination of Medina Embolic Device (MED) and intraluminal flow diversion, either sequentially (N = 9) or simultaneously (N = 16), using a jailing technique. Angiographic follow-up (mean = 9 mo) was available in 19 patients, with adequate occlusion achieved in 89% of cases, 3 of which had a neck remnant, and all were in the sequential group. The authors recommended this technique for giant aneurysms, nonspherical aneurysms carrying a high risk of neck remnant after MED-only treatment, and partially thrombosed aneurysms.26
In view of the available evidence, Medina is also well suited to wide-necked and bifurcation aneurysms. As opposed to WEB, which can be nimbly manoeuvred distally, Medina is probably best applied to the cavernous, supraclinoid, and paraopthlamic aneurysms. Furthermore, Medina could potentially be favored for larger aneurysms, whereas this is a downside of limited WEB sizes. The recent Karolinska experience suggests that Medina monotherapy is currently insufficient, and thus concomitant coiling or multiple devices may be required.30 Medina allows for this, which is also potentially relevant in the setting of recurrence.
Artisse
The Artisse (formerly called LUNA; Medtronic) is a self-expanding braided implant made from a double layer of nitinol wire mesh secured at proximal and distal ends and clearly marked with radiopaque platinum markers (Figures 2C and 2D and Table 1). It is delivered via a standard 027 microcatheter or larger.30 Similar to the WEB, it is intended to obviate dual antiplatelet therapy.7 A shape-memory alloy called thin-film nitinol endoluminal component is also in the early stages of being evaluated for improving the elasticity, with the aim of improving deployment in tortuous vessels and minimizing complications.31
The current model possesses ovoid and flared configurations; however, it is currently subject to re-prototyping. The long-term results of the European LUNA aneurysm embolization system trial were recently published,32 representing the largest data set available regarding the safety, efficacy, and procedural outcomes of this device, which are comparable but slightly less favorable than WEB (Table 2).
By further contrast, the profile of Artisse/Luna appears to permit safe deployment into sidewall lesions and small-necked bifurcation aneurysms such as anterior communicating. It remains to be seen if the same assertion can be made for vertebrobasilar sidewall lesions, but this is a natural extension of current successes.
Contour Neurovascular System
The Contour (Cerus Endovascular) is an intrasaccular flow diverter system, which is currently undergoing study in the EU with the aim of CE Mark approval. The device is constructed from a dual-layer radiopaque shape-memory mesh. While in fully unconstrained configuration, it has a flat disc-like shape. After deployment, the device assumes a tulip-like configuration, conforming to the wall of the lower hemisphere of the aneurysm and across the neck opening (Figure 3).
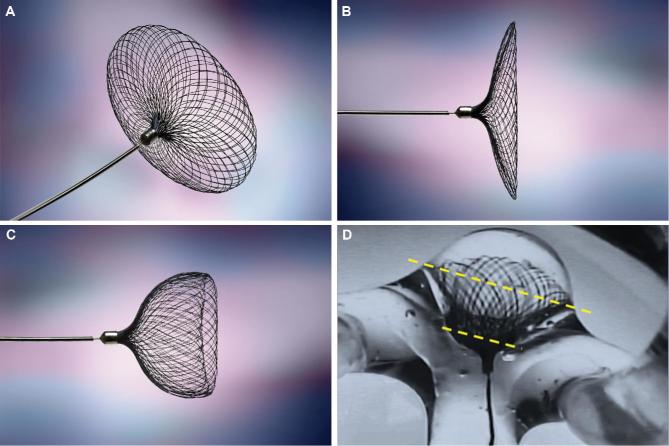
FIGURE 3.
A–C, Contour acts as both a neck and intrasaccular flow disruptor, the former of which is designed to promote neointimal growth without an intraluminal stabilizing component, and which D, aligns to the equatorial plane of the aneurysm.
The device is intentionally oversized to the neck and largest measured equatorial diameter of the aneurysm. The device is deployed through an 027 microcatheter and advanced with a pusher wire, and it exhibits controlled deployment as it “blooms” from the catheter tip. It can be reloaded and deployed a number of times, permitting an operator reposition across the neck of the aneurysm. The Contour is designed to seat across the neck with the marker position below the neck in the parent artery. The shape and configuration of the base of the device is designed to reconstruct the natural carina at the bifurcation. Hemodynamic flow and blood pressure at the base of the device transmits through the device and theoretically aids with stabilization within the lower hemisphere of the aneurysm. As the device is oversized to the largest-measured aneurysm diameter, it is constrained from opening further from any pressure applied at the device base. Once optimally positioned, it can be easily detached electrolytically by using any commercially available power supply and leads. The 6-mo and 12-mo data currently collected in an ongoing pilot study show promising results, with satisfactory operator feedback in regard to safety profile and ease of use. All aneurysms treated with the Contour have been unruptured aneurysms; however, as experience and understanding of the device grows, it is anticipated it will be able to treat some ruptured aneurysms.
Cerus Intrasaccular Stent
The design of the Cerus Intrasaccular Stent (Cerus Endovascular) derives from Contour, with the major difference being a radiopaque memory wire that has a 50% larger diameter and approximately 30 to 40% of the number of wires in its double-layer mesh construction compared to Contour. This is mainly to allow access through the mesh or between the mesh and aneurysm wall by a coiling microcatheter (Figure 4). Proceeding through or around the mesh is largely dictated by the size and shape of aneurysm and the corresponding device selected. The more the device is oversized to the aneurysm, the more the mesh at the neck of the device is constrained, and the operator at that point would access the aneurysm sac between the device mesh and aneurysm wall. In both animal studies and a number of clinical compassionate cases, either approach has proven successful.
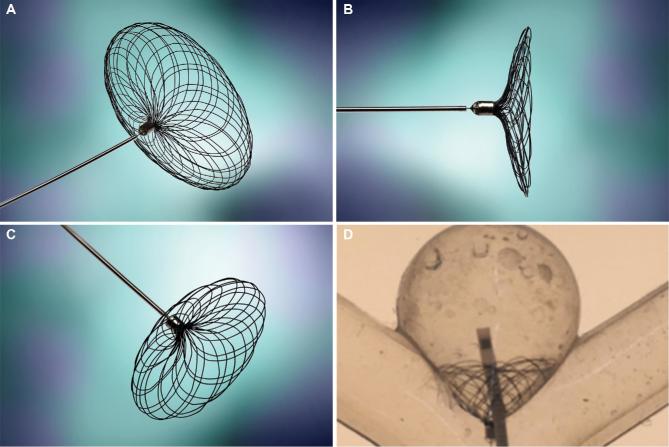
FIGURE 4.
Cerus Intrasaccular Stent A–C, is designed to treat a wider range of aneurysm morphologies, requiring only neck sizing without a proprietary cathether. With a smaller intrasaccular profile, it permits D, adjunctive same-session coiling.
Unlike Contour, this device is only oversized to the neck opening of the aneurysm to provide coverage across the aneurysm neck and prevent device movement down into the parent artery as the aneurysm is being filled with coils. The coil mass within the aneurysm sac prevents the upward movement of the device into the sac of the aneurysm. Though the mesh density of this device is less than Contour, it also affords a degree of flow disruption at its neck. It is believed that this will serve to attenuate the water-hammer effect on the coil mass at the bifurcation, minimizing compaction and recurrence over time. Being completely intrasaccular, this device will serve to treat ruptured and unruptured aneurysms, differentiating it from other coil adjunctive devices on the market, which all have a significant parent vessel stabilizing component, requiring dual antiplatelet, and limiting their utility in treating ruptured aneurysms. Moreover, they do not inherently afford flow disruption properties. Similar to the Contour, it is detached electrolytically once the final coil is deployed in the aneurysmal sac.
While the Cerus systems do not have enough human data for a confident assertion, compassionate cases show promise at the internal carotid artery bifurcation and basilar apex. While countless flow modifiers and coil-support systems have entered the market for the basilar apex, the Contour and the Cerus Intrasaccular Stent may offer a nonendoluminal option focused on this region for unruptured and ruptured cases, respectively.
CONCLUSION
The neurointerventional community has rapidly entered an era of flow diversion and disruption, with the latter being looked to for its independence of dual antiplatelet therapy. This may be a boon particularly in ruptured aneurysms, and safety in unruptured lesions is promising. Limited data likely reflect a learning curve for some devices, and outcomes are improving with experience. Further studies are needed to relate technical specifications to device performance and outcomes, and hence optimize usage, particularly for the most challenging aneurysm presentations.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
The authors provide a review of endosaccular devices that have been developed primarily for the treatment of unruptured wide-neck and bifurcation aneurysms. These devices are of interest, as they are designed to be wholly intra-aneurysmal, avoiding the parent artery and obviating the need for dual anti-platelet therapy. The WEB device is the best studied of the 5 devices and, as the authors explain, is currently part of an IDE study in the US and has been available in Europe since 2011. The other devices are currently being studied in Europe. While it is understood that little is known about these devices, especially long-term, the authors provide a thoughtful discussion regarding the details of each device. As the devices are used more frequently, we will learn more about efficacy, how recurrences are treated, and whether the devices lend themselves to reaccessing the aneurysm to treat recurrences. We know little about which aneurysms are best suited for each device, but if at some point many or all of these devices are commercially available, a very nuanced approach will be required to choose the right device for the right aneurysm. It is unlikely that just 1 of these devices will work for all aneurysms.
Mark R. Harrigan
Birmingham, Alabama
REFERENCES
- 1. Molyneux AJ, Kerr RS, Yu LM et al.. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. [DOI] [PubMed] [Google Scholar]
- 2. Wiebers DO, Whisnant JP, Huston J 3rd et al.. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. [DOI] [PubMed] [Google Scholar]
- 3. Crobeddu E, Lanzino G, Kallmes DF, Cloft HJ. Review of 2 decades of aneurysm-recurrence literature, part 1: reducing recurrence after endovascular coiling. AJNR Am J Neuroradiol. 2013;34(2):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crobeddu E, Lanzino G, Kallmes DF, Cloft HJ. Review of 2 decades of aneurysm-recurrence literature, part 2: managing recurrence after endovascular coiling. AJNR Am J Neuroradiol. 2013;34(3):481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014;270(2):394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding YH, Lewis DA, Kadirvel R, Dai D, Kallmes DF. The Woven EndoBridge: a new aneurysm occlusion device. AJNR Am J Neuroradiol. 2011;32(3):607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munich SA, Chen M. Endovascular advances in the treatment of cerebral aneurysms: an overview of the development of new neuroendovascular techniques and technology for the treatment of cerebral aneurysms. Endovasc Today. 2017;2(16):66–70. [Google Scholar]
- 8. Rajah G, Narayanan S, Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus. 2017;42(6):E2. [DOI] [PubMed] [Google Scholar]
- 9. Pierot L, Costalat V, Moret J et al.. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg. 2016;124(5):1250–1256. [DOI] [PubMed] [Google Scholar]
- 10. Pierot L, Gubucz I, Buhk JH et al.. Safety and efficacy of aneurysm treatment with the WEB: results of the WEBCAST 2 study. AJNR Am J Neuroradiol. 2017;38(6):1151–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pierot L, Moret J, Turjman F et al.. WEB Treatment of intracranial aneurysms: clinical and anatomic results in the French observatory. AJNR Am J Neuroradiol. 2016;37(4):655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papagiannaki C, Spelle L, Januel A-C et al.. WEB intrasaccular flow disruptor—prospective, multicenter experience in 83 patients with 85 aneurysms. AJNR Am J Neuroradiol. 2014;35(11):2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clajus C, Strasilla C, Fiebig T, Sychra V, Fiorella D, Klisch J. Initial and mid-term results from 108 consecutive patients with cerebral aneurysms treated with the WEB device. J Neurointervent Surg. 2017;9(4):411–417. [DOI] [PubMed] [Google Scholar]
- 14. Mine B, Goutte A, Brisbois D, Lubicz B. Endovascular treatment of intracranial aneurysms with the Woven EndoBridge device: mid term and long term results. J Neurointervent Surg. 2018;10(2):127–132. [DOI] [PubMed] [Google Scholar]
- 15. Caroff J, Mihalea C, Klisch J et al.. Single-layer WEBs: intrasaccular flow disrupters for aneurysm treatment—feasibility results from a European study. AJNR Am J Neuroradiol. 2015;36(10):1942–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lawson A, Molyneux A, Sellar R et al.. Safety results from the treatment of 109 cerebral aneurysms using the Woven EndoBridge technique: preliminary results in the United Kingdom. J Neurosurg. 2018;128(1):144–153. [DOI] [PubMed] [Google Scholar]
- 17. van Rooij SBT, Peluso JP, Sluzewski M, Kortman HG, van Rooij WJ. The new low-profile WEB 17 system for treatment of intracranial aneurysms: first clinical experiences. AJNR Am J Neuroradiol. 2018;39(5):859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tau N, Sadeh-Gonik U, Aulagner G, Turjman F, Gory B, Armoiry X. The Woven EndoBridge (WEB) for endovascular therapy of intracranial aneurysms: update of a systematic review with meta-analysis. Clin Neurol Neurosurg. 2018;166:110–115. [DOI] [PubMed] [Google Scholar]
- 19. Fiorella D, Molyneux A, Coon A et al.. Demographic, procedural and 30-day safety results from the WEB intra-saccular therapy study (WEB-IT). J Neurointervent Surg. 2017;9(12):1191–1196. [DOI] [PubMed] [Google Scholar]
- 20. Liebig T, Kabbasch C, Strasilla C et al.. Intrasaccular flow disruption in acutely ruptured aneurysms: a multicenter retrospective review of the use of the WEB. AJNR Am J Neuroradiol. 2015;36(9):1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Rooij WJ, Peluso JP, Bechan RS, Sluzewski M. WEB treatment of ruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2016;37(9):1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Popielski J, Berlis A, Weber W, Fischer S. Two-center experience in the endovascular treatment of ruptured and unruptured intracranial aneurysms using the WEB device: a retrospective analysis. AJNR Am J Neuroradiol. 2018;39(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asnafi S, Rouchaud A, Pierot L, Brinjikji W, Murad MH, Kallmes DF. Efficacy and safety of the Woven EndoBridge (WEB) device for the treatment of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37(12):2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabbasch C, Goertz L, Siebert E et al.. Treatment strategies for recurrent and residual aneurysms after Woven Endobridge implantation. J Neurointervent Surg. published online ahead of print: August 28, 2018. (doi:10.1136/neurintsurg-2018-014230).. [DOI] [PubMed] [Google Scholar]
- 25. Bhogal P, AlMatter M, Hellstern V et al.. The combined use of intraluminal and intrasaccular flow diversion for the treatment of intracranial aneurysms: report of 25 cases. Neurointervention. 2018;13(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aguilar Perez M, Bhogal P, Martinez Moreno R, Bazner H, Ganslandt O, Henkes H. The Medina Embolic Device: early clinical experience from a single center. J Neurointervent Surg. 2017;9(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turk AS, Maia O, Ferreira CC, Freitas D, Mocco J, Hanel R. Periprocedural safety of aneurysm embolization with the Medina Coil System: the early human experience. J Neurointervent Surg. 2016;8(2):168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sourour NA, Vande Perre S, Maria FD et al.. Medina(R) Embolization device for the treatment of intracranial aneurysms: safety and angiographic effectiveness at 6 months. Neurosurgery. 2018;82(2):155–162. [DOI] [PubMed] [Google Scholar]
- 29. Bhogal P, Brouwer PA, Yeo L, Svensson M, Soderman M. The Medina Embolic Device: Karolinska experience. Interv Neuroradiol. 2018;24(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwon SC, Ding YH, Dai D, Kadirvel R, Lewis DA, Kallmes DF. Preliminary results of the luna aneurysm embolization system in a rabbit model: a new intrasaccular aneurysm occlusion device. AJNR Am J Neuroradiol. 2011;32(3):602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Howe C, Lee Y, Cheon S, Yeo WH, Chun Y. Microstructured thin film nitinol for a neurovascular flow-diverter. Sci Rep. 2016;6:23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piotin M, Biondi A, Sourour N et al.. The LUNA aneurysm embolisation system for intracranial aneurysm treatment: short-term, mid-term and long-term clinical and angiographic results. J Neurointervent Surg. 2018;10(12):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]